Abstract

The reaction of either 2-aminophenol or 2-(N-methylamino)phenol with 1,2-difluoro-4,5-dinitrobenzene and sodium carbonate in EtOH gives 2,3-dinitrophenoxazines. One nitro group, conjugated to the aryl ether, was displaced from 2,3-dinitro-10-methylphenoxazine with different nucleophiles: BuNH2, KOEt, and KOH. The reaction of 2-aminothiophenol with 1,2-difluoro-4,5-dinitrobenzene under the same conditions gives 2,3-dinitrophenothiazine. This reacted with BuNH2 forming 2-butylamino-3-nitrophenothiazine. The dihedral angles of the different compounds are compared.
Introduction
Pentacene 1 has been investigated in organic electronics and device physics1,2 with fundamental studies on electronic3−7 and optical8−11 properties (Figure 1). However, it suffers from long-term stability because of its photo-oxidation and low thermal stability.12 To overcome these drawbacks and to provide alternative molecules with improved film stability, morphology, and processing properties, heteroatom-containing derivatives of pentacene, broadly known as N-heteroacenes13−15 and NOS-heteroacenes,16−18 have been investigated. There is much early work on the heterocyclic analogues of pentacene and related compounds (Figures 2–4).19−25
Figure 1.
Pentacene.
Figure 2.
Heterocyclic derivative of tetracene.
Figure 4.
Heterocyclic derivative of pentacene for potential material applications.28
Figure 2 shows a one-step heterocyclic synthesis of an analogue of tetracene using compound 2 and 2-aminophenol 3.26
Figure 3 shows a one-pot oxidation of 2-aminophenol 3 with K3Fe(CN)6 in dilute HCl under reflux27,28 to give a heterocyclic analogue of pentacene 5.27,28 The clean product crystals were filtered off from the aqueous system. This method and others give a product in which the polar amino and hydroxyl groups are condensed into the ring system or the middle part is capped on both ends. Further methods for making compound 5 involve the FeCl3 oxidation of phenoxazine followed by reaction with 2-aminophenol 3,29 2-aminophenol 3 with hydroxybenzoquinone,30 benzoquinone,31 2,5-dibromobenzoquinone,32 and dichloronaphthoquinone.33
Figure 3.
Heterocyclic derivative of pentacene for potential material applications.28
A similar method for making compound 5 has been reported for making compound 7 and its derivatives for organic electroluminescent materials and optoelectronic devices (Figure 4).28 Materials in this field are often exploited as organic transistors34−38 and light-emitting diodes.39,40
Results and Discussion
Previously, we reported the synthesis of the poorly stable phenazine derivative 10 using 4,5-difluoro-1,2-dinitrobenzene 9.41,42 This reaction failed with ortho-phenylenediamine 11 but worked with N-methyl-o-phenylenediamine 8 because the alkylated amine is more nucleophilic, which gets the reaction started followed by a cyclization (Figure 5).
Figure 5.
4,5-Difluoro-1,2-dinitrobenzene 9 for phenazine heterocyclic synthesis.
This article reports the first synthesis of phenoxazine and phenothiazine derivatives using 4,5-difluoro-1,2-dinitrobenzene 9. Poorly soluble heterocyclic acenes are difficult to make and characterize, so the compounds prepared in this paper are smaller and easier to work with. Figure 6 shows a reaction of 2-hydroxyaniline 3 and 2-hydroxy-N-methylaniline 12 with building block 9 in hot ethanol with Na2CO3 as a base. Phenoxazine 13 was formed in a lower yield of 32%, and methylated phenoxazine 14 was formed in a higher yield of 82%. The difference in yield suggests that the mechanistic pathway for these two reactions may be different. This is explained below.
Figure 6.
Synthesis of dinitrophenoxazines 13 and 14.
The use of Na2CO3 as a stirred suspension of base is similar to the Williamson ether synthesis using K2CO3 stirred in acetone to alkylate phenols.43 For compound 3, the phenol as phenoxide probably displaces fluoride first, followed by a fast intramolecular cyclization of the primary amine. For compound 12, the more nucleophilic N-methylamine probably displaces fluoride first, followed by a fast intramolecular cyclization of the phenol as phenoxide.
Compound 13 crystallizes in the orthorhombic space group P212121 with one molecule in the asymmetric unit (Figure 7). The fused-ring system is slightly puckered, and the dihedral angle between the C1–C6 and C7–C12 rings is 3.027 (14)°. Both the N2 and N3 nitro groups are significantly twisted away from the C1–C6 ring plane [dihedral angles = 43.64 (17)° and 31.67 (17)°, respectively], presumably to minimize steric repulsion between them [the dihedral angle between the nitro groups is 50.6 (5)°]. The C1–C6 and C3–C4 bond lengths (mean = 1.370 Å) are shorter than C5–C6 and C2–C3 (mean = 1.394 Å), possibly indicating some conjugation of the N1 lone pair electrons with the ring, and C2–N3 [1.450 (5) Å] is shorter than C1–N2 [1.469 (5) Å] for the same reason, although the effect is quite small. In the extended structure of 13, weak bifurcated N–H···(O,O) hydrogen bonds to the O atoms in the N2 nitro group link the molecules into [010] chains.
Figure 7.
Molecular structure of compound 13 showing 50% displacement ellipsoids.
The molecular structure of compound 14, which also crystallizes in the space group P212121 with one molecule in the asymmetric unit, is shown in Figure 8. Its fused-ring system shows significantly more puckering than that in 13, with a dihedral angle of 10.461 (16)° between the C1 and C7–C12 rings. The central phenoxazine ring is well described as a shallow boat, with N1 and O1 displaced from the mean plane of C4/C5/C7/C8 (r.m.s. deviation = 0.002 Å) by −0.161 (2) and −0.147 (2) Å, respectively. The N2 nitro group is rotated from its attached ring by 44.08 (9)°, and the N3 nitro group is even more twisted [dihedral angle = 76.64 (15)°]: the dihedral angle between the nitro groups is 46.05 (15)°. In the extended structure of 14, some weak C–H···O hydrogen bonds may help to consolidate the packing.
