Abstract
Background
Perinatal stroke refers to a diverse but specific group of cerebrovascular diseases that occur between 20 weeks of fetal life and 28 days of postnatal life. Acute treatment options for perinatal stroke are limited supportive care, such as controlling hypoglycemia and seizures. Stem cell‐based therapies offer a potential therapeutic approach to repair, restore, or regenerate injured brain tissue. Preclinical findings have culminated in ongoing human neonatal studies.
Objectives
To evaluate the benefits and harms of stem cell‐based interventions for the treatment of stroke in newborn infants compared to control (placebo or no treatment) or stem‐cell based interventions of a different type or source.
Search methods
We searched CENTRAL, PubMed, Embase, and three trials registries in February 2023. We planned to search the reference lists of included studies and relevant systematic reviews for studies not identified by the database searches.
Selection criteria
We attempted to include randomized controlled trials, quasi‐randomized controlled trials, and cluster trials that evaluated any of the following comparisons.
• Stem cell‐based interventions (any type) versus control (placebo or no treatment) • Mesenchymal stem/stromal cells (MSCs) of a specifictype (e.g. number of doses or passages) or source (e.g. autologous/allogeneic or bone marrow/cord) versus MSCs of another type or source • Stem cell‐based interventions (other than MSCs) of a specific type (e.g. mononuclear cells, oligodendrocyte progenitor cells, neural stem cells, hematopoietic stem cells, or induced pluripotent stem cell‐derived cells) or source (e.g. autologous/allogeneic or bone marrow/cord) versus stem cell‐based interventions (other than MSCs) of another type or source • MSCs versus stem cell‐based interventions other than MSCs
We planned to include all types of transplantation regardless of cell source (bone marrow, cord blood, Wharton's jelly, placenta, adipose tissue, peripheral blood), type of graft (autologous or allogeneic), and dose.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were all‐cause neonatal mortality, major neurodevelopmental disability, and immune rejection or any serious adverse event. Our secondary outcomes included all‐cause mortality prior to first hospital discharge, seizures, adverse effects, and death or major neurodevelopmental disability at 18 to 24 months of age. We planned to use GRADE to assess the certainty of evidence for each outcome.
Main results
We identified no completed or ongoing randomized trials that met our inclusion criteria. We excluded three studies: two were phase 1 trials, and one included newborn infants with conditions other than stroke (i.e. cerebral ischemia and anemia).
Among the three excluded studies, we identified the first phase 1 trial on the use of stem cells for neonatal stroke. It reported that a single intranasal application of bone marrow‐derived MSCs in term neonates with a diagnosis of perinatal arterial ischemic stroke (PAIS) was feasible and apparently not associated with severe adverse events. However, the trial included only 10 infants, and follow‐up was limited to three months.
Authors' conclusions
No evidence is currently available to evaluate the benefits and harms of stem cell‐based interventions for treatment of stroke in newborn infants. We identified no ongoing studies.
Future clinical trials should focus on standardizing the timing and method of cell delivery and cell processing to optimize the therapeutic potential of stem cell‐based interventions and safety profiles. Phase 1 and large animal studies might provide the groundwork for future randomized trials. Outcome measures should include all‐cause mortality, major neurodevelopmental disability and immune rejection, and any other serious adverse events.
Keywords: Female; Humans; Infant; Infant, Newborn; Pregnancy; Infant Mortality; Seizures; Seizures/therapy; Stem Cell Transplantation; Stem Cell Transplantation/adverse effects; Stroke; Stroke/therapy
Plain language summary
Stem cell‐based therapies for stroke in newborns
Review question
Can stem cell‐based therapies save the lives or improve the long‐term development of newborns who have a stroke?
Background
Newborns sometimes develop stroke, which occurs when blood supply to part of the brain is blocked or when a blood vessel in the brain bursts. Babies with less severe stroke may make a full recovery or may have only mild intellectual problems. If the stroke is very big it can lead to death or to severe neurodevelopmental problems later in life. Some of these babies develop intellectual disabilities, behavioral problems, concentration difficulties, socialization problems, and cerebral palsy. Currently, there is no treatment for stroke.
What did we want to find out?
The aim of this Cochrane Review was to assess whether stem cell‐based therapies could reduce death and improve the long‐term development of newborns with stroke. We also wanted to know if this treatment had any unwanted effects. During stem cell‐based therapy, stem cells are implanted into the baby (e.g. by injection) so that they can repair the brain cells that have been damaged by stroke. These stem cells may have come from humans or animals and may have been taken from cord blood, bone marrow, or other parts of the body.
What did we do?
We searched medical databases for clinical trials that looked at stem cell‐based therapies for stroke in newborns.
Key results
We were unable to include any studies in our review. We identified three studies, but we excluded them all because they had an ineligible design or because they included newborns without stroke.
How current is the evidence?
The evidence is current to February 2023.
Background
Description of the condition
Perinatal stroke refers to a diverse but specific group of cerebrovascular diseases that occur between 20 weeks of fetal life and 28 days of postnatal life (Dunbar 2018; Raju 2007). Timing of the insult and of the clinical presentation vary. Perinatal stroke can be primarily hemorrhagic or ischemic, and related to arterial or venous occlusion (Dunbar 2018). Acute presentations include neonatal arterial ischemic stroke (NAIS), neonatal cerebral sinovenous thrombosis (CSVT), and neonatal hemorrhagic stroke (NHS; Dunbar 2018). Delayed presentations include arterial presumed perinatal ischemic stroke (APPIS), periventricular venous infarction (PVI), and presumed perinatal hemorrhagic stroke (PPHS; Dunbar 2018). Newborns with acute symptomatic perinatal strokes typically present with focal seizures or encephalopathy within the first days of postnatal life. Other commonly used terms are perinatal arterial ischemic stroke (PAIS) and perinatal ischemic stroke (PIS), but these are not interchangeable (Mailo 2021). While PAIS/PIS include strokes that occur between 20 weeks of gestation and the first seven days of postnatal life, NAIS covers the time from birth until the 28th day of postnatal life (Mailo 2021).
