Abstract
In this work, TiO2 nanoparticles (NPs) were effectively synthesized by a green method using the Impatiens rothii Hook.f. leaf (IL) extract as a capping and reducing agent. The as-synthesized TiO2 NPs were characterized by different characterization methods such as the Brunauer–Emmett–Teller (BET) analysis, high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), diffused reflectance spectroscopy (DRS), and X-ray diffraction (XRD) and Raman spectroscopy. The specific surface area from BET analysis was found to be 65 m2/g. The average crystallite size from XRD analysis and average particle size from SEM analysis were found to be ∼11 and ∼25 nm, respectively. The Raman spectroscopy and XRD results showed that the biosynthesized (IL–TiO2) nanoparticles were purely anatase phase. XPS analysis illustrated the formation of Titania with an oxidation state of +4. The DRS study showcased that a blue-shifted intense absorption peak of IL–TiO2 (3.39 eV) compared to the bulk material reported in the literature (3.2 eV). HRTEM micrograph showed the presence of grain boundary with d spacings of 0.352, 0.245, and 0.190, which correspond to the lattice planes of (101), (004), and (200), respectively. From the EDX analysis, the weight percents of titanium and oxygen were found to be 54.33 and 45.67%, respectively. The photoinduced degradation of methylene blue (MB) dye was investigated in the presence of biosynthesized IL-TiO2 NPs photocatalyst. The effect of parameters like catalyst dosage (30 mg/L), initial concentration of MB (15 ppm), pH (10.5), and contact time (100 min) on the removal efficiency was optimized. The maximum photodegradation efficiency under the optimized conditions was found to be 98%.
1. Introduction
The tremendous environmental effects, such as climate change, water pollution, and depletion of natural resources caused by industrialization, are linked with an increasing socio-economic demand. The natural world is being severely contaminated by the uncontrolled discharge of many hazardous substances including liquid, semisolid, and solid wastes.1,2 Nowadays, one of our world challenges is the opportunity to get pure water due to pollution. Among the causes of water contamination include energy byproducts, mining operations, insect sides and chemical fertilizers, radioactive wastes, infrastructural activities, and industrial effluents including dyes.3 Numerous sectors, including those in the leather, textile, printing, cosmetics, and pharmaceuticals, release synthetic dyes, which are significant sources of water pollution.4 One toxic and nonbiodegradable organic chemical that is frequently utilized in the textile industries is methylene blue (MB). Additionally, due to its planar shape, it is very soluble in water.5 Therefore, the reprocessing and treatment of wastewater is the primary activity to reduce environmental pollution and avoid the shortage of a clean water supply.
There are a number of wastewater treatment methodologies whose primary objective is to reduce pollutants. These include physicochemical methods, electrochemical oxidation, biological processes, adsorption processes, and advanced oxidation processes (like photocatalysis).6−10 Among the methods mentioned, photocatalytic degradation is thought to be the most promising because it is simple to use, quick, clean, and inexpensive compared to other methods, which have high operating costs and take a long time to remove organic contaminants from water.11 Photocatalysis is the utilization of photon energy to initiate a given reaction process. Due to their capacities to destroy different organic contaminants found in water through a series of redox processes, semiconductor materials have drawn increased attention in the field of photocatalysis. The photocatalytic ability of semiconductors is related to the excitation of electrons from the valence band to the conduction band caused by the absorption of a photon with an appropriate wavelength.
There are four basic steps in the photocatalysis of pollutants: (1) absorption of light having an energy greater than the band gap of semiconductor material to form electron–hole pairs (excitons), (2) separation of excitons, (3) migration of electrons and holes to the surface of the semiconductor, and (4) the charged electrons and holes participating in the redox processes (electrons are utilized in reduction, and holes are utilized in oxidation process).12−14 In recent decades, transition metal oxides such as ZrO2,15 CuO,16 WO3,17 ZnO,18 and TiO219 are the well-known photocatalysts that generate the redox reactions at their surfaces when stimulated by the UV–visible light irradiation appropriate to their band gap energy.12,20,21 Among them, TiO2 is the most effective in photocatalytic splitting of water and photodegradation of organic pollutants and has good antibacterial activity.22
Due to its numerous benefits, such as great photocatalytic ability, high oxidizing power, low toxicity and biocompatibility, excellent chemical stability, and ease of accessibility, TiO2 is one of the best materials for photocatalytic processes.21,23 There are three crystallographic forms of TiO2, namely, anatase (tetragonal), rutile (tetragonal), and brookite (orthorhombic).24,25 In general, TiO2 is considered as an ecologically friendly and has been used in various industrial applications including gas sensors,26 corrosion protection,27 white pigment,28 dye-sensitized solar cells,29 remediation of the environment,30 hydrogen gas production,31 providing high dielectric constant and high electrical resistance,32 and reduction of carbon dioxide.33
Titanium dioxide NPs can be synthesized using a variety of methods, including sol–gel, sono-chemical, microwave-assisted,34 deposition, hydro/solvothermal, and oxidation methods.30,35 Biosynthesis of titanium dioxide NPs has gained wide attention among scholars due to its cost-effective, eco-friendly, and reproducible method.25 The green synthesis method of nanoparticles with the help of plants has many benefits, one of which is the elimination of the need for chemical stabilizing agents.36 Plant extracts can act as both reducing agents and stabilizers at the same time, and such a method also reduces the use of various chemical substances and makes use of natural substances, which further lowers the toxicity level of TiO2 nanoparticles.37,38
In the present study, we have successfully synthesized TiO2 NPs using Impatiens rothii Hook.f. leaf as a capping and reducing agent. Impatiens rothii Hook.f. is a flora classified under the family of Balsaminaceae.39 It is a tuberous species native to Ethiopia and used as local wound treatment, especially for fire burn skin. In addition to this, its root extract is used for nails and palm adornment due to its colorfulness and high adhesive property. To the best of our knowledge, this plant is not used for the synthesis of TiO2 nanomaterials. The resulting biosynthesized TiO2 nanoparticles are not also tested for photocatalytic degradation of potential pollutant methylene blue (MB). The biosynthesized IL-TiO2 showed good mesoporous behavior with a surface area of 56 m2/g. The SEM morphology also revealed almost uniform NPs with less agglomeration, which can be attributed to the capping ability of Impatiens rothii Hook.f. leaf extract during synthesis. Therefore, this mesoporous structure can contribute to enhancing the photocatalytic activity of IL-TiO2 against the MB dye.
2. Experimental section
2.1. Materials and Chemicals
The materials used in this work were titanium tetra isoprpoxide (TTIP, 97.5%, Merck, Germany), ethanol (99.9%, Luba, India), ascorbic acid (C6H8O6) (SRL, 99%), deionized water, sodium hydroxide (98%, Luba, India), methylene blue (C16H18ClN3S, Dallul Pharmaceuticals plc), and Impatiens rothii Hook.f. plant leaf
2.2. Extraction of Plant Leaves
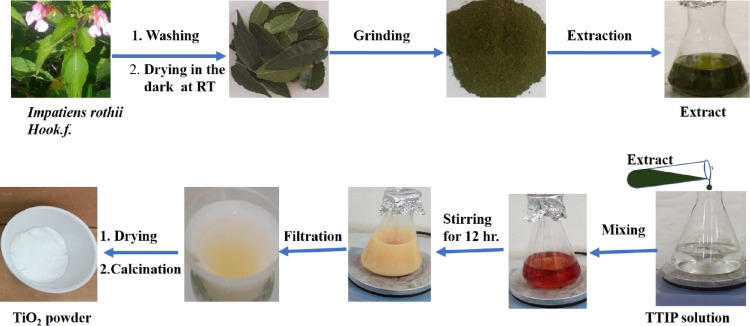
The extraction was done following the method reported by Basit and colleagues with modification.40 The fresh leaves of Impatiens rothii Hook.f. were collected from Menz Gera Midir, Amhara regional state, Ethiopia. The collected leaves undergo washing with deionized water to remove dust and suspended particles. Then, the leaves were dried in the dark at room temperature (RT) for 2 weeks. The dried leaves were ground with a mortar and pestle. An amount of 20 g of leaves powder was taken into 500 mL of deionized water and boiled at 60 °C for 4 h through stirring magnetically at stirring speed of 600 rpm. The resulting extracted solution was allowed to cool and was filtered by using Whatman filter paper No. 1. The filtrate was used to synthesize TiO2 nanoparticles as shown in Figure 1.
Figure 1.
Schematic representation of plant leaf extraction and green synthesis of TiO2. Photograph courtesy of “Getye Behailu Yitagesu”. Copyright 2023.