Figure 8.

Molecular structure of compound 14 showing 50% displacement ellipsoids.
Two ortho nitro groups activate each other to nucleophilic displacement.44 So far, in this synthesis, the fluoride groups are displaced first before the nitro groups. However, Figure 9 shows the selective displacement of just one of the ortho nitro groups with butylamine. The 2-nitro group is the most activated, and it is para to the aryl ether. The 3-nitro group is less easily displaced because it is conjugated to the electron-rich nitrogen lone pair.
Figure 9.
Synthesis of compound 15.
The crystal structure of compound 15, which was established from synchrotron data (Figure 10), shows the presence of two molecules (A containing C1 and B containing C18) in the asymmetric unit in the triclinic space group P1̅. The molecules have broadly similar conformations although the fused-ring system in A [dihedral angle between the outer rings = 10.08 (14)°] is rather more puckered than in B [4.93 (15)°]. The nitro group is close to the plane of its attached ring in both molecules [dihedral angles = 3.2 (2) and 6.9 (2)° for molecules A and B, respectively] and the n-butyl chain adopts an extended conformation in both A and B. In both molecules, an intramolecular N–H···O hydrogen bond closes an S(6) ring with H···O = 1.82 (4) Å and N–H···O = 142 (4)° for molecule A with equivalent data of 1.97 (4) Å and 139 (4)°, respectively, for molecule B. Some weak intermolecular C–H···O hydrogen bonds may help to consolidate the extended structure of 15.
Figure 10.

Molecular structure of compound 15 showing 50% displacement ellipsoids. The N–H···O hydrogen bonds are represented by double-dashed lines.
Not surprisingly, other nucleophiles displaced the 2-nitro group like butylamine did. Treatment of N-methyldinitrophenoxazine 14 with KOH/EtOH/H2O gave a high yield of compound 16 (Figure 11). TLC analysis showed no displacement by hydroxide to have occurred. This result was unexpected, as water is more acidic than EtOH and was expected to displace the fluoride atom. One explanation could be deactivation of the two nitro groups by the electron-rich benzene ring, so that an equilibrium amount of ethoxide is required for the reaction.
Figure 11.
Synthesis of compound 16.
Compound 16 crystallizes with two molecules (A containing C1 and B containing C16) in the asymmetric unit (Figure 12) in the monoclinic space group P21/n. Their conformations are similar to a dihedral angle of 2.74 (2)° between the C1–C6 and C7–C12 rings in molecule A and an equivalent angle of 6.372 (14)° for the C16–C21 and C22–C27 rings in molecule B. The plane of the nitro group lies close to its attached ring in both molecules [dihedral angles = 6.98 (5) and 17.08 (5)° for molecules A and B, respectively], and the C2–O2–C13-C14 and C17–O6–C28–C29 torsion angles are −173.24 (8) and −174.01 (8)°, respectively. In the extended structure of compound 16, some weak C–H···O hydrogen bonds link the molecules.
Figure 12.

Molecular structure of compound 16 showing 50% displacement ellipsoids.
Since in mixtures of KOH/EtOH/H2O, KOEt acted as a nucleophile toward compound 14, KOH/DMSO/H2O was used to favor KOH as a nucleophile (Figure 13). DMSO has a much higher pKa than EtOH, so KOH is more likely to be the soluble base. This proved to be the case, but the yield of compound 17 is much lower.
Figure 13.
Synthesis of compound 17.
Compound 17 crystallizes with one molecule in the asymmetric unit in the triclinic space group P1̅ (Figure 14). The dihedral angle between the outer rings is 4.23 (9)°, and the heterocyclic ring is a very shallow boat, with N1 and O1 displaced by 0.054 (4) and 0.070 (3) Å, respectively, from the four carbon atoms (rms deviation = 0.014 Å). The N2 nitro group is rotated by 6.7 (3)° from its attached ring, and an intramolecular O–H···O hydrogen bond closes an S(6) ring with H···O = 1.84 (3) Å and O–H···O = 154 (3)°. Some weak C–H···O hydrogen bonds occur in the extended structure.
Figure 14.

Molecular structure of compound 17 showing 50% displacement ellipsoids. The O–H···O hydrogen bond is indicated by a double-dashed line.
Thiols and thiolate anions are good nucleophiles, so we expected 2-aminothiophenol 18 to react efficiently with compound 9 provided the fluorine atoms displace first (Figure 15). The thiophenazine heterocycle 19 forms in an 81% yield. Presumably, the thiol or thiolate anion displaces fluorine first followed by an intramolecular cyclization of the primary amine.
Figure 15.
Synthesis of compound 19.
Compound 19 (Figure 16) crystallizes with one molecule in the asymmetric unit in the space group P212121. The phenothiazine fused-ring system in compound 19 is significantly puckered compared to those of the phenoxazines described above, with a dihedral angle of 23.13 (3)° between the C1–C6 and C7–C12 rings. This compares with a dihedral angle of 21.5° between the outer rings in phenothiazine, C12H8NS.45,46 The central heterocycle in compound 19 is a distorted boat with atoms N1 and S1 displaced by −0.245 (2) and −0.503 (2) Å, respectively, from C4/C5/C7/C8 (rms deviation = 0.007 Å). The N2 and N3 nitro groups are rotated by 21.02 (11) and 51.63 (6)°, respectively, with respect to their attached ring. In the crystal, a N–H···O hydrogen bond with H···O = 2.26 (2) Å and N–H···O = 166 (2)° generates [001] chains.
Figure 16.

Molecular structure of compound 19 showing 50% displacement ellipsoids.
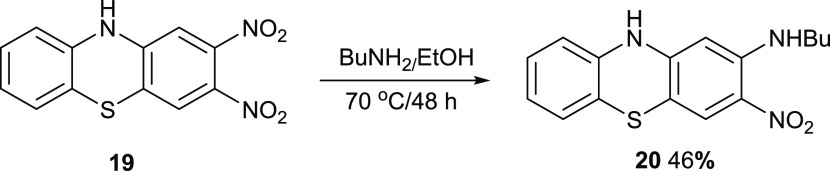
The 2-nitro group can be displaced with butylamine as for compound 14, but the heterocyclic ring system is more robust, so the reaction required 48 h (Figure 17).