The estimated birth prevalence of perinatal stroke is between 1:3000 and 1:1600, although these conditions are likely underdiagnosed (Laugesaar 2007; Nelson 2004). One study conducted in southern Alberta, Canada, estimated a higher prevalence of 1:1100, probably owing to the higher sensitivity of prospective methods and more specific classifications of perinatal stroke (Dunbar 2020). NAIS is the most common type of acute neonatal stroke, representing approximately 70% to 80% of all symptomatic perinatal stroke cases (Dunbar 2019). Neonates with NAIS often present with seizures in the first days after birth, sometimes accompanied by (asymmetric) hypotonia, lethargy, and apnea (Raju 2007).
Stroke in preterm infants is gaining attention in the literature, probably owing to the increased awareness of stroke in this population and more sophisticated neuroimaging techniques (Roy 2022). The estimated birth prevalence of stroke in preterm infants varies widely in the published literature, from 1:8000 to 1:1600, though these are probably underestimations (Dunbar 2020). The most common type of stroke observed in preterm infants is periventricular hemorrhagic infarction (PVHI), followed by PAIS and CSVT (Roy 2022). Stroke in preterm infants is unlikely to manifest as focal seizures and may be asymptomatic or have a nonspecific clinical presentation (Roy 2022).
The pathogenesis of perinatal stroke is not completely understood. Multifactorial interactions related to both maternal‐placental and fetal‐neonatal disorders may play a role (Dunbar 2018). Different studies have linked the pathogenesis of NAIS with several independent risk factors such as male sex, chorioamnionitis, multiple births, prematurity, and small weight for gestational age (Li 2017a; Sorg 2020).
Overall, outcomes from perinatal stroke are poor, with approximately 75% of survivors experiencing neurodevelopmental deficits (Dunbar 2018; Srivastava 2021). The most frequent complications include sensorimotor deficits, intellectual disabilities, language impairments, behavioral disorders, and epilepsy (Bolk 2022; Kirton 2013; Sundelin 2021). Perinatal stroke is the leading cause of unilateral or hemiparetic cerebral palsy. The location of the lesion determines whether the lower or upper extremities are more affected, and symptoms range from subtle deficits to severely restricted movements and posturing (Kirton 2021).
Acute treatment options for perinatal stroke are limited supportive care, such as controlling hypoglycemia and seizures (Ferriero 2019). As these treatments offer only symptomatic care and no cure, additional therapeutic strategies for perinatal stroke are urgently needed. One exception is the use of anticoagulants for CSVT (Moharir 2010).
Description of the intervention
Since the early 2010s, investigation into stem cells for the treatment of brain damage and bronchopulmonary dysplasia (BPD) in newborns has become one of the most rapidly increasing areas of neonatal research. Several stem cell types are implicated in different brain injury and BPD models. The most common type of stem cell used in animal studies and clinical trials is mesenchymal stem/stromal cells (MSCs; El‐Kadiry 2021). These cells are easy to isolate and have a good safety profile; they dampen the inflammation and oxidative stress, reduce cell death, and restore energy failure (Thébaud 2021; Trounson 2015). The criteria for defining MSCs include adherence to plastic in standard culture conditions, expression and absence of specific surface antigen markers, and capacity for multipotent differentiation (Dominici 2006). Other stem cell‐based therapies (also known as regenerative cells) include neural stem cells (NSCs), mononuclear cells, human amnion epithelial cells, hematopoietic stem cells, endothelial cells (colony‐forming and progenitor), bone marrow‐derived cells, human amniotic fluid stem cells, cord blood stem cells, and inducible pluripotent stem cell (iPSC)‐derived cells (Pimentel‐Coelho 2012; Thébaud 2021).
The efficacy of regenerative cells depends on the cell type, tissue source, and laboratory processing. Amniotic fluid, umbilical cord tissue/blood, or placental tissue can be collected during the perinatal period (Parolini 2014; Sanberg 2014; Srivastava 2018). These sources were once considered medical waste, but they offer a vast supply of regenerative cells, characterized by high proliferative rates/differentiation potential, the paracrine release of biological factors, and low likelihood of immunoreactivity following transplantation (Batsali 2013; Moreira 2019). Of note, the cord blood of preterm infants contains a larger amount of immature hematopoietic progenitors and MSCs with higher proliferative potential compared with the cord blood of term infants (Podesta 2015). Despite the low immunogenicity of regenerative cells, autologous transplantation is preferable as it entails a lower risk of infection and immune rejection (Gebler 2012). Furthermore, allogeneic transplantation offers considerable practical advantages, such as the rapid availability of disease‐free products that are generated in a cost‐effective manner (Hare 2017).
How the intervention might work
One systematic review and meta‐analysis showed that MSCs may improve sensorimotor and cognitive performance in an animal model of PAIS (Lehnerer 2022). MSCs exert their neuroprotective role via mitochondrial transfer, by modulating immune‐inflammatory response and regulating reactive oxygen species (ROS) production (Nair 2021). MSCs inhibit apoptosis by transporting mitochondria through tunneling nanotubules (TNTs) to rescue aerobic respiration (Liu 2014). The transfer of healthy mitochondria to injured cells via TNTs increases the selective autophagy of damaged mitochondria and thereby maintains cellular homeostasis and regulates oxidative stress (Hagberg 2014; Li 2017b). MCSs appear to improve neurogenesis through the paracrine release of factors such as basic fibroblast growth factor, insulin‐like growth factor 1, and anti‐inflammatory cytokines (Li 2002; van Velthoven 2010; van Velthoven 2011; van Velthoven 2012). Furthermore, MSCs seem to modulate the local immune response by regulating the function of immune cells, such as T‐cells and B‐cells, macrophages, and dendritic cells (Iyer 2008). In injured brain tissue, MSCs up‐regulate the expression of genes associated with cell proliferation (e.g. secreted phosphoprotein 1 [Spp1] and interleukin [IL]‐17), neurogenesis (neural cell adhesion molecule and nerve growth factor), migration (CXC chemokine receptor 4 [CXCR4]), neuronal survival (glial‐derived neurotrophic factor), and down‐regulation of genes involved in inflammation, such as IL‐1β (van Velthoven 2011). The systematic review and meta‐analysis of preclinical studies on PAIS showed that MSC treatment following stroke led to reduced lesion size (both in neuroimaging and in immunohistochemistry studies), increased neurogenesis and myelinization, and enhanced synaptic plasticity (Lehnerer 2022).