2.3. Synthesis of TiO2 Nanoparticles
An amount of 15 mL of titanium tetra isopropoxide (TTIP) was dissolved in 50 mL ethanol. Then, 50 mL of the plant extract solution was added to the precursor solution slowly with vigorous stirring for 12 h until yellowish-colored precipitate formed (Figure 1). After standing for a night (12 h), the precipitate was filtered by Whatman filter paper no. 1 followed by washing 3 times with deionized water and twice with ethanol. Next to filtration, the paste was dried at 80 °C for 3 h in a hot air oven. The dried powder was ground using mortar and pestle and calcined at 500 °C for 3 h in a muffle furnace with a heating rate of 10 °C/min.
2.4. Characterization of the As-Synthesized TiO2 NPs
The characteristics of IL–TiO2 NPs were studied by using various techniques. The thermogravimetric analysis/differential thermal analysis (TGA/DTA) curve was obtained on thermal analyzer (DTG-60H Shimadzu Co., South Korea) instrument in a temperature range of 0 to 800 °C at a heating rate of 10 °C/min. The X-ray diffraction (XRD) analysis was carried out using a Shimadzu 7000 X-ray diffractometer (Japan) equipped with Cu Kα1 radiation source (λ = 1.5406 Å) with an accelerating voltage of 40 kV and a source current of 30 mA at a scanning rate of 4° min–1 in a 2θ range of 10 to 80°. BET analysis of pore size, pore volume, and specific surface area distribution was evaluated by measuring nitrogen adsorption–desorption at 77.7 K using a Quantachrome v11.02, USA, instrument.
The Raman spectrum was analyzed by a Raman spectrophotometer (JASCO, NRS-5000, Japan). X-ray photoelectron spectroscopy (XPS) was measured by a Kratos Axis DLD X-ray photoelectron spectrometer (Shimadzu, Canada). The UV–vis DRS and dye absorbance were recorded on a UV–vis defused reflectance spectrophotometer (JASCO V-750 UV–vis). FTIR spectra were recorded in the range of 4000 to 400 cm–1 on an FTIR-6600 spectrophotometer (JACO International Co., Ltd., Tokyo, Japan). The morphology of IL-TiO2 NPs was studied by the SEM technique (400, FEG, USA). Energy-dispersive spectroscopy attached to SEM was used to analyze the elemental composition. High-resolution lattice images were achieved by means of a transmission electron microscope with an operating voltage of 300 kV (FEI, Titan, 80–300 kV).
2.5. Photocatalytic Performance Test
The photocatalytic degradation activity of IL–TiO2 NPs against methylene blue dye was tested under a 150 W tungsten-halogen lamp (Philips, China). An amount of 60 mL of aqueous solution of MB (5–20 ppm) was prepared in vessels, and an appropriate amount of IL–TiO2 NPs (10–50 mg) was added. The solutions were sonicated in dark for 10 min and allowed to stand for 30 min prior to irradiation for adequate adsorption–desorption equilibrium. In every 10 min interval, 4 mL of MB solution was taken from the reaction mixture and absorbance was measured by a UV–vis spectrophotometer. To study the effect of pH on the photocatalytic degradation ability of IL–TiO2 NPs, the point of zero charge was obtained (pHZPC = 7.32) by the salt addition method (Figure S1).41 Then, based on the pHZPC value, photocatalytic activity was done at different pH (8–11.5). The pH values were adjusted by adding 0.1 M NaOH solution. The percent removal of MB dye by IL–TiO2 NPs was calculated using the formula in eq 1.40
| 1 |
where C0 and A0 are dye concentration and absorbance, respectively, before irradiation. The parameters C and A are the concentration of MB dye and its absorbance, respectively, at any time after the irradiation has started. The pseudo first order kinetic model in eq 2 was used to investigate the rate of photocatalytic degradation of MB dye.
| 2 |
where C0 and C are initial concentrations of MB dye at the start of catalysis and after a given time t, respectively. The rate constant k is obtained from the slope of the curve ln(Co/C) against t.42,43
2.6. Reusability Test
The recyclability tests were done for IL–TiO2 after being used in the photocatalytic degradation of MB. After the first photocatalysis degradation, the solution was centrifuged three times with distilled water. Then, the washed IL–TiO2 was dried at 70 °C and reused. This procedure was repeated for four consecutive additional cycles of photocatalysis using fresh MB dye.
3. Results and Discussion
3.1. Characterization
3.1.1. TGA Analysis
The thermal decomposition property of IL–TiO2 NPs was evaluated between room temperature and 800 °C. Figure S2 demonstrates thermal differential analysis (DTA) coupled with thermogravimetric analysis (TGA). The TGA curve shows three main steps of mass loss. The first nearly 6% weight loss between 36 and 150 °C represented the loss of adsorbed water. The second approximately 4% mass loss that observed between 180 and 420 °C could be attributed to the volatilization of organic matters from TTIP precursor, the transformation of amorphous structure to a crystalline form, and the elimination of chemisorbed hydroxyl groups. The third weight loss in the temperature range of 580–610 °C and exothermic peaks at 590 °C can be attributed to the phase transformation of anatase to rutile polymorph.44
3.1.2. XRD Analysis
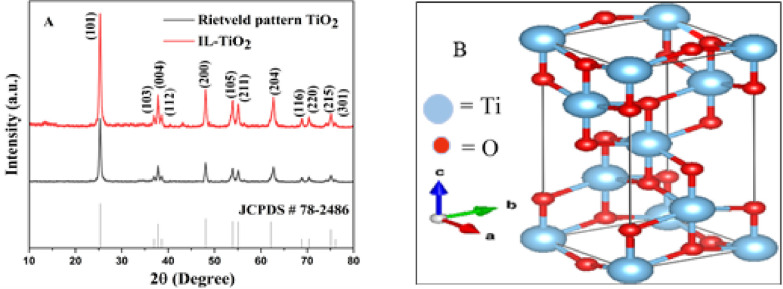
The XRD pattern of IL-TiO2 NPs was investigated with the Rietveld profile matching method with the help of Xpert High Score plus software. The Rietveld pattern fitting of IL–TiO2 NPs’ XRD, as shown in Figure 2A, demonstrates the high quality of fitting within space group of I41/amd anatase. The peaks obtained at 2θ values of 25.32, 37.07, 37.99, 38.58, 48.12, 53.97, 55.16, 62.79, 68.87, 70.42, 75.07, and 76.14° are indexed to crystal planes of (101), (103), (004), (112), (200), (105), (211), (204), (116), (220), (215), and (301), respectively, with a JCDPS card number of 78–2486.45 The tetragonal crystal structure of the anatase phase with lattice parameters a = b = 3.786 Å and c = 9.517 Å and α = β = γ = 90° was visualized using VESTA (Visualization for Electronic Structural Analysis) win64 software (Figure 2B). The XRD profiles of the IL–TiO2 NPs showed no signs of phase impurity. Using the Scherrer formula (eq 3), the average crystallite size of IL–TiO2 NPs was calculated from the broadening of the peaks.46 The calculated average crystallite size of IL-TiO2 nanoparticles was 11 nm.
| 3 |
where D represents the average crystalline size; K is Scherrer's shape factor (0.94); λ is the wavelength of the source X-ray (0.15406 nm); β is the full width at half-maxima; and θ is Bragg's X-ray diffraction angle.
Figure 2.
X-ray diffraction (A); 3D VESTA visualization (B) of IL–TiO2 NPs.
3.1.3. SEM-EDX and HRTEM Analysis
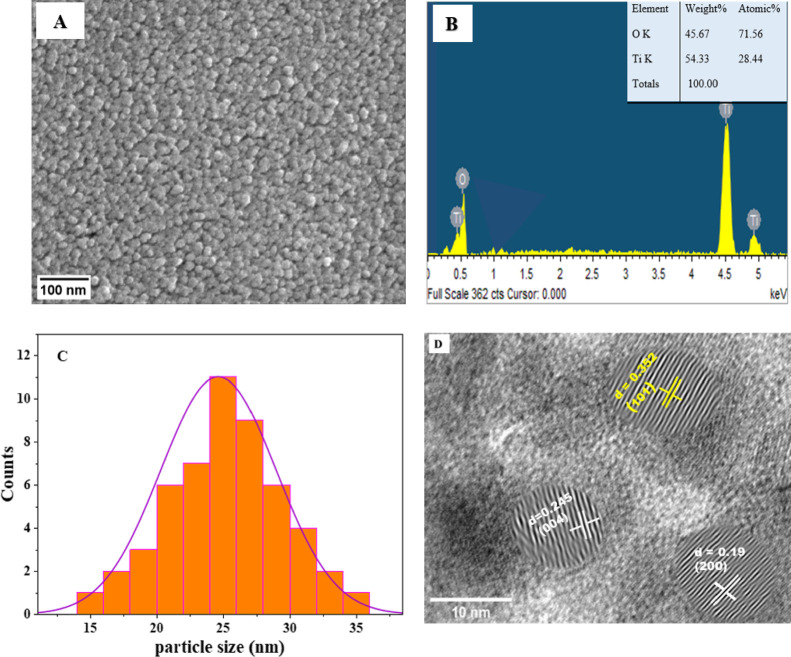
SEM analysis was used to determine the morphology of IL–TiO2 NPs. The obtained results as shown in Figure 3A reveal that the IL–TiO2 nanoparticles were in spherical structure and the average size was evaluated. Figure 3C showed the nanoparticle distribution, and it can be observed that particle size ranges from 15 to 35 nm with an average diameter of approximately 25 nm. The average particle size obtained from the SEM analysis is greater than the average crystallite size calculated using the Scherrer formula (eq 3) because the particle may contain two or more grains attached together due to agglomeration and aggregation effect. The elemental composition analysis of IL–TiO2 NPs was performed by EDX analysis. As presented in Figure 3B, the weight percents of titanium and oxygen were found to be 54.33 and 45.67%, respectively, which confirms the presence of pure TiO2 nanoparticles.