Figure 17.
Synthesis of compound 20.
Compound 20 crystallizes with two phenothiazine molecules (A containing C1 and B containing C17) in the asymmetric unit accompanied by a dichloromethane solvent molecule (Figure 18) in the monoclinic space group P21/c. The molecules have similar conformations, with a dihedral angle between the outer rings of 14.2 (3)° for A and 13.6 (3)° for B. The near-coplanarity of the nitro group with its attached ring [dihedral angles = 1.9 (6) and 1.7 (6)° for molecules A and B, respectively] is reinforced by the formation of an intramolecular N–H···O hydrogen bond with H···O = 1.97 Å for A and 1.95 Å for B and N–H···O = 131 and 130°, respectively. In the extended structure of compound 20, N–H···O hydrogen bonds link the molecules into [010] chains, which are reinforced by weak C–H···O links. The solvent molecules occupy small [100] channels.
Figure 18.

Molecular structure of compound 20 showing 50% displacement ellipsoids. The N–H···O hydrogen bonds are indicated by double-dashed lines.
Conclusions
The synthetic use of 1,2-difluoro-4,5-dinitrobenzene 9 for making heterocycles has been extended. 2,3-Dinitrophenoxazine 13 and 2,3-dinitrophenothiazine 19 are easily formed by reacting 2-aminophenol 3 or 2-aminothiophenol 18, respectively, with 1,2-difluoro-4,5-dinitrobenzene 9 using Na2CO3 as a base in hot EtOH. The use of 2-hydroxy-N-methylaniline 12 gave phenoxazine 14 in a high yield of 80%, presumably because the N-alkyl amino group is more nucleophilic, compared to aniline, and displaces fluorine rapidly. The two nitro groups of phenazine 14 or phenothiazine 19 activate each other, and one nitro group can be displaced with butylamine to give phenazine 15 and phenothiazine 20, respectively. The nitro group para to the aryl ether is displaced in preference to the one para to the more electron-rich amino group. Phenoxazine 14 gives an unusual reaction with KOH/EtOH/H2O, forming 2-ethoxy-3-nitro-10-methylphenoxazine 16. An equilibrium concentration of ethoxide must displace the 2-nitro group rather than hydroxide. With KOH/DMSO/H2O, hydroxide displaces the 2-nitro group from phenoxazine 14 as expected to give 2-hydroxy-3-nitro-10-methylphenoxazine 17. The dihedral angles for each compound are compared. For 2,3-dinitrophenothiazine, it is 23°, which is larger than that for the phenoxazines studied. These fall in the range of 3–10°.
Experimental Section
IR spectra were recorded on a thermoscientific Nicolet
Summit diamond-attenuated
total reflection (ATR) Fourier transform infrared (FTIR) spectrometer.
Ultraviolet (UV) spectra were recorded by using a PerkinElmer Lambda
25 UV–vis spectrometer with EtOH as the solvent. The term “sh”
means shoulder. 1H and 13C nuclear magnetic
resonance (NMR) spectra were recorded at 400 and 100.5 MHz, respectively,
by using a Bruker 400 spectrometer. Chemical shifts, δ, are
given in ppm and measured by comparison with the residual solvent.
Coupling constants, J, are given in Hz. A broad signal
is abbreviated as br. High-resolution mass spectra were obtained at
the University of Wales, Swansea, using an Atmospheric Solids Analysis
Probe (ASAP) (positive mode) instrument: Xevo G2-S ASAP. Melting points
were determined on a U.K. Cole-Palmer Stuart microscope.
2,3-Dinitro-10H-phenoxazine 13
4,5-Difluoro-1,2-dinitrobenzene
(100 mg, 0.49 mmol) in EtOH (30 mL) was mixed with 2-aminophenol (54
mg, 0.49 mmol) and Na2CO3 (500 mg) and then
stirred at 70 °C for 6 h. After cooling, the mixture was added
to water (200 mL), left standing for 1 h, filtered through a sinter
grade No. 4, and air-dried to give the title compound (43 mg, 32%) as pure red crystals; mp >245 °C (from dichloromethane:light
petroleum ether). λmax (EtOH)/nm 283 (log ε
4.3), 378 (3.8) and 468 (3.9); νmax (diamond)(cm–1) 3362w, 1633w, 1575s, 1514s, 1494s, 1450s, 1396s,
1314s, 1208s, 869s, 826s, 746s and 491s; δH (400
MHz; D7DMF) 6.80 (1H, d, J = 8.0), 6.89
(1H, d, J = 8.0), 6.97 (1H, t, J = 8.0 and 8.0), 7.04 (1H, t, J = 8.0 and 8.0),
7.12 (1H, s) and 7.51 (1H, s); δC (100.1 MHz; D7DMF) 108.0, 112.1, 115.2, 116.0, 123.8, 125.5, 129.3, 132.9,
140.0, 142.2, 142.7 and 144.9; m/z (Orbitrap ASAP) 274.0459 (M+ + H) C12H7N3O5H requires 274.0464.
2,3-Dinitro-N-methylphenoxazine 14
4,5-Difluoro-1,2-dinitrobenzene
(332 mg, 1.6 mmol) in EtOH
(30 mL) was mixed with 2-(methylamino)phenol (200 mg, 1.6 mmol) and
Na2CO3 (2.0 g) and then stirred at 70 °C
for 20 h. After cooling, the mixture was added to water (200 mL),
left standing for 1 h, filtered through a sinter grade No. 4, and
air-dried to give the title compound (383 mg, 82%)
as pure red crystals, mp 164–165 °C (from dichloromethane:light
petroleum ether). λmax (EtOH)/nm 283 (log ε
3.9), 373 (3.4) and 463 (3.5); νmax (diamond)(cm–1) 3091w, 1525s, 1491s, 1370s, 1329s, 1293s, 1220,
1202, 1064s, 870s, 824s and 741s; δH (400 MHz; CDCl3) 3.14 (3H, s), 6.67 (3H, d, J = 8.0), 6.91
(2H, J = 8.0) and 7.24 (1H, d, J = 8.0); δC (100.1 MHz; CDCl3) 31.8,
106.2, 111.6, 112.9, 116.1, 124.0, 125.4, 131.1, 134.6, 139.9, 141.2,
143.9 and 146.9; m/z (Orbitrap ASAP)
288.0612 (M+ + H, 100%) C13H9N3O5H requires 288.0620.