Mononuclear cells are another type of regenerative cell. In an adult rodent model of stroke, bone marrow‐derived mononuclear cells (BMMNCs) reduced the neurological deficit and cortical brain loss by decreasing CD4+ T‐cell and microglial infiltration to the injury site (McDonald 2018; Sato 2018), and attenuated the expression of several acute injury biomarkers, including active caspase‐3 and ED1 (Sato 2018). One phase 1 clinical trial in adults with acute stroke showed that BMMNC treatment was safe, and neuroimaging studies revealed enhanced fractional anisotropy two years after stroke, suggesting microstructural repair (Vahidy 2019).
NSCs are multipotent cells that are generally found in the ventricular‐subventricular zone of the forebrain lateral ventricles and the subgranular zone of the dentate gyrus of the hippocampus (Toda 2001). Through the paracrine action and secretion of neurotrophic factors such as brain‐derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), NSCs restore neuronal functions by enhancing neurogenesis and proliferation (Smith 2012). In addition, NSCs can differentiate into neurons and glial cells, and may help to restore neurogenesis and gliogenesis affected in perinatal stroke. Preclinical evidence for perinatal brain injury suggests that NSCs decrease brain inflammation assessed by either the number of microglia and macrophages or IL‐1β (Braccioli 2017; Ji 2015; Shin 2018). In addition, Smith and colleagues showed in their meta‐analysis that NSC administration decreases infarct size and improves motor and cognitive function (Smith 2021). This beneficial effect was also evident in preclinical models of adult stroke, where NSCs significantly reduced brain infarct size and improved functional deficits (Chen 2016; Huang 2018).
Another neural tissue regenerative cell type is oligodendrocyte progenitor cells (OPCs). OPCs administered to an experimental model of periventricular leukomalacia migrated to injured areas, matured to oligodendrocytes expressing myelin basic protein (MBP), and attenuated the loss of host MBP in the corpus callosum (Kim 2018). OPCs differentiate into myelin‐producing cells, and BDNF expression and B‐cell lymphoma 2 (Bcl‐2) expression appear to be mechanisms by which OPCs encourage learning and memory in neonatal asphyxia (Chen 2015; Wang 2021).
The neuroprotective effects of endothelial progenitor cells (EPCs) are ascribed to their ability to decrease neuroinflammation and cell apoptosis (Grandvuillemin 2017). Research suggests that EPCs can secrete protective cytokines and growth factors. These factors promote the self‐repair of injured endothelial cells and mediate neighboring injured endothelial cells with normal structure and function, extending into injured sites and performing the function of repair (Li 2015). In one study, intraperitoneal injection of human umbilical vein EPCs preserved microvessels and lessened apoptosis in the cortex of treated animals, via regulation of stromal cell‐derived factor 1 and CXCR4 (Wu 2013). Furthermore, in an adult rodent model of stroke, treatment with human EPCs reduced infarction volume three days after injury, reduced cortical atrophy, and improved neurobehavioral outcomes four weeks after injury (Fan 2010).
Human umbilical cord 34+ cells given 48 hours after middle cerebral artery occlusion in a neonatal rodent stroke model transiently increased cerebral blood flow and blood vessel diameter in the peri‐infarct area (Tsuji 2014). Although ipsilateral hemisphere volume loss was lower in animals treated with human umbilical cord 34+ cells at seven weeks after injury, there were no differences in neurobehavioral outcomes between intervention and control group animals (Tsuji 2014). These immunohistochemical findings can be attributed to the release of growth factors (i.e. VEGF and glial‐derived neurotrophic factor) known to stimulate neurogenesis and angiogenesis (Verina 2013).
There are controversial data on the neuroprotective effects of iPSC‐derived cells in adult rodent stroke models. This is mainly due to the type of iPSC‐derived cells; undifferentiated cells have been shown to cause tumors (Kawai 2010). The administration of iPSC‐derived neural progenitor and precursor cells has been shown to improve sensorimotor function (Lau 2018; Oh 2020), to reduce lesion volume (Oh 2020), and to promote neurogenesis and angiogenesis (Oh 2020). Different effects have been reported for the administration of iPSC‐derived NSCs. While one study showed improved neurological function due to immunomodulatory and anti‐inflammatory effects (Eckert 2015), another observed no reduction in infarct volume or improvement in behavior (Jensen 2013). Positive effects have been reported for iPSC‐derived MSCs (Xia 2020) and neuroepithelial‐like stem cells (Oki 2012).
Considered together, regenerative cells may exert their therapeutic benefit through multiple routes to establish a favorable environment for tissue regeneration, which ultimately leads to better functional outcomes following perinatal stroke.
Why it is important to do this review
Several preclinical studies have provided evidence for the therapeutic effect of regenerative cell‐based therapy in the neonatal period, particularly on the condition of BPD. Two published reviews of preclinical trials showed a significant therapeutic benefit of MSC therapy on several outcome measures and suggested that MSCs are the most effective therapy for BDP (Augustine 2020; Augustine 2017). Other studies have indicated that MSCs have a significant positive effect on neurobehavioral outcomes for neonates with hypoxic ischemic encephalopathy (HIE; Archambault 2017; Serrenho 2021), and are effective in repairing brain tissue and attenuating brain damage following PAIS and intraventricular hemorrhage (IVH; Ahn 2016; Park 2017; Wagenaar 2018). The tissue most frequently used to derive regenerative cells is the umbilical cord (Ahn 2016; Mitsialis 2016). While most research has focused on MSCs, a few trials are evaluating neural progenitor cells, mononuclear cells, and placenta/cord blood cells (Chen 2021; NCT02854579; Zhou 2022).
To date, clinical trials in the USA studying the safety, feasibility, or efficacy of regenerative cells have been registered for BPD, HIE, PAIS, hypoplastic left heart syndrome, congenital hydrocephalus, and IVH (Zhou 2022). Liau 2020 identified 29 registered studies for neonatal diseases. Four studies are currently ongoing for neonatal brain diseases (NCT01962233; NCT02854579; NCT02890953; NCT03635450).