Figure 3.
SEM micrograph (A), EDX (B), particle size histogram from SEM (C) and HRTEM micrograph (D) of IL–TiO2.
Figure 3D reveals the HRTEM micrograph of IL–TiO2 nanoparticles. The parallel lattice fringes of the nanoparticles are clearly visible, confirming their single-crystallinity. The lattice fringes spacing 0.352, 0.245, and 0.19 nm are consistent with the (101), (004), and (200) planes of tetragonal anatase, respectively.
3.1.4. BET Study
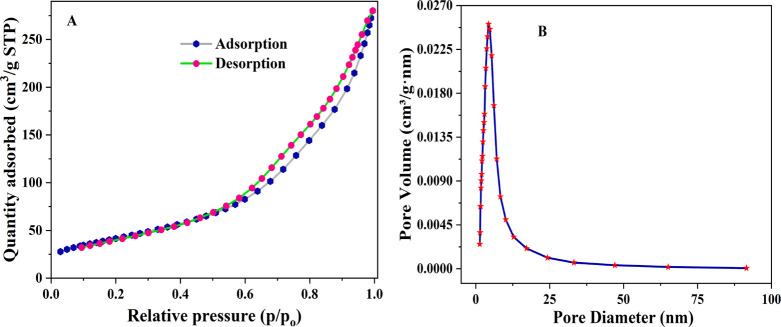
The Brunauer–Emmett–Teller (BET) surface area analyzer was used for the surface area analysis of the as-synthesized TiO2 NPs. In Figure 4A, a typical plot of the N2 adsorption–desorption isotherm and showed a type IV with the hysteresis H3 loops type in the relative pressure range of 0.57–0.9, which indicates that the synthesized nanoparticles were mesoporous.47 The corresponding pore size distribution curve of IL–TiO2 NPs is shown in Figure 4B. Barrett–Joyner–Halenda analysis revealed that the IL–TiO2 NPs showed a high surface area of 65 m2/g with the pore diameter and pore volume of 7.45 nm and 0.026 cm3/g, respectively. A higher surface area provides more surface active parts for the adsorption of pollutants, resulting in a more efficient photocatalytic degradation process that occurs.48 This BET result revealed that the IL–TiO2 NPs were promising for the photocatalytic degradation of the MB dye.
Figure 4.
Nitrogen adsorption desorption isotherms (A), pore size distribution curve (B) of IL–TiO2 NPs.
3.1.5. XPS Analysis
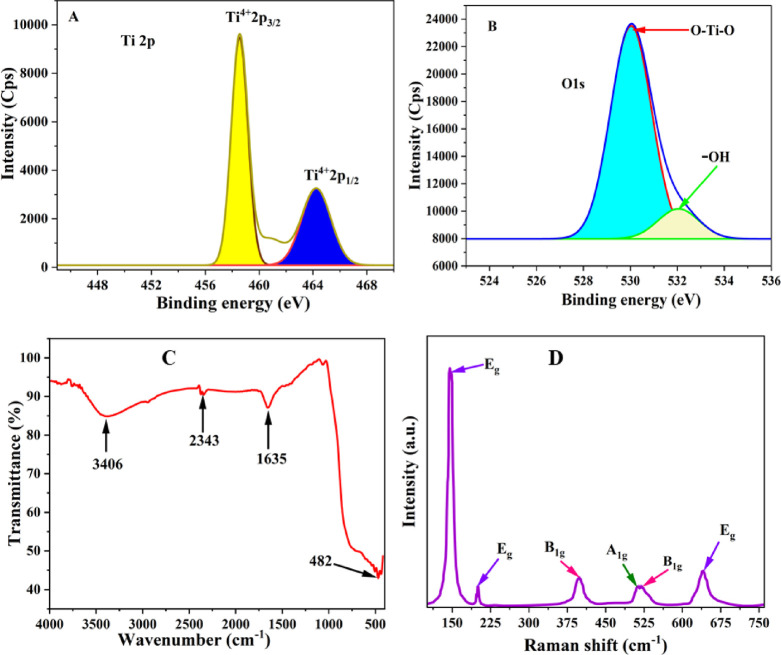
The X-ray photoelectron spectroscopy technique was used to confirm the oxidation state of the IL–TiO2 nanoparticles. As shown Figure 5A, the binding energies at 458.66 and 464.14 eV resemble Ti 2p3/2 and Ti 2p1/2, respectively. The area ratio of Ti4+2p1/2 to Ti4+2p3/2 was 0.65 approximately. The difference in binding energy due to the spin orbit coupling, ΔEb = Eb (464.14 eV) – Eb (458.66 eV) was 5.7 eV, which is well-matched with the reported values.49,50 The O 1s spectra of IL–TiO2 showed two peaks at binding energies of 530.5 eV and a minor peak at 532.014 eV (Figure 5B), which are attributed to oxygen anions (O–Ti–O) and surface hydroxyl (−OH) species, respectively.51
Figure 5.
Deconvoluted Ti 2p XPS spectra (A) and O 1s spectra (B). FTIR spectra (C), and Raman spectra of IL-TiO2 (D).
3.1.6. FTIR Spectroscopic Analysis
As shown in (Figure 5C) the FTIR spectrum of IL–TiO2 consists of characteristic peaks of TiO2. The broad band in the range of 3600–3200 cm–1 is attributed to the hydroxyl group of the water molecule’s intermolecular interaction with the surface of TiO2.52,53 The absorption peak at 1635 cm–1 was ascribed to the bending mode of O–H bond adsorbed on the IL–TiO2 NP surface. The strong absorption peak in the range of 850–420 cm–1 was attributed to the stretching band of the O–Ti–O bond.54,55 The vibrational band at 2343 cm–1 is related to adsorption of atmospheric CO2 on the surface of NPs.55−58
3.1.7. Raman Spectroscopic Studies
The Raman spectroscopy technique was used for structural identification of the IL–TiO2 NPs. The Raman spectroscopy analysis of IL–TiO2 (Figure 5D) showed tetragonal anatase Raman active modes of A1g, 2B1g, and 3Eg vibrational spectrum centered at 515, 399, 519, 144, 197, and 639 cm–1.59−63
3.1.8. Optical Band Gap Analysis
The Tauc plot method was used to determine the band gap energy of IL–TiO2 based on the expression in eq 4.64
| 4 |
F(R) is the Kubelka–Munk function expressed in eq 5; h is Plank's constant; v is the frequency of photon energy; the exponent n depends on the type of the electron transition; n = 1/2 for direct and n= 2 for indirect transition band gaps. A is a constant; Eg is the band gap energy.
| 5 |
where R, K, and S are the reflectance, absorption coefficient, and scattering coefficient, respectively.
The UV–visible diffused reflectance spectrum of IL–TiO2 is shown in Figure 6A. The IL–TiO2 showed an intense absorption peak at a wavelength of 366 nm. When compared to the bulk material of titanium oxide, the biosynthesized TiO2 showed a blue shift. This shift in wavelength was attributed to the decrease in size of nanoparticles.65
Figure 6.
UV–vis DRS spectra (A); Tauc plot (B) of IL–TiO2.
Figure 6B displays the Tauc plot of IL–TiO2. The band gap energy was estimated from the intersection point of energy-axis with the Tauc plot’s linear portion. The band gap energy value was estimated to be 3.39 eV. The band gap energy of IL–TiO2 exceeded by about 0.19 eV than that of the bulk one (3.2 eV)19 due to the size reduction in the IL–TiO2 nanoparticles.
3.2. Photocatalytic Performance Test
The photocatalytic degradation efficiency of IL–TiO2 was evaluated against the methylene blue dye. The effect of parameters like catalyst dose, initial dye concentration, initial pH of dye solution, and contact time were evaluated and optimized.