2-Butylamino-3-nitro-N-methylphenoxazine 15
N-Methyl-3,4-dinitrophenoxazine
(50 mg, 0.17 mmol) in EtOH (30 mL) was mixed with butylamine (61 mg,
0.69 mmol) and heated at 70 °C for 20 h. After cooling, the mixture
was mixed with water (170 mL) and filtered, and the precipitate was
purified by chromatography. Elution with DCM gave the title
compound (18 mg, 34%) as red crystals, mp 147–148
°C (from dichloromethane). λmax (EtOH)/nm 244
(log ε 3.2), 284 (2.6), 394 (2.5) and 484 (2.8); νmax (diamond)(cm–1) 2956w, 2931w, 2865w,
1939s, 1608s, 1575s, 1484s, 1470s, 1400s, 1335s, 1248s, 1225s, 1195s,
1169s, 1041s and 733s; δH (400 MHz; D7DMF) 0.97 (3H, t, J = 7.0), 1.46 (2H, s, J = 7.0), 1.72 (2H, q, J = 7.0), 3.35 (3H,
s), 3.46 (2H, q, J = 7.0), 6.12 (1H, s), 6.86 (1H,
d, J = 8.0), 6.92 (1H, t, J = 8.0
and 8.0), 6.98–7.07 (2H, m), 7.33 (1H, s) and 8.69 (1H, s,
br, NH); δC (100.1 MHz; D7DMF) 13.3, 20.1,
30.9, 31.5, 42.4, 93.6, 109.1, 113.9, 115.6, 122.4, 123.2, 124.1,
130.8, 135.6, 142.9, 144.1, and 146.4; m/z (Orbitrap ASAP) 314.1510 (M+ + H, 100%) C17H19N3O3H requires 314.1505.
2-Ethoxy-3-nitro-N-methylphenoxazine 16
(20 mg, 0.0697
mmol) was mixed with EtOH (18 mL)
and an aqueous base (90 mg of KOH in 1 mL of water). The mixture was
heated at 70 °C for 20 h with stirring. After cooling, the mixture
was neutralized with dilute hydrochloric acid (5 mL of cHCl in 100
mL water) and then filtered to give the title compound (18 mg, 90%) as orange crystals; mp 175–176 °C (from
dichloromethane:light petroleum ether). The NMR data had minor traces
of impurities, so for NMR, the sample was purified by chromatography
and eluted with DCM. λmax (EtOH)/nm 224 (log ε
3.7), 282 (3.4), and 438 (3.4); νmax (diamond)(cm–1) 2987w, 1634s, 1579s, 1532s,1489s, 1367s, 1323s,
1263s, 1078s, 1046s, 1023s, 922s, 870s, 806s and 733s; δH (400 MHz; D7DMF) 1.58 (3H, t, J = 8.0), 3.46 (1H, s), 4.47 (2H, q, J = 8.0), 6.74
(1H, s), 6.98 (1H, dd, J = 8.0 and 2.0), 7.06 (1H,
dt, J = 8.0 and 2.0), 7.09–7.17 (2H, m) and
7.50 (1H, s); δC (100.1 MHz; CDCl3) 14.3,
31.6, 65.9, 98.5, 111.4, 113.4, 115.4, 123.2, 124.6, 129.6, 132.1,
137.2, 141.7, 144.5 and 152.7; m/z (Orbitrap ASAP) 287.1027 (M+ + H, 100%) C15H14N2O4H requires 287.1032.
2-Hydroxy-3-nitro-N-methylphenoxazine 17
N-Methyl-3,4-dinitrophenoxazine
(50 mg, 0.17 mmol) was mixed with DMSO (5 mL) and water (1 mL of water
with 20 mg of KOH) and then heated at 70 °C for 1.5 h with stirring.
The mixture was diluted with water (200 mL), extracted with DCM (50
mL), backwashed with water (100 mL), dried over MgSO4,
and purified by chromatography. Elution with DCM gave the title compound (12 mg, 27%) as red crystals; mp 210–211
°C (from dichloromethane:light petroleum ether). λmax (EtOH)/nm 226 (log ε 4.4), 285(3.6) and 475(3.8);
νmax (diamond)(cm–1) 2926w,1640w,
1607w, 1574w, 1488s, 1419s, 1398s, 1349s, 1242s, 1197s, 1116s, 1084s,
1039s, 855s, 811s, 742s, 698s, 677s, 652s, 458s and 438s; δH (400 MHz; D7DMF) 3.48 (3H, s), 6.62 (1H, s), 7.02
(1H, d, J = 8.0), 7.10 (1H, td, J = 8.0 and 4.0), 7.15–7.22 (2H, m), 7.42 (1H, s) and 11.48–11.41
(1H, s, br); δC (100.1 MHz; D7DMF) 31.9,
100.1, 108.5, 114.2, 115.7, 123.8, 124.5, 125.4, 130.8, 138.1, 144.0,
144.3 and 155.4; m/z (Orbitrap ASAP)
259.0716 (M+ + H, 100%) C13H10N2O4H requires 259.0719.