One meta‐analysis including eight studies (321 adults) across different disciplines evaluated the safety of MSCs and found no association between the intervention and acute infusional toxicity, organ system complications, infection, death, or malignancy (Lalu 2012). Safety is a critical concern, particularly with regard to the unwanted differentiation of the transplanted cells and their potential to suppress antitumor immune response and generate new blood vessels that could promote tumor growth and metastasis (Volarevic 2018). However, the risk of potential tumorigenicity related to MSC‐based interventions needs to be further elucidated (Barkholt 2013). Cochrane Reviews have investigated MSCs and other stem cell‐based interventions for the prevention and treatment of different conditions in infants: BDP (Pierro 2017), HIE (Bruschettini 2020), and IVH (Romantsik 2023). This Cochrane Review will focus on the treatment of stroke in newborn infants.
Objectives
To evaluate the benefits and harms of stem cell‐based interventions for the treatment of stroke in newborn infants compared to control (placebo or no treatment) or stem‐cell based interventions of a different type or source.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs.
Types of participants
We planned to include preterm and term infants of postmenstrual age (PMA) up to 46 weeks and 0 days, irrespective of their gestational age at birth, with any type of perinatal stroke. We planned to address studies that included both newborn infants and older infants by contacting study authors to obtain separate outcome data for the newborn infants (if not available in the publication). If we could not obtain this information, we planned to exclude the study if the mean PMA of the included participants was above 46 weeks and 0 days.
Types of interventions
The following comparisons were eligible.
Stem cell‐based interventions (any type) versus control (placebo or no treatment)
MSCs of a specifictype (e.g. number of doses or passages) or source (e.g. autologous/allogeneic or bone marrow/cord) versus MSCs of another type or source
Stem cell‐based interventions (other than MSCs) of a specific type (e.g. mononuclear cells, OPCs, NSCs, hematopoietic stem cells, or iPSCs) or source (e.g. autologous/allogeneic or bone marrow/cord) versus stem cell‐based interventions (other than MSCs) of another type or source
MSCs versus stem cell‐based interventions other than MSCs
We planned to include all types of transplantation regardless of cell source (bone marrow, cord blood, Wharton's jelly, placenta, adipose tissue, peripheral blood), type of graft (autologous or allogeneic), and dose. We excluded stem cell‐derived cerebral organoids (Di Lullo 2017).
For further information about the characteristics of the interventions and co‐interventions (e.g. cooling), see Subgroup analysis and investigation of heterogeneity.
Types of outcome measures
Outcome measures did not form part of our eligibility criteria.
Primary outcomes
All‐cause neonatal mortality (before 28 days' chronological age)
-
Major neurodevelopmental disability (presence of any of the following conditions). We planned to perform separate analyses for children aged 18 to 24 months and children aged three to five years.
Cerebral palsy
Developmental delay (Bayley Mental Developmental Index [BMDI] or Griffiths Mental Development Scale [GMDS] score greater than 2 standard deviations [SDs] below the mean; Bayley 1993; Bayley 2006; Griffiths 1954)
Intellectual impairment (intelligence quotient [IQ] greater than 2 SDs below the mean)
Blindness (vision less than 6/60 in both eyes)
Sensorineural deafness requiring amplification (Jacobs 2013)
Immune rejection or any serious adverse event (certain, probable, or possible, according to the World Health Organization [WHO] probability scale). We planned to consider post‐hoc analyses for any unexpected adverse effects reported by the studies.
Secondary outcomes
All‐cause mortality prior to first hospital discharge
-
Seizures (suspected clinically or identified by electroencephalogram [EEG] or amplitude‐integrated electroencephalogram [aEEG])
Seizures during neonatal period (after MSC administration)
Seizures or need for anticonvulsants at 12 to 14 months' chronological age
Adverse effects (e.g. significant decrease or increase in systemic/diastolic blood pressure or heart rate; vomiting; diarrhea; exanthema; or systemic infection)
Death or major neurodevelopmental disability (as defined in Primary outcomes) at 18 to 24 months' chronological age
-
Each component of major neurodevelopmental disability. We planned to report these long‐term outcome components for all studies that evaluated children after 18 months' chronological age. We planned to perform separate analyses for children aged 18 to 24 months and those aged three to five years.
Cerebral palsy
-
Developmental delay
BMDI or GMDS score greater than 2 SDs below the mean
Neuromotor development (Bayley Scales of Infant Development – Psychomotor Development Index [BSID‐PDI]) assessed in survivors
Cognitive development (Bayley Scales of Infant Development – Mental Development Index [BSID‐MDI]) assessed in survivors
Intellectual impairment (IQ greater than 2 SD below the mean)
Blindness (vision less than 6/60 in both eyes)
Sensorineural deafness requiring amplification
Stroke volume on cerebral magnetic resonance imaging (MRI)
Duration of hospital stay (days)
Tumor formation (any type, any location) detected by MRI or computed tomography (CT) when assessing the risk of tumorigenicity of donor MSCs
Search methods for identification of studies
The Cochrane Sweden Information Specialist developed a draft search strategy for PubMed (National Library of Medicine) in consultation with the review authors (Appendix 1). An Information Specialist peer reviewed this strategy using the PRESS Checklist (McGowan 2016a; McGowan 2016b). We translated the PubMed strategy for other databases using appropriate syntax.
We used a population filter developed by Cochrane Neonatal. The Cochrane Sweden Information Specialist adapted the RCT search filter for Ovid MEDLINE to the syntax of PubMed for identification of randomized and quasi‐randomized studies. We performed searches for eligible trials without language, publication year, publication type, or publication status restrictions.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2023, Issue 2) in the Cochrane Library (searched 23 February 2023)
PubMed (1946 to 23 February 2023)
Embase.com via Elsevier (1974 to 24 February 2023)
Searching other resources
We identified trial registration records using CENTRAL and by independent searches of the following registries.
ISRCTN registry (www.isrctn.com, searched 24 February 2023)
US National Institutes of Health (NIH) Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 24 February 2023)
WHO International Clinical Trials Registry Platform (ICTRP; trialsearch.who.int; searched 24 February 2023)
In addition, we planned to search the reference lists of included studies and relevant systematic reviews for studies not identified by the database searches. However, we identified no eligible trials or relevant systematic reviews.