3.3. Effect of IL-TiO2 Dosage
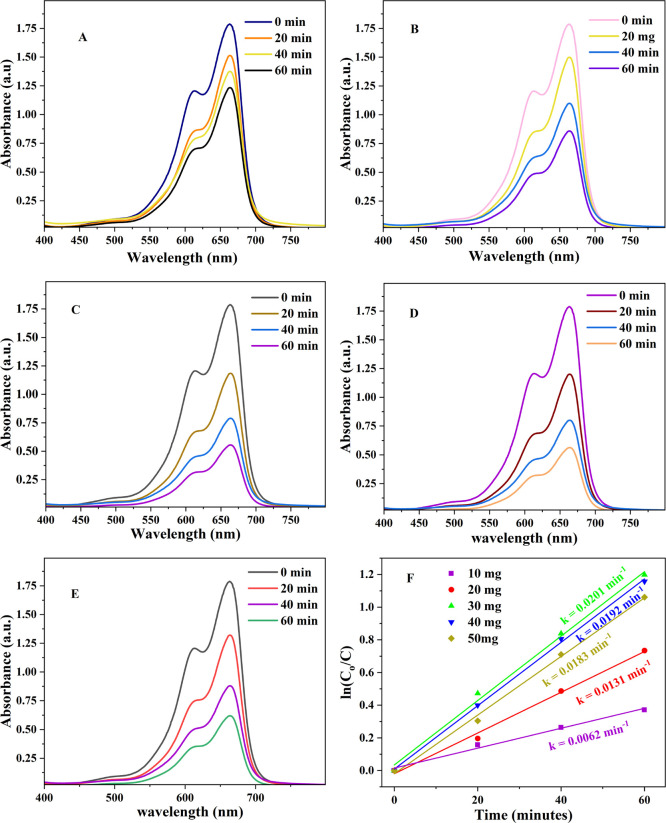
In order to study the effect of loading ability on the dye degradation efficiency, different amounts of IL–TiO2 NPs in the range of 10–50 mg were used for degrading 10 ppm of MB at pH of 9.5 with a contact time of 60 min as shown in Figure 7A–E. The percent of photodegradation of the dye was increased with increasing the dosage of TiO2 up to 30 mg (68.89%), and after that, it became almost a constant value (Figure S3). As the amount of IL–TiO2 increases, the total active sites on the surface of the catalyst increase up to some extent. However, increasing the amount of TiO2 decreases the penetration of solar light as a consequence of the increase in turbidity of the solution. Hence, a higher amount of catalyst is not useful due to the aggregation of catalyst and reduction of the radiation field due to light scattering.66 The highest rate constant for the degradation of MB with 30 mg of catalyst was found to be 0.0201 min–1 (Figure 7F). Therefore, all the experiments were performed with a 30 mg catalyst dose.
Figure 7.
UV–vis absorption spectra of (A) 10, (B) 20, (C) 30, (D) 40, and (E) 50 mg of IL–TiO2; (F) pseudo-first order model plots of (A–E).
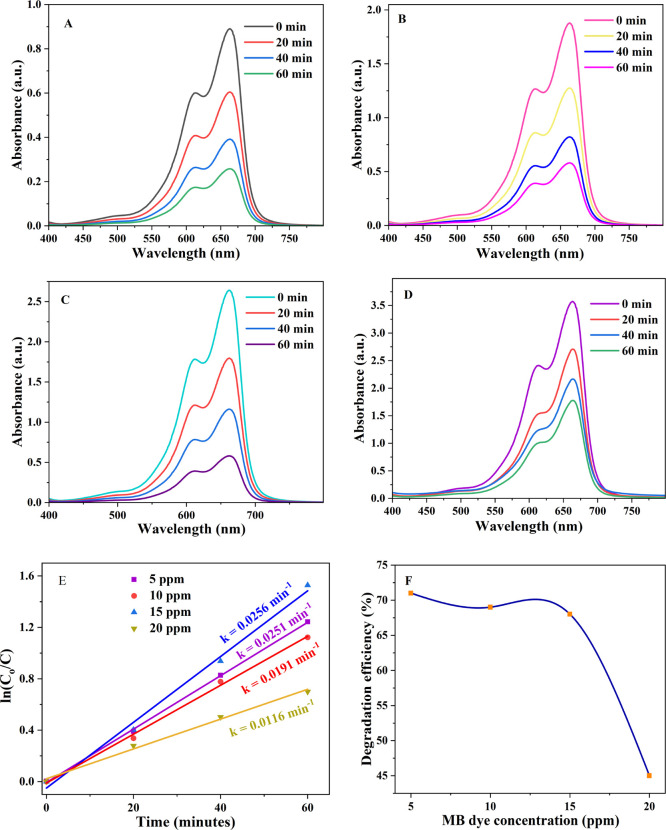
3.4. Effect of Initial Dye Concentration
The concentration of MB dye was varied from 5 to 20 ppm with an optimum IL–TiO2 dose of 30 mg. Figure 8A–D clearly shows the difference in absorption peaks as a result of concentration increment. The rate of degradation of MB was evaluated when the concentration of MB dye was increased from 5 to 20 ppm with a constant catalyst dose of 30 mg/L. As shown in Figure 8F, 15 ppm of dye concentration resulted in higher rate of photodegradation with a rate constant of 0.020 min–1. The percent of photodegradation at this concentration was recorded as 68%. A sudden decrease of photodegradation efficiency to 45.46% (Figure 8F) as the concentration of MB dye was increased to 20 ppm was attributed to the accumulation of MB dye on the IL–TiO2 surface that prevents the direct contact of light irradiation to the catalyst surface.67
Figure 8.
UV–vis absorption spectra of (A) 5, (B) 10, (C) 15, and (D) 20 ppm of MB dye; (E) pseudo-first order model plots of (A–D); (F) degradation efficiency of (A–D).
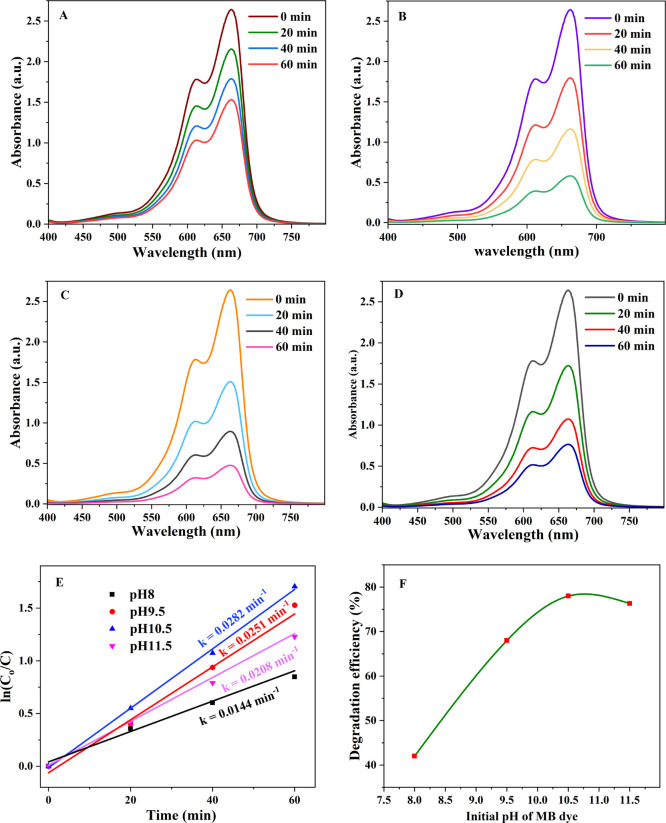
3.5. Effect of Initial pH
The pH of the solution is another important factor in the photocatalytic degradation of pollutants. Photocatalyst surface charge is significantly affected by the pH of the dye solution. The pH of the MB dye solution was adjusted through adding 1 M NaOH solution. As indicated in Figure S1, the point of zero charge of IL–TiO2 was found approximately 7.32 in which its surface is negatively charged above this point it attracts the cationic MB dye for efficient photodegradation. Then, the effect of the pH of solution on the photocatalytic degradation activity was studied within the range of 8–11.5 at optimized dye concentration (15 ppm) and photocatalyst amount (30 mg/L) as shown in Figure 9F. An increase in the photodegradation of MB dye was observed by increasing the pH of solution from 8 to 10.5 for optimized IL-TiO2 NPs dosage as well as optimized dye concentration.
Figure 9.
UV–vis absorption spectra of 15 ppm of MB dye with a catalyst dose of 30 mg at pH values of (A) 8, (B) 9.5, (C) 10.5, and (D) 11.5; (E) pseudo-first order model plot of (A–D); (F) degradation efficiency of (A–D).