2,3-Dinitrophenothiazine 19
4,5-Difluoro-1,2-dinitrobenzene 9 (110 mg, 0.539 mmol) in EtOH (30 mL) was mixed with 2-aminothiophenol 15 (68 mg, 0.54 mmol) and Na2CO3 (500
mg) and then stirred at 70 °C for 20 h. After cooling, the mixture
was added to water (200 mL), left standing for 1 h, filtered through
a sinter grade No. 4, and air-dried to give the title compound (126 mg, 81%) as dark red crystals, mp 226–227 °C (from
dichloromethane:light petroleum ether). λmax (EtOH)/nm
233 (log ε 4.1), 296 (3.9) and 476 (3.3); νmax (diamond)(cm–1) 3327s, 1600s, 1568s,
1527s, 1568s, 1527s, 1500s, 1474s, 1354s, 1295s, 1264s, 1230s, 1150s,
1106s, 883s, 847s, 821s and 753s; δH (400 MHz; CDCl3) 6.93 (1H, d, J = 8.0), 7.08–7.17
(2H, m), 7.25 (1H, d, J = 8.0), 7.28 (1H, s), 7.97
(1H, s) and 10.04 (1H, NH, br); δC (100.1 MHz; D7DMF) 108.9, 115.2, 116.4, 122.1, 123.6, 124.9, 126.7, 128.9,
134.4, 138.5, 144.3 and 147.8; m/z (Orbitrap ASAP) 290.0243 (M+ + H, 100%) C12H7N3O4SH requires 290.0236.
2-Butylamino-3-nitrophenothiazine 20
3,4-Dinitrophenothiazine 19 (80 mg, 0.27 mmol) in EtOH (30 mL) was mixed with butylamine (61 mg, 0.69 mmol) and heated at 70 °C for 48 h. After cooling, the mixture was mixed with water (170 mL) and filtered, and the precipitate was purified by chromatography. Elution with DCM gave the title compound (39 mg, 46%) as dark red crystals, mp 242–243 °C (from dichloromethane:light petroleum ether). λmax (EtOH)/nm 261(log ε 4.3), 306(3.8) 403sh(3.5) and 481(3.8); νmax (diamond)(cm–1) 3280w, 3199w, 3147w, 2952w, 2928w, 2861w, 1616s, 1591s, 1553s, 1464s, 1451s, 1418s, 1406s, 1330s, 1298s, 1262s, 1231s, 1169s, 1067s, 877s, 832s, 729s, 674s, 532s and 489s; δH (400 MHz; CDCl3) 1.13 (3H, t, J = 8.0), 1.60 (2H, s, J = 8.0), 1.84 (2H, q, J = 8.0), 3.49 (2H, q, J = 8.0), 6.41 (1H, s), 7.02 (1H, d, J = 8.0), 7.07 (1H, t, J = 8.0), 7.21 (1H, d, J = 8.0), 7.25 (1H, t, J = 8.0), 7.86 (1H, s), 8.63 (1H, s, br, NH) and 9.91 (1H, s, NH); δC (100.1 MHz; D7DMF) 13.5, 20.1, 30.8, 42.5, 95.4, 106.0, 115.9, 116.9, 123.1, 123.3, 126.2, 126.3, 127.8, 138.3, 147.6, and 148.7 (one aliphatic peak is missing); m/z (Orbitrap ASAP) 316.1118 (M+ + H, 100%) C16H17N3O2SH requires 316.1120.
Crystal Structures
The crystal structures of compounds 13–17, 19, and 20 were established using intensity data collected on a Rigaku CCD diffractometer at 100 K. The structures were routinely solved by dual-space methods using SHELXT,47 and the structural models were completed and optimized by refinement against |F|2 with SHELXL-2019.48 The O- and N-bound hydrogen atoms (if any) were located in difference maps, and their positions were freely refined, except for compound 20, where they were geometrically placed. The C-bound hydrogen atoms were placed in idealized locations (C–H = 0.95–0.99 Å) and were refined as riding atoms. The methyl groups (if any) were allowed to rotate, but not to tip, to best fit the electron density. The constraint Uiso(H) = 1.2Ueq(carrier) or 1.5Ueq(methyl carrier) was applied in all cases. The data quality for compound 20 is poor, but the structure has been unambiguously established. Full details of the structures and refinements are available in the deposited cifs.
Crystal data for compound 13 C12H7N3O5, red plate 0.23 × 0.14 × 0.03 mm3, Mr = 273.21, orthorhombic, space group P212121 (No. 19), a = 6.6145 (4) Å, b = 11.2990 (6) Å, c = 14.7681 (9) Å, V = 1103.73 (11) Å3, Z = 4, T = 100 K, Cu Kα radiation, λ = 1.54178 Å, μ = 1.132 mm–1, ρcalc = 1.644 g cm–3, 5772 reflections measured (9.9 ≤ 2θ ≤ 140.1°), 2001 unique (RInt = 0.073), R(F) = 0.064 [1722 reflections with I > 2σ(I)], wR(F2) = 0.177 (all data), Δρmin,max (e Å–3) = −0.33, + 0.45, Flack absolute structure parameter 0.0 (4), CCDC deposition number 2291273.
Crystal data for compound 14 C13H9N3O5, orange lath 0.30 × 0.08 × 0.02 mm3, Mr = 287.23, orthorhombic, space group P212121 (No. 19), a = 6.73506 (4) Å, b = 10.46527 (8) Å, c = 16.90115 (15) Å, V = 1191.263 (16) Å3, Z = 4, T = 100 K, Cu Kα radiation, λ = 1.54178 Å, μ = 1.079 mm–1, ρcalc = 1.602 g cm–3, 35898 reflections measured (9.9 ≤ 2θ ≤ 153.8°), 2411 unique (RInt = 0.067), R(F) = 0.030 [2384 reflections with I > 2σ(I)], wR(F2) = 0.086 (all data), Δρmin,max (e Å–3) = −0.23, + 0.15, Flack absolute structure parameter 0.09 (7), CCDC deposition number 2291274.