We planned to search for errata and retraction notices published on PubMed for included studies (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
For each included study, we planned to collect information regarding the method of randomization, blinding, interventions, stratification, and whether the trial was single‐center or multicenter. We planned to analyze the clinical outcomes listed in Types of outcome measures.
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management software and removed duplicates. Two review authors (OR, AR) independently screened the remaining titles and abstracts and excluded the records they considered clearly ineligible. Two review authors (OR, AR) then assessed the full‐text articles of the remaining records. When disagreements arose at any point during the screening process, we resolved them by discussion or by consulting a third review author (MB). We documented the reasons for excluding studies during the full‐text review in the Characteristics of excluded studies table (reasons for exclusion were the absence of one or more PICO‐S elements; where a study omitted more than one PICO‐S element, we documented only one). We collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We also planned to provide any information we could obtain about ongoing studies. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
Two review authors (OR, AR) planned to independently extract data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care (EPOC) Group data collection checklist (Cochrane EPOC Group 2017). We planned to pilot the form within the review team using a sample of included studies.
We planned to extract the following characteristics from each included study.
Administrative details: study author(s), publication status, year of publication, dates and duration of trial, funding source(s), presence of vested interests, details of other relevant papers cited
Study characteristics: study registration, study design, study setting, number and location of study centers, informed consent, ethics approval, details of any run‐in period (if applicable), completeness of follow‐up (e.g. greater than 80%)
Participants: number randomized, number lost to follow‐up/withdrawn, number analyzed, mean gestational age, gestational age range, mean chronological age, chronological age range, sex, severity of condition, diagnostic criteria, inclusion and exclusion criteria
Interventions: initiation, dose, and duration of administration
Outcomes as listed in Types of outcome measures
We planned to resolve any disagreements by discussion.
We planned to describe ongoing studies identified by our search and document available information such as the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date, and record them in the Characteristics of ongoing studies table.
We planned to contact study investigators/authors if we had any queries or required additional data. Two review authors (OR, AR) would have used Cochrane statistical software for data entry (RevMan Web 2022). We planned to replace any standard error of the mean (SEM) by the corresponding SD.
Assessment of risk of bias in included studies
Had we identified any eligible trials, two review authors (OR, AR) would have independently assessed the risk of bias (low, high, or unclear) of all included trials using the original Cochrane risk of bias tool (RoB 1), which covers the following domains (Higgins 2017).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other potential sources of bias
We planned to resolve any disagreements by discussion or by consulting a third review author (MB). See Appendix 2 for a more detailed description of risk of bias for each domain. We planned to assign an overall risk of bias rating as follows.
Low overall risk of bias: all domains at low risk of bias
Unclear overall risk of bias: one or more domains at unclear risk of bias and no domains at high risk of bias
High overall risk of bias: at least one domain at high risk of bias
Measures of treatment effect
For dichotomous data, we planned to present results using risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs). We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs if the RD showed a statistically significant reduction (or increase).
For continuous data, we planned to use mean differences (MDs) for outcomes measured in the same way across trials, and standardized mean differences (SMDs) to combine data from trials that measured the same outcome using different methods. Had we identified trials that reported continuous data as median and interquartile range (IQR) and the data passed the test of skewness, we would have converted the median to the mean and estimated the SD as IQR/1.35.
Had we found RCTs that reported data in a format that could not be entered directly into a meta‐analysis, we would have converted the data to the required format using the information inChapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
Unit of analysis issues
We planned to perform the primary analysis per individual randomized.
For cluster‐randomized trials, we would have extracted information on the study design and unit of analysis for each study, indicating whether clustering of observations was present due to allocation to the intervention at the group level or clustering of individually randomized observations (e.g. patients within clinics). We would have extracted the available statistical information needed to account for the implications of clustering on the estimation of outcome variances, such as design effects or intracluster correlation coefficients (ICCs), and record whether the study had adjusted results for the correlations in the data. Where a study had not accounted for clustering, we planned to make appropriate adjustments to the effective sample size following Cochrane guidance (Higgins 2020). Where possible, we planned to derive the ICC for these adjustments from the trial itself, or from a similar trial. If an appropriate ICC was unavailable, we would have conducted sensitivity analyses to investigate the potential effect of clustering by imputing a range of values of ICC.
Had we identified any trials that compared multiple eligible arms to the same control condition, we would have either combined groups to create a single pair‐wise comparison, or selected one pair of interventions and excluded the other(s). We planned to list all treatment arms in the Characteristics of included studies table, even if they were not used in the review.
Dealing with missing data
We planned to carry out analyses on an intention‐to‐treat basis for all outcomes wherever feasible (i.e. we planned to analyze all participants in the treatment group to which they were randomized, regardless of the actual treatment received). Had we identified important missing data (in the outcomes) or unclear data, we would have contacted the original investigators to request the missing data or clarifications. We planned to make explicit the assumptions of any methods used to deal with missing data. We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in the undertaken assumptions. We would have addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
Had we included any studies, we would have described the clinical diversity and methodological variability of the evidence narratively and in tables. The tables would have included data on study characteristics such as design features, population characteristics, and intervention details.
To assess statistical heterogeneity, we planned to visually inspect forest plots and describe the direction and magnitude of effects and the degree of overlap between CIs. We planned to also consider the heterogeneity statistics generated in forest plots. We would have used the I² statistic to quantify inconsistency among the trials in each analysis, and we would also have considered the P value from the Chi² test to assess if this heterogeneity was significant (P < 0.1). If we identified substantial heterogeneity, we planned to report the finding and explore possible explanatory factors using prespecified subgroup analysis.
We planned to interpret the degree of heterogeneity as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
We planned to use this rough guideline to interpret the I² value rather than a simple threshold, and our interpretation would have taken into account an understanding that measures of heterogeneity (I² and Tau) are estimated with high uncertainty when the number of studies is small (Deeks 2020).
Assessment of reporting biases
We planned to assess reporting bias by comparing the prespecified primary and secondary outcomes with the reported outcomes. Where study protocols were available, we planned to compare these to the full publications to determine the likelihood of reporting bias. If any eligible studies did not report any of our primary or secondary outcomes, we planned to describe them in the Characteristics of included studies table.