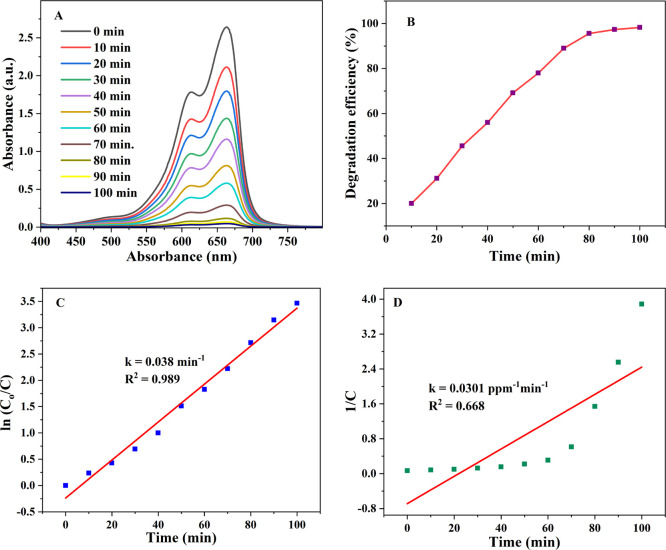
3.6. Effect of Contact Time
The rate of MB degradation on the IL-TiO2 NP surface is equivalent to the time duration of adsorption. Figure 10A shows the contact time effect on the removal of MB at optimum conditions (30 mg of TiO2, 15 ppm of MB, at pH 10.5) in aqueous solution. The result shows that the rapid photodegradation of methylene blue (MB) was observed in the first 80 min. As shown in Figure 10B, the removal efficiency of MB using the IL-TiO2 catalyst was approximately 98% after an irradiation time duration of 100 min. It was found that as the time of light irradiation prolonged the percent photodegradation efficiency was increased and get hold of a maximum after 100 min. The degradation efficiency of our work revealed good agreement with that of other works reported in Table 1.
Figure 10.
UV–vis absorption spectra of MB for 100 min at the optimized conditions (A); (B) degradation efficiency of (A); (C) pseudo-first order model plot of (A); (D) pseudo-second order model plot of (A).
Table 1. Comparison of the Photodegradation Efficiency of IL-TiO2 with Other Recently Reported Works.
| plant | photocatalyst | Dye | Catalyst dosage | time (minutes) | efficiency (%) | references |
|---|---|---|---|---|---|---|
| Acalypha indica | TiO2 | MB | 50 mg | 360 | 98.5 | (68) |
| Commelina benghalensis | TiO2 | MB | 30 mg | 120 | 65 | (69) |
| Cannabis sativa | TiO2 | MB | 10 mg | 80 | 98.2 | (36) |
| Cestrum nocturnum | TiO2 | MB | 8 mg | 40 | 80 | (70) |
| Ceaspina pulcherrima | TiO2 | MB | 10 mg | 40 | 86 | (71) |
| Nervila aragona | TiO2 | MB | 10 mg | 40 | 75 | (71) |
| Manihot esculante | TiO2 | MB | 10 mg | 40 | 56 | (71) |
| Cochlospermum Gossypium | TiO2 | MB | 15 mg | 120 | 99 | (72) |
| Citrus Limetta | TiO2 | MB | 70 mg | 80 | 90 | (73) |
| Impatiens rothii Hook.f. | TiO2 | MB | 30 mg | 100 | 98 | this work |
The kinetic models of both pseudo-first (Figure 10C) and second (Figure 10D) orders were used to study the rate of photodegradation efficiency at optimized conditions. The small value of correlation coefficient (R2 = 0.668) showed the inconsistency of the pseudo-second order kinetic model. The higher value of correlation coefficient (R2 = 0.989) (Table 2) with a rate constant of 0.038 min–1 shows the good fitness of the pseudo-first order kinetic model with good photodegradation efficiency.42,43
Table 2. Summary of Degradation Efficiency under Optimized Conditions.
| IL-TiO2 dosage | MB dye concentration | pH | time | fitted model | rate constant (k) | correlation constant (R2) |
|---|---|---|---|---|---|---|
| 30 mg | 15 ppm | 10.5 | 100 min | pseudo-first order | 0.038 min–1 | 0.989 |
| pseudo-second order | 0.03 ppm–1min–1 | 0.668 |
3.7. Scavenging Test
The photostimulated surface properties of the as-synthesized IL-TiO2 nanoparticles were studied for the capability of photo degradation effect on MB dye. Reactive species such as holes (h+), superoxide radicals (O2–•), electrons (e–), and hydroxyl radicals (OH•) play a vital role in the photodegradation of organic pollutants.74 Therefore, the photocatalytic activity of IL-TiO2 NPs on the degradation of MB dye was studied in the presence of silver nitrate (AgNO3), Ethylenediaminetetraacetic acid disodium (EDTA-2Na),11 isopropanol (IPA),75 and acetonitrile76 as scavengers for e–, h+, OH•, and O2–•, respectively, to quantify the relative contribution of reactive species. Figure 11 shows the light-assisted percent degradation of MB using IL-TiO2 NPs in the presence of the above-mentioned radical scavengers. The scavenger tests were done using the optimum conditions. The results revealed that the hydroxyl free radicals (OH•) were the dominant reactive species as the addition of IPA resulted in a decrease of degradation efficiency approximately to 38 (from 98% without a scavenger). Upon the addition of acetonitrile, the degradation efficiency decreased to 47%, which indicates that superoxide radicals (O2–•) played a role in photodegradation of MB. The decrement of degradation efficiency to 54% as a result of addition of holes scavenger (EDTA-2Na) revealed good participation of holes (h+) in the degradation mechanism. In general, the results appear to confirm the photocatalytic activity of IL-TiO2,against MB dye driven by the reactive species in a sequence of OH• > O2•– > h+ > e–, and similar results were reported by Saharudin et al.76
Figure 11.

Effect of radical scavengers on the photodegradation ability of IL-TiO2.
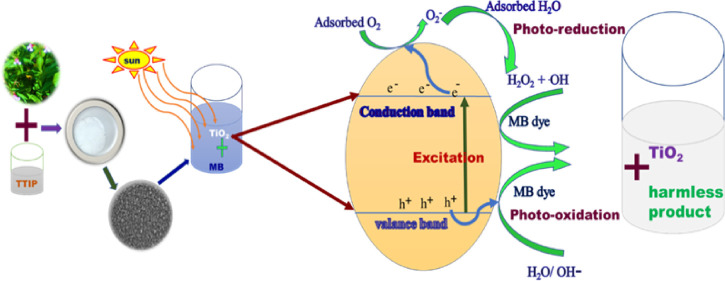
3.8. Mechanism of Photocatalysis
Upon irradiation of light energy that exceeds the band gap of IL-TiO2, the electrons excite to the conduction band leaving holes in the valence band as shown in Figure 12 and given by eq 6.77 The electrons generated in the conduction band of IL-TiO2 are captured by O2 adsorbed on the surface of the photocatalyst to produce very reactive superoxide anion radicals (eq 7), contributing to both direct MB degradation and indirect MB removal through reactions with H2O to generate hydroxyl free radicals. The positively charged holes in the valence band react with water molecules or hydroxyl anions, resulting in hydrogen cation ions (H+) and hydroxyl radicals (OH•). Hydroxyl radicals, which are powerful oxidants, can react with organic pollutants and degrade them to environmentally harmless products.18 Generally, the following steps take place in photocatalysis of organic pollutants (1) absorption of photon energy and excitation of electron to the conduction band, (2) reduction, (3) oxidation, and (4) pollutant degradation as given in eqs 6–13.5,78−80
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
Figure 12.
Proposed photodegradation mechanism of methylene blue by IL-TiO2.
3.9. Reusability Test
The photocatalyst recyclability and stability are key properties in cost minimization while maintaining durable and efficient photocatalytic performances in pollutants removal. The reusability test of IL–TiO2 NPs showed good stability, and the percent removal values for the first, second, third, and fourth cycles were 96, 93.4, 91.7, and 88.3%, respectively, relative to the initial degradation efficiency (Figure 13).
Figure 13.
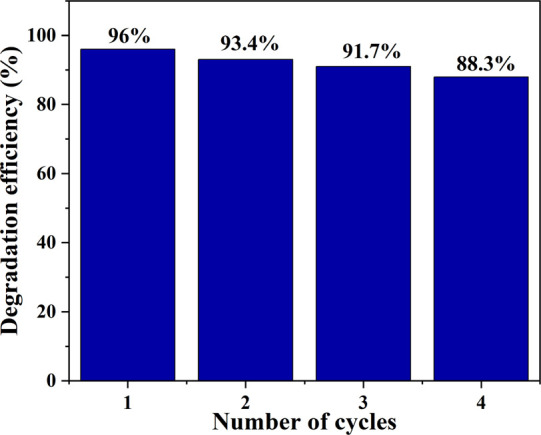
Recyclability test of IL–TiO2 NPs for the photodegradation of MB dye.