Crystal data for compound 15 C17H19N3O3, orange lath 0.08 × 0.05 × 0.03 mm3, Mr = 313.35, triclinic, space group P1̅ (No. 2), a = 7.2750 (3) Å, b = 12.8080 (5) Å, c = 16.1101 (6) Å, α = 78.021 (3)°, β = 87.636 (3)°, γ = 85.439 (3)°, V = 1463.30 (10) Å3, Z = 4, T = 100 K, synchrotron radiation, λ = 0.6889 Å, μ = 0.093 mm–1, ρcalc = 1.422 g cm–3, 16071 reflections measured (3.2 ≤ 2θ ≤ 47.0°), 4712 unique (RInt = 0.169), R(F) = 0.090 [2866 reflections with I > 2σ(I)], wR(F2) = 0.259 (all data), Δρmin,max (e Å–3) = −0.37, + 0.32, CCDC deposition number 2291275.
Crystal data for compound 16 C15H14N2O4, orange slab 0.32 × 0.19 × 0.05 mm3, Mr = 286.28, monoclinic, space group P21/n (No. 14), a = 13.67695 (14) Å, b = 13.86021 (15) Å, c = 13.70349 (13) Å, β = 98.2282 (10)°, V = 2570.97 (5) Å3, Z = 8, T = 100 K, Cu Kα radiation, λ = 1.54178 Å, μ = 0.909 mm–1, ρcalc = 1.479 g cm–3, 74062 reflections measured (8.5 ≤ 2θ ≤ 149.5°), 5214 unique (RInt = 0.022), R(F) = 0.033 [4809 reflections with I > 2σ(I)], wR(F2) = 0.100 (all data), Δρmin,max (e Å–3) = −0.26, + 0.29, CCDC deposition number 2291276.
Crystal data for compound 17 C13H10N2O4, red plate 0.12 × 0.05 × 0.02 mm3, Mr = 258.23, triclinic, space group P1̅ (No. 2), a = 7.8187 (8) Å, b = 8.3024 (9) Å, c = 9.1432 (8) Å, α = 79.273 (8)°, β = 81.368 (8)°, γ = 67.723 (10)°, V = 537.56 (10) Å3, Z = 2, T = 100 K, Cu Kα radiation, λ = 1.54178 Å, μ = 1.018 mm–1, ρcalc = 1.595 g cm–3, 9116 reflections measured (11.6 ≤ 2θ ≤ 149.7°), 2139 unique (RInt = 0.038), R(F) = 0.060 [1702 reflections with I > 2σ(I)], wR(F2) = 0.186 (all data), Δρmin,max (e Å–3) = −0.38, + 0.38, CCDC deposition number 2291277.
Crystal data for compound 19 C12H7N3O4S, black prism 0.59 × 0.19 × 0.11 mm3, Mr = 289.27, orthorhombic, space group P212121 (No. 19), a = 6.83300 (12) Å, b = 11.01073 (17) Å, c = 15.6038 (2) Å, V = 1173.97 (3) Å3, Z = 4, T = 100 K, Mo Kα radiation, λ = 0.71073 Å, μ = 0.294 mm–1, ρcalc = 1.637 g cm–3, 38956 reflections measured (4.5 ≤ 2θ ≤ 66.4°), 4233 unique (RInt = 0.055), R(F) = 0.030 [4038 reflections with I > 2σ(I)], wR(F2) = 0.076 (all data), Δρmin,max (e Å–3) = −0.24, + 0.40, Flack absolute structure parameter 0.40 (6), CCDC deposition number 2291278.
Crystal data for compound 20 2(C16H17N3O2S)·CH2Cl2, orange plate 0.39 × 0.31 × 0.04 mm3, Mr = 715.70, monoclinic, space group P21/c (No. 14), a = 7.4727 (2) Å, b = 15.1810 (5) Å, c = 29.2495 (10) Å, β = 94.148 (3)°, V = 3309.46 (18) Å3, Z = 4, T = 100 K, Cu Kα radiation, λ = 1.54178 Å, μ = 3.344 mm–1, ρcalc = 1.436 g cm–3, 30254 reflections measured (6.1 ≤ 2θ ≤ 147.7°), 6518 unique (RInt = 0.073), R(F) = 0.117 [5139 reflections with I > 2σ(I)], wR(F2) = 0.326 (all data), Δρmin,max (e Å–3) = −0.88, + 1.49, CCDC deposition number 2291279.
Acknowledgments
The authors thank the U.K. EPSRC National Mass Spectrometry Service Centre for mass spectrometric data and the U.K. National Crystallography Centre (University of Southampton) for the X-ray data collections. M.J.P. performed all synthesis and obtained the characterization data, and W.T.A.H. solved the crystallographic data sets. Data sets were obtained free of charge from the National Crystallography Center, Southampton University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06461
The proton and carbon NMR data for all compounds in the Experimental Section (Figures S1–S19) (PDF)
Accession Codes
CCDC 2291273 (compound 13), CCDC 2291274 (compound 14), CCDC 2291275 (compound 15), CCDC 2291276 (compound 16), CCDC 2291277 (compound 17), CCDC 2291278 (compound 19), and CCDC 2291279 (compound 20) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data request/cif.
The authors declare no competing financial interest.