If we had included more than 10 studies in a single meta‐analysis, we would have used funnel plots to screen for publication bias. Where visual assessment suggested significant funnel plot asymmetry, we planned to incorporate this into our assessment of certainty of the evidence (Egger 1997). The capacity of funnel plots to detect publication bias decreases with the number of studies included; where a meta‐analysis includes 10 or fewer studies, we planned to simply note our inability to rule out possible publication bias or small‐study effects.
Data synthesis
Had we identified multiple studies that we considered sufficiently similar, we would have performed meta‐analysis using Review Manager Web (RevMan Web 2022). For categorical outcomes, we planned to calculate the typical estimates of RR and RD, each with its 95% CI; for continuous outcomes, we planned to calculate the MD or the SMD, each with its 95% CI. We planned to use a fixed‐effect model to combine data where it was reasonable to assume that studies had estimated the same underlying treatment effect. If we judged meta‐analysis to be inappropriate, we planned to analyze and interpret individual trials separately. If there was evidence of clinical heterogeneity, we planned to try to explain this based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We planned to interpret the results of tests for subgroup differences with caution given the potential for confounding with other study characteristics and the observational nature of the comparisons, as recommended in Section 10.10.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020). In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain a valid difference in effects, and we would not have included these analyses in our results. When subgroup comparisons were possible, we planned to perform stratified meta‐analysis and a formal statistical test for interaction to examine subgroup differences that could account for effect heterogeneity (e.g. Cochran's Q test, meta‐regression; Borenstein 2013; Higgins 2022).
Given the potential differences in the effects of interventions related to gestational age, source of the stem cell‐based interventions, and dose, as discussed in the Background section, we planned to conduct the following subgroup analyses.
Gestational age: term infants (37 weeks' gestation or more), moderately preterm infants (32 to 36 weeks' gestation), very preterm infants (less than 32 weeks' gestation), extremely preterm infants (less than 28 weeks' gestation)
MSCs source: bone marrow, cord blood, Wharton's jelly, placenta, adipose tissue, peripheral blood
MSCs dose: less than 2 × 10⁷/kg versus 2 × 10⁷/kg or greater
Number of doses: multiple versus single administration
We planned to perform subgroup analyses of the primary outcomes if sufficient studies reported the outcome (at least five studies per subgroup).
Sensitivity analysis
If we identified substantial heterogeneity, we planned to conduct sensitivity analysis to determine whether the findings were affected by the inclusion of only those trials considered to have used adequate methodology (at low risk of selection, performance, and reporting bias). We planned to report the results of sensitivity analyses for primary outcomes only.
As there is no formal statistical test for sensitivity analysis, we planned to provide informal comparisons between the different ways of estimating the effect under different assumptions. Changes in the P values should not be used to judge whether there is a difference between the main analysis and sensitivity analysis, because statistical significance may be lost with fewer studies included.
Had we performed any sensitivity analyses, we would have reported the results in tables rather than forest plots.
Summary of findings and assessment of the certainty of the evidence
If we had identified any eligible studies, we would have used the GRADE approach, as outlined in the GRADE Handbook, to assess the certainty of evidence for the following (clinically relevant) outcomes (Schünemann 2013; Schünemann 2020).
All‐cause neonatal mortality (before 28 days' chronological age)
Major neurodevelopmental disability
Immune rejection or any serious adverse event
All‐cause mortality prior to first hospital discharge
Seizures
Adverse effects
Death or major neurodevelopmental disability at 18 to 24 months of age
Two review authors (OR, MB) would have independently assessed the certainty of the evidence for each outcome. We planned to use GRADEpro GDT software to create a summary of findings table for each of the comparisons listed in Types of interventions. We planned to consider evidence from RCTs as high certainty to begin with, downgrading by one level for serious (or two levels for very serious) limitations related to design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We planned to report our GRADE ratings in the summary of findings tables.
We planned to interpret the GRADE ratings as follows.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Figure 1 shows the study selection process in a PRISMA diagram.
1.
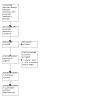
PRISMA flow diagram.
Results of the search
The search identified 214 references. We excluded 210 references based on their title/abstract and reviewed four full‐text articles. Two articles were from the same study (Baak 2022). We excluded all three studies (Baak 2022; NCT02460484; NCT03352310), and we identified no completed or ongoing studies for inclusion in this review. For details of the excluded studies, see the Characteristics of excluded studies table.
Included studies
We identified no trials that matched our inclusion criteria.
Excluded studies
We excluded three studies during full‐text review: two were not randomized trials (Baak 2022; NCT02460484), and one included infants with cerebral ischemia and anemia rather than stroke (NCT03352310).
Baak 2022 enrolled 10 neonates with MRI‐confirmed PAIS in the middle cerebral artery region. The median gestational age was 40.3 weeks (IQR 39.9 to 41.1), and the median birth weight was 3415 g (IQR 3140 to 3834). The neonates received one dose of 45 ×106 to 50 ×106 bone marrow‐derived MSCs (from a healthy 12‐year‐old male donor) intranasally within seven days of presenting signs of PAIS. The primary endpoints were acute and subacute safety outcomes, assessed by vital signs at the time of treatment, blood sampling before and after MSC treatment, and the occurrence of any adverse event or toxicity in the first three months after treatment. The occurrence of unexpected cerebral abnormalities at three months of age was a secondary endpoint. One participant developed a fever one hour after MSC administration, but there were no serious adverse events or other signs of toxicity reported after treatment or during the three‐month follow‐up. No unexpected structural cerebral abnormalities were observed on the repeat MRI scan at three months of follow‐up. All 10 participants had signs of pre‐Wallerian degeneration of the corticospinal tract on the initial MRI scan. Although the study could not conclude on the efficacy of MSC treatment, the follow‐up MRI scan at three months showed asymmetry of the corticospinal tract in only four of 10 participants.
NCT02460484 is a suspended study. It was initially designed to test the safety of intravenous autologous human umbilical cord blood (hUCB) given to children aged six weeks to six years after a PAIS. The primary outcome was safety, including hemodynamic, pulmonary, renal, and neurological status.