4. Conclusions
In the present study, TiO2 nanoparticles were effectively synthesized via a green synthetic method using Impatiens rothii Hook.f leaf extract as a reducing, capping, and stabilizing agent. Extensive evaluation of structural, thermal, optical, and crystallinity properties of the synthesized TiO2 NPs has been performed using a number of characterization methods such as the Brunauer–Emmett–Teller (BET) analysis, high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy(SEM), energy dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), diffused reflectance spectroscopy (DRS), X-ray diffraction (XRD), and Raman spectroscopy. The specific surface area from BET analysis was found to be 65 m2/g. The average crystallite size and particle size of synthesized NPs were found to be 11 and 25 nm, respectively. DRS analysis showed that the band gap of IL-TiO2 was found to be 3.39 eV. The HRTEM micrograph showed the presence of grain boundary with d spacings of 0.352, 0.245, and 0.190, which correspond to the lattice planes of (101), (004), and (200), respectively. From the EDX analysis, weight percents of titanium and oxygen were found to be 54.33 and 45.67%, respectively, which confirms the presence of pure IL-TiO2 nanoparticles. The synthesized NPs were found effective in the degradation of methylene blue in aqueous medium under a photocatalytic process. The removal efficiency of methylene blue under photocatalytic conditions was found to be 98% at the optimized parameters (initial MB dye concentration of 15 ppm, pH = 10.5, catalyst dosage of 30 mg, in the course of 100 min contact time). The kinetic analysis of photocatalytic degradation of MB showed that it was well fitted in a pseudo-first-order kinetic model with a correlation factor of 0.989. The reusability test showed good stability of IL–TiO2 NPs.
Acknowledgments
The authors acknowledge Adama Science and Technology University, Ethiopia and Wachemo University, Ethiopia for their financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06142.
Experimental procedures for the preparation of plant extract and phytochemical analysis; (Figure S1) zero point charge determination of IL–TiO2; (Figure S2) TGA-DTA for IL–TiO2; (Figure S3) effect of IL–TiO2 dose on photodegradation efficiency; (Figure S4) SEM image; (Figures S5–S7) photos of the solutions at different conditions; and (Table S1) phytochemical screening test summary (PDF)
Author Contributions
Writing of the original draft and laboratory work was done by G.B. Writing of the review and editing of the paper was done by G.A. and D.T. All authors read the final revised manuscript in detail and approved it.
The authors declare no competing financial interest.
Supplementary Material
References
- Pavel M.; Anastasescu C.; State R.-N.; Vasile A.; Papa F.; Balint I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: assessment of practical application potential for water and air cleaning. Catalysts 2023, 13 (2), 380. 10.3390/catal13020380. [DOI] [Google Scholar]
- Al-Musawi T. J.; Yilmaz M.; Mohebi S.; Balarak D. Ultraviolet radiation/persulfate/hydrogen peroxide treatment system for the degradation of acid blue 80 dye from a batch flow chemical reactor: effects of operational parameters, mineralization, energy consumption, and kinetic studies. Energy, Ecology and Environment 2022, 7 (6), 630–640. 10.1007/s40974-022-00250-9. [DOI] [Google Scholar]
- Crini G.; Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters 2019, 17, 145–155. 10.1007/s10311-018-0785-9. [DOI] [Google Scholar]
- Ghazy N. M.; Ghaith E. A.; Abou El-Reash Y.; Zaky R. R.; Abou El-Maaty W. M.; Awad F. S. Enhanced performance of hydroxyl and cyano group functionalized graphitic carbon nitride for efficient removal of crystal violet and methylene blue from wastewater. RSC Adv. 2022, 12 (55), 35587–35597. 10.1039/D2RA07032D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem B. B.; Essalah G.; Ameur S. B.; Duponchel B.; Guermazi H.; Guermazi S.; Leroy G. Synthesis and comparative study of the structural and optical properties of binary ZnO-based composites for environmental applications. RSC Adv. 2023, 13 (9), 6287–6303. 10.1039/D2RA07837F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagar S.; Singh S. K.; Gupta M. K. Economics of advanced technologies for wastewater treatment: Evidence from pulp and paper industry. Frontiers in Environmental Science 2022, 10, 960639. 10.3389/fenvs.2022.960639. [DOI] [Google Scholar]
- Abdel-Raouf M. E.; Maysour N. E.; Farag R. K.; Abdul-Raheim M. A. R. Wastewater treatment methodologies, review article. Int. J. Environ. Agric. Sci. 2019, 3, 018. [Google Scholar]
- Salama R. S.; El-Sayed E.-S. M.; El-Bahy S. M.; Awad F. S. Silver nanoparticles supported on UiO-66 (Zr): as an efficient and recyclable heterogeneous catalyst and efficient adsorbent for removal of Indigo Carmine. Colloids Surf., A 2021, 626, 127089. 10.1016/j.colsurfa.2021.127089. [DOI] [Google Scholar]
- Sillanpää M.; Mahvi A. H.; Balarak D.; Khatibi A. D. Adsorption of Acid orange 7 dyes from aqueous solution using Polypyrrole/nanosilica composite: Experimental and modelling. International Journal of Environmental Analytical Chemistry 2023, 103 (1), 212–229. 10.1080/03067319.2020.1855338. [DOI] [Google Scholar]
- Rezaei M.; Mengelizadeh N.; Berizi Z.; Salehnia S.; Asgari M.; Balarak D. Synthesis of MMT– CuFe2O4 Composite as a Peroxymonosulfate Activator for the Degradation of Reactive Black 5. ChemistrySelect 2023, 8 (2), e202201729. 10.1002/slct.202201729. [DOI] [Google Scholar]
- Bakry A. M.; Alamier W. M.; El-Shall M. S.; Awad F. S. Facile synthesis of amorphous zirconium phosphate graphitic carbon nitride composite and its high performance for photocatalytic degradation of indigo carmine dye in water. Journal of Materials Research and Technology 2022, 20, 1456–1469. 10.1016/j.jmrt.2022.07.161. [DOI] [Google Scholar]
- Landi S. Jr; Segundo I. R.; Freitas E.; Vasilevskiy M.; Carneiro J.; Tavares C. J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid state communications 2022, 341, 114573. 10.1016/j.ssc.2021.114573. [DOI] [Google Scholar]
- Zhu S.; Wang D. Photocatalysis: basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7 (23), 1700841. 10.1002/aenm.201700841. [DOI] [Google Scholar]
- Yilmaz M.; Mengelizadeh N.; Saloot M. k.; shahbaksh S.; Balarak D. Facile synthesis of Fe3O4/ZnO/GO photocatalysts for decolorization of acid blue 113 under solar, visible and UV lights. Mater. Sci. Semicond. Process. 2022, 144, 106593. 10.1016/j.mssp.2022.106593. [DOI] [Google Scholar]
- Chelliah P.; Wabaidur S. M.; Sharma H. P.; Majdi H. S.; Smait D. A.; Najm M. A.; Iqbal A.; Lai W.-C. Photocatalytic Organic Contaminant Degradation of Green Synthesized ZrO2 NPs and Their Antibacterial Activities. Separations 2023, 10 (3), 156. 10.3390/separations10030156. [DOI] [Google Scholar]
- Vinothkanna A.; Mathivanan K.; Ananth S.; Ma Y.; Sekar S. Biosynthesis of copper oxide nanoparticles using Rubia cordifolia bark extract: characterization, antibacterial, antioxidant, larvicidal and photocatalytic activities. Environmental Science and Pollution Research 2023, 30 (15), 42563–42574. 10.1007/s11356-022-18996-4. [DOI] [PubMed] [Google Scholar]
- Abbaspoor M.; Aliannezhadi M.; Tehrani F. S. High-performance photocatalytic WO3 nanoparticles for treatment of acidic wastewater. J. Sol-Gel Sci. Technol. 2023, 105 (2), 565–576. 10.1007/s10971-022-06002-9. [DOI] [Google Scholar]
- K K S.; P M P. N.; Vasundhara M. Enhanced photocatalytic activity in ZnO nanoparticles developed using novel Lepidagathis ananthapuramensis leaf extract. RSC Adv. 2023, 13 (3), 1497–1515. 10.1039/D2RA06967A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbood H. K.; Ali N. A.. Studying of the structural and optical properties of titanium dioxide nanoparticles prepared by chemical method J. Opt. 20231–6. DOI: 10.1007/s12596-023-01393-6. [DOI] [Google Scholar]