Supplementary Material
References
- Klauk H.; Zschieschang U.; Pflaum J.; Halik M. Ultralow-power organic complementary circuits. Nature 2007, 445, 745–748. 10.1038/nature05533. [DOI] [PubMed] [Google Scholar]
- Kitamura M.; Arakawa Y. Pentacene-based organic field-effect transistors. J. Phys.: Condens. Matter 2008, 20, 184011 10.1088/0953-8984/20/18/184011. [DOI] [Google Scholar]
- Brédas J. L.; Calbert J. P.; da Silva Filho D. A.; Cornil J. Organic semiconductors: a theoretical characterization of the basic parameters governing charge transport. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 5804–5809. 10.1073/pnas.092143399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C.; Silbey R. J.; da Silva Filho D. A.; Calbert J. P.; Cornil J.; Bredas J. L. Three-dimensional band structure and band like mobility in oligoacene single crystals: A theoretical investigation. J. Chem. Phys. 2003, 118, 3764. 10.1063/1.1539090. [DOI] [Google Scholar]
- Hummer K.; Ambrosch-Draxl C. Electronic properties of oligoacenes from first principles. Phys. Rev. B: Condens. Matter Mater. Phys. 2005, 72, 205205 10.1103/PhysRevB.72.205205. [DOI] [Google Scholar]
- Nabok D.; Puschnig P.; Ambrosch-Draxl C.; Werzer O.; Resel R.; Smilgies D.-M. Crystal and electronic structures of pentacene thin films from grazing-incidence X-Ray diffraction and first principles calculations. Phys. Rev. B: Condens. Matter Mater. Phys. 2007, 76, 235322 10.1103/PhysRevB.76.235322. [DOI] [Google Scholar]
- Sharifzadeh S.; Biller A.; Kronik L.; Neaton J. B. Quasiparticle and optical spectroscopy of the organic semiconductors pentacene and PTCDA from first principles. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 85, 125307 10.1103/PhysRevB.85.125307. [DOI] [Google Scholar]
- Tiago M. L.; Northrup J. E.; Louie S. G. Ab initio calculation of the electronic and optical properties of solid pentacene. Phys. Rev. B: Condens. Matter Mater. Phys. 2003, 67, 115212 10.1103/PhysRevB.67.115212. [DOI] [Google Scholar]
- Hummer K.; Ambrosch-Draxl C. Oligoacene exciton binding energies: their dependence on molecular size. Phys. Rev. B: Condens. Matter Mater. Phys. 2005, 71 (R), 081202 10.1103/PhysRevB.71.081202. [DOI] [Google Scholar]
- Ambrosch-Draxl C.; Nabok D.; Puschnig P.; Meisenbichler C. The role of polymorphism in organic thin films: oligoacenes investigated from first principles. New J. Phys. 2009, 11, 125010 10.1088/1367-2630/11/12/125010. [DOI] [Google Scholar]
- Rangel T.; Berland K.; Sharifzadeh S.; Brown-Altvater F.; Lee K.; Hyldgaard P.; Kronik L.; Neaton J. B. Structural and excited state properties of oligoacene crystals from first principles. Phys. Rev. B 2016, 93, 115206 10.1103/PhysRevB.93.115206. [DOI] [Google Scholar]
- Maliakal A.; Raghavachari K.; Katz H.; Chandross E.; Siegrist T. Photochemical stability of pentacene and a substituted pentacene in solution and in thin films. Chem. Mater. 2004, 16, 4980–4986. 10.1021/cm049060k. [DOI] [Google Scholar]
- Kollmann B.; Chen Z.; Lüftner D.; Siri O.; Puschnig P. Synthesis and combined experimental and theoretical characterization of dihydro-tetraaza-acenes. J. Phys. Chem. C 2018, 122, 6475–6482. 10.1021/acs.jpcc.8b00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q.; Nguyen T.-Q.; Someya T.; Blanchet G. B.; Nuckolls C. Synthesis, assembly, and thin film transistors of dihydrodiazapentacene: an isostructural motif for pentacene. J. Am. Chem. Soc. 2003, 125, 10284–10287. 10.1021/ja036466+. [DOI] [PubMed] [Google Scholar]
- Bunz U. H. F. The larger N-heteroacenes. Pure Appl. Chem. 2010, 82, 953–968. 10.1351/PAC-CON-09-09-17. [DOI] [Google Scholar]
- Guo H.-S.; Liu M.-F.; Han Y.; Han S.; Chen Y.-L. Synthesis and characterisation of S,N-heteroacenes by Bischler-Napieralski reaction. Chin. J. Polym. Sci. 2016, 34, 1319–1329. 10.1007/s10118-016-1846-9. [DOI] [Google Scholar]
- Vogt A.; Henne F.; Wetzel C.; Mena-Osteritz E.; Bauerle P. Synthesis and characterization of S,N-heterotetracenes. Beilstein J. Org. Chem. 2020, 16, 2636–2644. 10.3762/bjoc.16.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier E.; Wetzel C.; Bauer M.; Mena-Osteritz E.; Wunderlin M.; Bauerle P. S,N-Heteroacenes up to a tridecamer. Chem. Mater. 2019, 31, 7007–7023. 10.1021/acs.chemmater.9b01652. [DOI] [Google Scholar]
- Fischer O.; Hepp E. Ueber die fluorindine. Ber. Dtsch. Chem. Ges. 1890, 23, 2789–2793. 10.1002/cber.189002302190. [DOI] [Google Scholar]
- Fischer G. Ueber einen neuen farbstoff aus orthoamidophenol. J. Prakt. Chem. 1879, 19, 317–321. 10.1002/prac.18790190129. [DOI] [Google Scholar]
- Fischer O.; Trost J. Ueber oxydation von aminbasen mit natriumsuperoxyd. Ber. Dtsch. Chem. Ges. 1893, 26, 3083–3085. 10.1002/cber.189302603149. [DOI] [Google Scholar]
- Fischer O.; Jonas O. Beitrag zur oxydation der aromatischen orthodiamine und orthoamidophenole. Ber. Dtsch. Chem. Ges. 1894, 27, 2782–2785. 10.1002/cber.18940270329. [DOI] [Google Scholar]
- Fischer O.; Hepp E. Ueber die fluorindine II. Ber. Dtsch. Chem. Ges. 1895, 28, 293–301. 10.1002/cber.18950280170. [DOI] [Google Scholar]
- Mills C. XCIII.-Some new azo-compounds. J. Chem. Soc., Trans. 1895, 67, 925–933. 10.1039/CT8956700925. [DOI] [Google Scholar]
- Diepolder E. Ueber methyl-o-anisidin, methyl-o aminophenol und dessen oxydationsproduct (N-methylphenoxazin-o-Chinon). Ber. Dtsch. Chem. Ges. 1899, 32, 3514–3528. 10.1002/cber.189903203131. [DOI] [Google Scholar]
- Tauer E.; Grellmann K.-H.; Kaufmann E.; Noltemeyer M. The condensation product of 2-aminophenol and glyoxal. Structure and photochemistry. Ber. Dtsch. Chem. Ges. 1986, 119, 3316–3325. 10.1002/cber.19861191111. [DOI] [Google Scholar]
- Seidel P. Triphendioxazin als oxydationsproduct des orthoamidophenols. Ber. Dtsch. Chem. Ges. 1890, 23, 182–189. 10.1002/cber.18900230136. [DOI] [Google Scholar]
- Xiangcheng W.; Ying L.; Hongyang R.; Wei H.; Chen L.. Organic electroluminescent material and organic optoelectronic device. US2017256717A1 and CN106831817A.