NCT03352310 is an ongoing study investigating the feasibility and safety of autologous umbilical cord blood transfusion to treat newborn infants with clinical signs of neonatal HIE and anemia.
Risk of bias in included studies
No studies met the eligibility criteria of this review.
Discussion
Summary of main results
We found no published or ongoing RCTs addressing the benefits or harms of stem cell‐based therapies for prevention or treatment of stroke in newborn infants.
Overall completeness and applicability of evidence
Among the excluded studies, we identified the first clinical trial on the use of stem cells for the treatment of neonatal stroke (Baak 2022). In this phase 1 trial, conducted in the Netherlands, term neonates with a diagnosis of PAIS were treated with a single intranasal application of bone marrow‐derived MSCs. The administration was feasible and apparently not associated with adverse events. However, the sample size was very small (10 infants) and the follow‐up was limited to three months. The results should be interpreted with caution. We did not include this study in our review because it is a phase 1 trial.
In adults, only two RCTs with over 100 participants are registered, but neither has been completed (Negoro 2019). Currently, no effective therapies for neonatal stroke are available. Cell‐based therapies are significant contenders for treatment, as paracrine factors can act on the multifactorial mechanisms implicated in brain injury. Although exciting progress has been noted in animal studies (Lehnerer 2022), successful translation into the clinic requires an improved understanding of key fundamental questions in stem cell biology.
Potential biases in the review process
We used the standard methods of Cochrane Neonatal to conduct this systematic review, and we applied no language restrictions. It is unlikely that our search strategy missed relevant trials, so we are confident that this systematic review summarizes all the available RCT evidence on stem cell‐based interventions for stroke in newborn infants.
Agreements and disagreements with other studies or reviews
Cochrane Reviews have evaluated stem‐cell based interventions for other indications: hypoxic‐ischemic encephalopathy (Bruschettini 2020), IVH (Romantsik 2023), and BPD (Pierro 2017). In animal models of PAIS, MSCs may improve sensorimotor and cognitive performance (Lehnerer 2022).
Authors' conclusions
Implications for practice.
No evidence is currently available to evaluate the benefits and harms of stem cell‐based interventions for treatment of stroke in newborn infants. We identified no ongoing studies.
Implications for research.
Clinical trials should focus on standardizing the timing and method of cell delivery and cell processing to optimize the therapeutic potential of stem cell‐based interventions and safety profiles. Phase 1 studies and large animal studies might provide the groundwork for future randomized trials. Outcome measures should include all‐cause mortality, major neurodevelopmental disability and immune rejection, or any other serious adverse events.
History
Protocol first published: Issue 2, 2023
Acknowledgements
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Matthias Bank (Library and ICT services, Lund University) designed and ran the literature searches.
Souvik Mitra (Departments of Pediatrics, Community Health & Epidemiology, Dalhousie University & IWK Health Centre, Halifax, Canada) and Beatriz Fernandez‐Munoz (Departamento de Farmacia y Tecnología Farmacéutica, Facultad de Farmacia, Universidad de Sevilla, Sevilla, Spain) peer‐reviewed the review.
We thank Julia Turner (Cochrane Central Production Service) for copy‐editing the review.
We would like to thank the following members of Cochrane Neonatal for their support: Michelle Fiander and Jane Cracknell (Managing Editors), Roger Soll (Joint Co‐ordinating Editor), and Bill McGuire (Joint Co‐ordinating Editor).
Appendices
Appendix 1. Search strategies
Information specialist: Matthias Bank
Affiliation: Lund University, Faculty of Medicine, Library & ICT, Sweden
PubMed (National Library of Medicine)
Date of search: 23 February 2023
No publication date limitations or language limitations were used.
Search filters: The Cochrane Neonatal search filter for RCTs was used and converted to the PubMed syntax. The Cochrane Neonatal filter for neonates was used and converted to the PubMed syntax.
#1 "Stem Cells"[Mesh] OR "Stem Cell Transplantation"[Mesh] OR “Fetal Blood”[Mesh] OR "Stromal Cells"[Mesh] 365,673
#2 “stem cell”[TW] OR “stem cells”[TW] OR “mesenchymal cell”[TW] OR “mesenchymal cells” [TW] OR “mononuclear cell”[TW] OR “mononuclear cells”[TW] OR “progenitor cell” [TW] OR “progenitor cells” [TW] OR “cord blood” [TW] OR “cord blood cell”[TW] OR “regenerative cell” [TW] OR “regenerative cells” [TW] OR "stromal cell"[TW] OR "stromal cells"[TW] OR “Wharton* jelly cell*”[TW] OR telopode*[TW] OR telocyte*[TW] OR “interstitial cajal‐like cell*”[TW] OR “fetal blood”[TW] OR “mother cell”[TW] OR “mother cells”[TW] OR “progenitor cell*”[TW] OR “colony forming unit*”[TW] 644,727
#3 #1 OR #2 659,648 STEM‐CELL BASED INTERVENTIONS
#4 ("stroke"[Mesh]) OR stroke[TW] OR AIS[TW] OR NAIS[TW] OR PAIS[TW] OR cerebral sinovenous thrombosis[TW] OR CSVT[TW] OR “hemorrhagic stroke”[Mesh] OR APPIS[TW] OR periventricular venous infarction[TW] OR PPHS[TW] 398,062
STROKE
#5 (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) 5,664,013
#6 quasirandom*[tw] or quasi‐random*[tw] or randomi*[tw] or randomly[tw] 1,297,556
#7 control*[tw] AND (group[tw] OR groups[tw] OR random[tw] OR trial[tw] OR trials[tw] OR study[tw]) 3,826,235
#8 #5 OR #6 OR #7 7,524,074
#9 (animals [mh] NOT humans [mh]) 5,093,871
#10 #8 NOT #9 6,451,829
RANDOMIZED CONTROLLED TRIALS
#11 (infant, newborn[Mesh]) OR (intensive care, neonatal[Mesh]) OR (intensive care units, neonatal[Mesh]) OR (gestational age[Mesh]) 716,615
#12 babe[TW] OR babes[TW] baby*[TW] OR babies[TW] OR gestational age[TW] OR gestational ages[TW] OR infant[TW] OR infants[TW] OR infant s[TW] OR infant's[TW] OR infantile[TW] OR infancy[TW] OR low birth weight[TW] OR low birthweight[TW] OR neonat*[TW] OR neo‐nat*[TW] OR newborn*[TW] OR new born[TW] OR new borns[TW] OR newly born[TW] OR premature[TW] OR prematures[TW] OR pre‐mature*[TW] OR prematurity[TW] OR pre‐maturity[TW] OR preterm[TW] OR preterms[TW] OR pre term[TW] OR pre terms[TW] OR preemie[TW] OR preemies[TW] OR premies[TW] OR premie[TW] OR VLBW[TW] OR VLBWI[TW] OR VLBW‐I[TW] OR VLBWs[TW] OR LBW[TW] OR LBWI[TW] OR LBWs[TW] OR ELBW[TW] OR ELBWI[TW] OR ELBWs[TW] OR NICU[TW] OR NICUs[TW] 1,788,548
#13 #11 OR #12 1,788,548
NEONATES
#9 #3 AND #4 AND #10 AND #13 71
INTERVENTIONS, STROKE, RCT AND NEONATES
CENTRAL via Cochrane Library Online (Issue 2 of 12, February 2023)
Date of search: 23 February 2023 No publication date limitations or language limitations were used.