- Danish M. S. S.; Estrella L. L.; Alemaida I. M. A.; Lisin A.; Moiseev N.; Ahmadi M.; Nazari M.; Wali M.; Zaheb H.; Senjyu T. Photocatalytic applications of metal oxides for sustainable environmental remediation. Metals 2021, 11 (1), 80. 10.3390/met11010080. [DOI] [Google Scholar]
- Ali I.; Suhail M.; Alothman Z. A.; Alwarthan A. Recent advances in syntheses, properties and applications of TiO 2 nanostructures. RSC Adv. 2018, 8 (53), 30125–30147. 10.1039/C8RA06517A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagadevan S.; Imteyaz S.; Murugan B.; Anita Lett J.; Sridewi N.; Weldegebrieal G. K.; Fatimah I.; Oh W.-C. A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Processing and Synthesis 2022, 11 (1), 44–63. 10.1515/gps-2022-0005. [DOI] [Google Scholar]
- Luthfiah A.; Permana M. D.; Deawati Y.; Firdaus M. L.; Rahayu I.; Eddy D. R. Photocatalysis of nanocomposite titania–natural silica as antibacterial against Staphylococcus aureus and Pseudomonas aeruginosa. RSC Adv. 2021, 11 (61), 38528–38536. 10.1039/D1RA07043F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamukamba P.; Okoh O.; Mungondori H.; Taziwa R.; Zinya S.; Yang D. Synthetic methods for titanium dioxide nanoparticles: a review. Titanium Dioxide-Mater. Sustainable Environ. 2018, 8, 151–175. 10.5772/intechopen.75425. [DOI] [Google Scholar]
- Waghmode M. S.; Gunjal A. B.; Mulla J. A.; Patil N. N.; Nawani N. N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appli. Sci. 2019, 1 (4), 310. 10.1007/s42452-019-0337-3. [DOI] [Google Scholar]
- Nunes D.; Fortunato E.; Martins R. Flexible nanostructured TiO2-based gas and UV sensors: A review. Discover Mater. 2022, 2 (1), 2. 10.1007/s43939-022-00023-5. [DOI] [Google Scholar]
- Das R.; Ambardekar V.; Bandyopadhyay P. P., Titanium dioxide and its applications in mechanical, electrical, optical, and biomedical fields. Titanium Dioxide-Advances Applications 2021. 10.5772/intechopen.98805 [DOI] [Google Scholar]
- Wu X., Applications of titanium dioxide materials. Titanium Dioxide—Advances and Applications 2022. 10.5772/intechopen.99255 [DOI] [Google Scholar]
- Jayachithra J.; Elampari K.; Meena M. Fabrication of TiO2 based Dye-Sensitized Solar Cell using Nerium oleander as a sensitizer. OP Conf. Ser.: Mater. Sci. Eng. IOP Publishing 2022, 1263, 012018. 10.1088/1757-899X/1263/1/012018. [DOI] [Google Scholar]
- Arun J.; Nachiappan S.; Rangarajan G.; Alagappan R. P.; Gopinath K.; Lichtfouse E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environmental Chemistry Letters 2023, 21 (1), 339–362. 10.1007/s10311-022-01503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldoni A.; Altomare M.; Zoppellaro G.; Liu N.; Kment S.; Zboril R.; Schmuki P. Photocatalysis with reduced TiO2: from black TiO2 to cocatalyst-free hydrogen production. ACS catalysis 2019, 9 (1), 345–364. 10.1021/acscatal.8b04068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkerud J.; Zimmermann C.; Weiser P. M.; Vines L.; Monakhov E. V., On the dielectric constant of titanium dioxide. 2021 10.21203/rs.3.rs-301495/v1. [DOI] [PMC free article] [PubMed]
- Rehman Z. U.; Bilal M.; Hou J.; Butt F. K.; Ahmad J.; Ali S.; Hussain A. Photocatalytic CO2 reduction using TiO2-based photocatalysts and TiO2 z-scheme heterojunction composites: A review. Molecules 2022, 27 (7), 2069. 10.3390/molecules27072069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekru A. G.; Tufa L. T.; Zelekew O. A.; Goddati M.; Lee J.; Sabir F. K. Green Synthesis of a CuO–ZnO Nanocomposite for Efficient Photodegradation of Methylene Blue and Reduction of 4-Nitrophenol. ACS omega 2022, 7 (35), 30908–30919. 10.1021/acsomega.2c02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinle A.; Hüsing N. Liquid-Phase Synthesis of Metal Oxide Nanoparticles. Metal Oxide Nanoparticles: Formation, Functional Properties, and Interfaces 2021, 1, 109–150. 10.1002/9781119436782.ch4. [DOI] [Google Scholar]
- BinSabt M.; Sagar V.; Singh J.; Rawat M.; Shaban M. Green synthesis of CS-TiO2 NPs for efficient photocatalytic degradation of methylene blue dye. Polymers 2022, 14 (13), 2677. 10.3390/polym14132677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M.; Venkatesan M.; Arumugam V.; Natesan G.; Saravanan N.; Murugesan S.; Ramachandran S.; Ayyasamy R.; Pugazhendhi A. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochemistry 2019, 80, 197–202. 10.1016/j.procbio.2019.02.010. [DOI] [Google Scholar]
- Narayanan M.; Devi P. G.; Natarajan D.; Kandasamy S.; Devarayan K.; Alsehli M.; Elfasakhany A.; Pugazhendhi A. Green synthesis and characterization of titanium dioxide nanoparticles using leaf extract of Pouteria campechiana and larvicidal and pupicidal activity on Aedes aegypti. Environmental Research 2021, 200, 111333. 10.1016/j.envres.2021.111333. [DOI] [PubMed] [Google Scholar]
- Yohannis S. W.; Asfaw Z.; Kelbessa E. Ethnobotanical study of medicinal plants used by local people in menz gera midir district, north shewa zone, amhara regional state, Ethiopia. J. Med. Plants Res. 2018, 12 (21), 296–314. 10.5897/JMPR2018.6616. [DOI] [Google Scholar]
- Basit R. A.; Abbasi Z.; Hafeez M.; Ahmad P.; Khan J.; Khandaker M. U.; Al-Mugren K. S.; Khalid A. Successive photocatalytic degradation of methylene blue by ZnO, CuO and ZnO/CuO synthesized from coriandrum sativum plant extract via green synthesis technique. Crystals 2023, 13 (2), 281. 10.3390/cryst13020281. [DOI] [Google Scholar]
- Al-Maliky E. A.; Gzar H. A.; Al-Azawy M. G. Determination of point of zero charge (PZC) of concrete particles adsorbents. IOP Conf. Ser.: Mater. Sci. Eng. IOP Publishing 2021, 1184, 012004. 10.1088/1757-899X/1184/1/012004. [DOI] [Google Scholar]
- Akerdi A. G.; Bahrami S. H.; Pajootan E. Modeling and optimization of Photocatalytic Decolorization of binary dye solution using graphite electrode modified with Graphene oxide and TiO 2. Journal of Environmental Health Science and Engineering 2020, 18, 51–62. 10.1007/s40201-019-00437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Wang X.; Niu Y. Visible Light Driven Photocatalytic Degradation of Norfloxacin Using 3D Supramolecular Compounds. J. Cluster Sci. 2023, 34, 2643–2652. 10.1007/s10876-023-02412-z. [DOI] [Google Scholar]
- Zhu X.; Han S.; Feng W.; Kong Q.; Dong Z.; Wang C.; Lei J.; Yi Q. The effect of heat treatment on the anatase–rutile phase transformation and photocatalytic activity of Sn-doped TiO 2 nanomaterials. RSC Adv. 2018, 8 (26), 14249–14257. 10.1039/C8RA00766G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi A. K.; Ahmed H. M.; Wahab M.; Katheria S.; Wabaidur S. M.; Eldesoky G. E.; Islam M. A.; Rane K. P. Synthesis and applications of green synthesized TiO2 nanoparticles for photocatalytic dye degradation and antibacterial activity. J. Nanomater. 2022, 2022, 7060388. 10.1155/2022/7060388. [DOI] [Google Scholar]
- Abebe B. Synergetic and charge transfer properties of a metal oxide heterojunction: Photocatalytic activities. Frontiers in Catalysis 2022, 2, 950384. 10.3389/fctls.2022.950384. [DOI] [Google Scholar]
- Toncón-Leal C. F.; Villarroel-Rocha J.; Silva M. T. P. d.; Braga T. P.; Sapag K. Characterization of mesoporous region by the scanning of the hysteresis loop in adsorption–desorption isotherms. Adsorption 2021, 27 (7), 1109–1122. 10.1007/s10450-021-00342-8. [DOI] [Google Scholar]
- Baudys M.; Berthet E.; Macak J. M.; Lhotka M.; Krýsa J. Photocatalytic degradation of gaseous pollutants on nanostructured TiO2 films of various thickness and surface area. Photochem. Photobiol. Sci. 2023, 22, 883–892. 10.1007/s43630-022-00359-0. [DOI] [PubMed] [Google Scholar]