- Khalifa M. J. Chem. Soc. 1960, 2779–2780. [Google Scholar]
- Kehrmann F.; Cherpillod F. Neue synthesen in der gruppe der Chinon-imid-farbstoffe. V. uber synthesen ausgehend von oxybenzochinon. Helv. Chim. Acta 1924, 7, 973–980. 10.1002/hlca.192400701122. [DOI] [Google Scholar]
- Bolognese A.; Scherillo G.; Schaefer W. Reaction of o-aminophenol and p-benzoquinone in acetic acid. J. Heterocycl. Chem. 1986, 23, 1003–1006. 10.1002/jhet.5570230406. [DOI] [Google Scholar]
- Okoro U. C.; Ezeokonkwo M. A.; Utime A.; Godwin-Nwakwasi E. U.; Ibeanu F. N.; Okafor S. N. Synthesis of functionalized triphenodioxazines via palladium catalyzed cross-coupling reactions. Asian J. Chem. 2018, 30, 2317–2321. 10.14233/ajchem.2018.21477. [DOI] [Google Scholar]
- Agarwal N. L.; Schaefer W. Quinone chemistry. Reaction of 2,3-dichloro-1,4-naphthoquinone with o-aminophenols under various conditions. J. Org. Chem. 1980, 45, 2155–2161. 10.1021/jo01299a024. [DOI] [Google Scholar]
- Wang N.; Zhao Z.; Ding T.; Liu W.; Ahmed A. S.; Wang Z.; Tian M.; Sun X. W.; Zhang Q. Improving interfacial charge recombination in planar heterojunction perovskite photovoltaics with small molecule as electron transport layer. Adv. Energy Mater. 2017, 7, 1700522 10.1002/aenm.201700522. [DOI] [Google Scholar]
- Gu P.-Y.; Wang N.; Wang C.; Zhou Y.; Long G.; Tian M.; Chen W.; Sun X. W.; Kanatzidis M. G.; Zhang Q. Pushing up the efficiency of planar perovskite solar cells to 18.2% with organic small molecules as the electron transport layer. J. Mater. Chem. A 2017, 5, 7339–7344. 10.1039/C7TA01764B. [DOI] [Google Scholar]
- Gu P.-Y.; Wang N.; Wu A.; Wang Z.; Tian M.; Fu Z.; Sun X. W.; Zhang Q. An azaacene derivative as promising electron-transport layer for inverted perovskite solar cells. Chem. - Asian J. 2016, 11, 2135–2138. 10.1002/asia.201600856. [DOI] [PubMed] [Google Scholar]
- Miao Q. Ten years of N-heteropentacenes as semiconductors for organic thin-film transistors. Adv. Mater. 2014, 26, 5541–5549. 10.1002/adma.201305497. [DOI] [PubMed] [Google Scholar]
- Endres A. H.; Schaffroth M.; Paulus F.; Reiss H.; Wadepohl H.; Rominger F.; Kramer R.; Bunz U. H. F. Coronene-containing N-heteroarenes: 13 rings in a row. J. Am. Chem. Soc. 2016, 138, 1792–1795. 10.1021/jacs.5b12642. [DOI] [PubMed] [Google Scholar]
- Biegger P.; Stolz S.; Intorp S. N.; Zhang Y.; Engelhart J. U.; Rominger F.; Hardcastle K. I.; Lemmer U.; Qian X.; Hamburger M.; Bunz U. H. F. Soluble diazaiptycenes: materials for solution-processed organic electronics. J. Org. Chem. 2015, 80, 582–589. 10.1021/jo502564w. [DOI] [PubMed] [Google Scholar]
- Tonzola C. J.; Alam M. M.; Kaminsky W.; Jenekhe S. A. New n-type organic semiconductors: synthesis, single crystal structures, cyclic voltammetry, photophysics, electron transport, and electroluminescence of a series of diphenylanthrazolines. J. Am. Chem. Soc. 2003, 125, 13548–13558. 10.1021/ja036314e. [DOI] [PubMed] [Google Scholar]
- Plater M. J.; Harrison W. T. A. A potential iterative approach to 1,4-dihydro-N-heteroacene arrays. ChemistryOpen 2022, 11 (1 of 4), e202100150 10.1002/open.202100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater M. J.; Harrison W. T. A. Potential building blocks for 1,4-dihydro-N-heteroacenes. ChemistryOpen 2022, 11 (1 of 7), e202200092 10.1002/open.202200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 4th ed.; March J.; Ed.; John Wiley & Sons, Inc., 1992, pp 1–1512. [Google Scholar]
- Chiacchiera S. M.; Singh J. O.; Anunziata J. D.; Silber J. J. Kinetics of the reactions between 1,2-dinitrobenzene and aliphatic primary amines in benzene. A probable mechanism for the observed mild acceleration. J. Chem. Soc., Perkin Trans. 2 1988, 11, 1585–1589. 10.1039/p29880001585. [DOI] [Google Scholar]
- McDowell J. J. H. The crystal and molecular structure of phenothiazine. Acta Crystallogr. 1976, 32, 5–10. 10.1107/S0567740876002215. [DOI] [Google Scholar]
- Holzapfel M.; Lambert C.; Selinka C.; Stalke D. Organic mixed valence compounds with N,N-dihydrodimethylphenazine redox centres. J. Chem. Soc., Perkin Trans. 2 2002, 1553–1561. 10.1039/b204392k. [DOI] [Google Scholar]
- Sheldrick G. M. SHELXT – Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.