- Bharti B.; Kumar S.; Lee H.-N.; Kumar R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6 (1), 32355. 10.1038/srep32355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeez F.; Al-Hetlani E.; Arafa M.; Abdelmonem Y.; Nazeer A. A.; Amin M. O.; Madkour M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8 (1), 7104. 10.1038/s41598-018-25673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouk Z.; Islam M.; Gutiérrez M.; Pireaux J.-J.; Achour A. X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films. Nanomaterials 2022, 12 (20), 3692. 10.3390/nano12203692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandiyanto A. B. D.; Oktiani R.; Ragadhita R. How to read and interpret FTIR spectroscope of organic material. Indonesian Journal of Science and Technology 2019, 4 (1), 97–118. 10.17509/ijost.v4i1.15806. [DOI] [Google Scholar]
- Alamier W. M.; DY Oteef M.; Bakry A. M.; Hasan N.; Ismail K. S.; Awad F. S. Green Synthesis of Silver Nanoparticles Using Acacia ehrenbergiana Plant Cortex Extract for Efficient Removal of Rhodamine B Cationic Dye from Wastewater and the Evaluation of Antimicrobial Activity. ACS Omega 2023, 8, 18901. 10.1021/acsomega.3c01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. A.; Farha A.; Kotkata M. Sol–Gel Synthesized Co-Doped Anatase TiO 2 Nanoparticles: Structural, Optical, and Magnetic Characterization. Journal of Inorganic and Organometallic Polymers and Materials 2019, 29, 1375–1382. 10.1007/s10904-019-01102-6. [DOI] [Google Scholar]
- Aravind M.; Amalanathan M.; Mary M. S. M. Synthesis of TiO 2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 1–10. 10.1007/s42452-021-04281-5. [DOI] [Google Scholar]
- Ali H. M.; Ibrahim S. M.; Zeid E. F. A.; Al-Hossainy A. F.; Abd El-Aal M. A comparative study of Cu-anchored 0D and 1D ZnO nanostructures for the reduction of organic pollutants in water. RSC Adv. 2022, 12 (26), 16496–16509. 10.1039/D2RA02515A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D.; Zhi J.; Wang Q.; Yan W.; Guo Y.; Han Y.; Dong C.; Xiao Y.; Bai H.; Liang W.; Fan L. Efficient photoelectrochemical aptasensing of di-2-ethylhexyl phthalate in environmental samples based on N, S co-doped graphene quantum dots/TiO2 nanorods. Anal. Chim. Acta 2023, 1271, 341477. 10.1016/j.aca.2023.341477. [DOI] [PubMed] [Google Scholar]
- Farooq U.; Ahmed J.; Alshehri S. M.; Ahmad T. High-surface-area sodium tantalate nanoparticles with enhanced photocatalytic and electrical properties prepared through polymeric citrate precursor route. ACS omega 2019, 4 (21), 19408–19419. 10.1021/acsomega.9b02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych A.; Bobowska I.; Tracz M.; Opasinska A.; Kadlubowski S.; Krzywania-Kaliszewska A.; Grobelny J.; Wojciechowski P. Dielectric properties and characterisation of titanium dioxide obtained by different chemistry methods. J. Nanomater. 2014, 2014, 1–9. 10.1155/2014/124814. [DOI] [Google Scholar]
- El-Deen S.; Hashem A.; Abdel Ghany A.; Indris S.; Ehrenberg H.; Mauger A.; Julien C. Anatase TiO 2 nanoparticles for lithium-ion batteries. Ionics 2018, 24, 2925–2934. 10.1007/s11581-017-2425-y. [DOI] [Google Scholar]
- Raguram T.; Rajni K. Synthesis and analysing the structural, optical, morphological, photocatalytic and magnetic properties of TiO 2 and doped (Ni and Cu) TiO 2 nanoparticles by sol–gel technique. Appl. Phys. A: Mater. Sci. Process. 2019, 125, 1–11. 10.1007/s00339-019-2581-1. [DOI] [Google Scholar]
- Kundu A.; Mondal A. Photodegradation of methylene blue under direct sunbeams by synthesized anatase titania nanoparticles. SN Appl. Sci. 2019, 1, 1–17. 10.1007/s42452-019-0280-3. [DOI] [Google Scholar]
- Al-Taweel S. S.; Saud H. R. New route for synthesis of pure anatase TiO2 nanoparticles via utrasound-assisted sol-gel method. J. Chem. Pharm. Res. 2016, 8 (2), 620–626. [Google Scholar]
- Makuła P.; Pacia M.; Macyk W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. ACS Publications 2018, 9, 6814–6817. 10.1021/acs.jpclett.8b02892. [DOI] [PubMed] [Google Scholar]
- Praveen P.; Viruthagiri G.; Mugundan S.; Shanmugam N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles–Synthesized via sol–gel route. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2014, 117, 622–629. 10.1016/j.saa.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Mortazavian S.; Saber A.; James D. E. Optimization of photocatalytic degradation of acid blue 113 and acid red 88 textile dyes in a UV-C/TiO2 suspension system: application of response surface methodology (RSM). Catalysts 2019, 9 (4), 360. 10.3390/catal9040360. [DOI] [Google Scholar]
- Reza K. M.; Kurny A.; Gulshan F. Parameters affecting the photocatalytic degradation of dyes using TiO 2: a review. Applied Water Science 2017, 7, 1569–1578. 10.1007/s13201-015-0367-y. [DOI] [Google Scholar]
- Rao K. G.; Ashok C.; Rao K. V.; Chakra C.; Tambur P. Green synthesis of TiO2 nanoparticles using Aloe vera extract. Int. J. Adv. Res. Phys. Sci. 2015, 2 (1A), 28–34. [Google Scholar]
- Bopape D. A.; Tetana Z. N.; Mabuba N.; Motaung D. E.; Hintsho-Mbita N. C. Biosynthesis of TiO2 nanoparticles using Commelina benghanlensis for the photodegradation of methylene blue dye and antibiotics: effect of plant concentration. Results in Chemistry 2023, 5, 100825. 10.1016/j.rechem.2023.100825. [DOI] [Google Scholar]
- Amanulla A. M.; Sundaram R. Green synthesis of TiO2 nanoparticles using orange peel extract for antibacterial, cytotoxicity and humidity sensor applications. Mater. Today: Proc. 2019, 8, 323–331. 10.1016/j.matpr.2019.02.118. [DOI] [Google Scholar]
- Rathi V. H.; Jeice A. R. Green fabrication of titanium dioxide nanoparticles and their applications in photocatalytic dye degradation and microbial activities. Chemical Physics Impact 2023, 6, 100197. 10.1016/j.chphi.2023.100197. [DOI] [Google Scholar]
- Saranya K. S.; Vellora Thekkae Padil V.; Senan C.; Pilankatta R.; Saranya K.; George B.; Wacławek S.; Černík M. Green synthesis of high temperature stable anatase titanium dioxide nanoparticles using gum kondagogu: characterization and solar driven photocatalytic degradation of organic dye. Nanomaterials 2018, 8 (12), 1002. 10.3390/nano8121002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi G.; Majid A.; Riaz A.; Alharbi T.; Kamran M. A.; Al-Habardi M. Green synthesis of spherical TiO2 nanoparticles using Citrus Limetta extract: Excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun. 2021, 129, 108618. 10.1016/j.inoche.2021.108618. [DOI] [Google Scholar]
- Mugumo R.; Ichipi E.; Tichapondwa S. M.; Chirwa E. M. N., Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using CuS/ZnS PN Heterojunction Nanocomposite under Visible Light Irradiation. 2023 10.20944/preprints202306.1272.v1. [DOI]
- Salesi S.; Nezamzadeh-Ejhieh A. An experimental design study of photocatalytic activity of the Z-scheme silver iodide/tungstate binary nano photocatalyst. Environ. Sci. Pollut. Res. 2023, 30, 105440–105456. 10.1007/s11356-023-29730-z. [DOI] [PubMed] [Google Scholar]
- Saharudin K. A.; Sreekantan S.; Basiron N.; Khor Y. L.; Harun N. H.; Mydin R. B. S. M. N.; Md Akil H.; Seeni A.; Vignesh K. Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios. Polymers 2018, 10 (8), 878. 10.3390/polym10080878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Miao B.; Chen Q.; Bai Z.; Cao Y.; Davaa B. Synthesis, structure, and photocatalytic activity of TiO2-montmorillonite composites. Catalysts 2022, 12 (5), 486. 10.3390/catal12050486. [DOI] [Google Scholar]
- Kumari H.; Sonia; Suman; Ranga R.; Chahal S.; Devi S.; Sharma S.; Kumar S.; Kumar P.; Kumar S.; Kumar A.; Parmar R. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water, Air, Soil Pollut. 2023, 234 (6), 349. 10.1007/s11270-023-06359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir M. B.; Sohaib M.; Sagir M.; Rafique M. Role of nanotechnology in photocatalysis. Encyclopedia of Smart Materials 2022, 578. 10.1016/B978-0-12-815732-9.00006-1. [DOI] [Google Scholar]
- Kermani M.; Mostafapour A.; Sabouri Z.; Gheibihayat S. M.; Darroudi M. The photocatalytic, cytotoxicity, and antibacterial properties of zinc oxide nanoparticles synthesized using Trigonella foenum-graecum L extract. Environmental Science and Pollution Research 2023, 30 (7), 19313–19325. 10.1007/s11356-022-23518-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.