Search filters: The Cochrane Neonatal filter for Cochrane Library, neonatal populations, was used.
#1 MeSH descriptor: [Stem Cells] explode all trees 1,108
#2 MeSH descriptor: [Stem Cell Transplantation] explode all trees 2,855
#3 MeSH descriptor: [Fetal Blood] explode all trees 765
#4 MeSH descriptor: [Stromal Cells] explode all trees 314
#5 ("stem cell" OR "stem cells" OR "mesenchymal cell" OR “mesenchymal cells” OR “mononuclear cell” OR “mononuclear cells” OR “progenitor cell” OR “progenitor cells” OR “cord blood” OR “cord blood cell”):ti,ab,kw OR (“regenerative cell” OR “regenerative cells” OR "stromal cell" OR "stromal cells" OR “Wharton jelly cell*” OR telopode* OR telocyte* OR “interstitial cajal‐like cell*” OR “fetal blood”):ti,ab,kw OR (“mother cell” OR “mother cells” OR “colony forming unit*”):ti,ab,kw (Word variations have been searched) 25,694
#6 #1 OR #2 OR #3 OR #4 OR #5 25,802 STEM‐CELL BASED INTERVENTIONS
#7 MeSH descriptor: [Stroke] explode all trees 14,102
#8 (stroke OR AIS OR NAIS OR PAIS OR "cerebral sinovenous thrombosis" OR CSVT OR APPIS OR "periventricular venous infarction" OR PPHS):ti,ab,kw (Word variations have been searched) 74,642
#9 MeSH descriptor: [Hemorrhagic Stroke] explode all trees 29
#10 #7 OR #8 OR #9 75,127 STROKE
#11 MeSH descriptor: [Infant, Newborn] explode all trees 20,204
#12 MeSH descriptor: [Intensive Care, Neonatal] explode all trees 375
#13 MeSH descriptor: [Intensive Care Units, Neonatal] explode all trees 1,014
#14 MeSH descriptor: [Gestational Age] explode all trees 3,120
#15 ("babe" or "babes" or baby* or "babies" or "gestational age" or "gestational ages" or infant? or "infantile" or infancy or "low birth weight" OR "low birth weights" or "low birthweight" or "low birthweights" or neonat* or "neo‐nat*" or newborn* or "new born?" or "newly born" or "premature" or "pre‐mature" or "pre‐matures" or prematures or prematurity or "pre‐maturity" or "preterm" or "preterms" or "pre term?" or "preemie" or "preemies" or "premies" or "premie" or "VLBW" or "VLBWI" or "VLBW‐I" or "VLBWs" or "LBW" or "LBWI" or "LBWs" or "ELBW" or "ELBWI" or "ELBWs" or "NICU" or "NICUs"):ti,ab,kw (Word variations have been searched) 106,409
#16 #11 OR #12 OR #13 OR #14 OR #15 106,409
NEONATES
#17 #6 AND #10 AND #16 45
Embase.com (Elsevier, 1947‐present)
Date of search: 24 February 2023. No publication date limitations or language limitations were used.
Search filters: The Cochrane Neonatal filter for RCTs for OVID Embase was adapted to the syntax of Embase.com. The Cochrane Neonatal filter for neonatal populations for OVID Embase was adapted to the syntax of Embase.com.
#1. 'stem cell'/exp OR 'stem cell transplantation'/exp OR 'fetus blood'/exp OR 'stroma cell'/exp 649,516
#2. 'stem cell':ti,ab,kw OR 'stem cells':ti,ab,kw OR 'mesenchymal cell':ti,ab,kw OR 'mesenchymal cells':ti,ab,kw OR 'mononuclear cell':ti,ab,kw OR 'mononuclear cells':ti,ab,kw OR 'progenitor cell':ti,ab,kw OR 'progenitor cells':ti,ab,kw OR 'cord blood':ti,ab,kw OR 'cord blood cell':ti,ab,kw OR 'regenerative cell':ti,ab,kw OR 'regenerative cells':ti,ab,kw OR 'stromal cell':ti,ab,kw OR 'stromal cells':ti,ab,kw OR 'wharton jelly cell*':ti,ab,kw OR telopode*:ti,ab,kw OR telocyte*:ti,ab,kw OR 'interstitial cajal‐like cell*':ti,ab,kw OR 'fetal blood':ti,ab,kw OR 'mother cell':ti,ab,kw OR 'mother cells':ti,ab,kw OR 'colony forming unit*':ti,ab,kw 781,825
#3 #1 OR #2 926,830 STEM‐CELL BASED INTERVENTIONS
#4. 'cerebrovascular accident'/exp OR 'hemorrhagic stroke'/exp 549,271
#5. stroke:ti,ab,kw OR ais:ti,ab,kw OR nais:ti,ab,kw OR pais:ti,ab,kw OR 'cerebral sinovenous thrombosis':ti,ab,kw OR csvt:ti,ab,kw OR appis:ti,ab,kw OR 'periventricular venous infarction':ti,ab,kw OR pphs:ti,ab,kw 502,788
#6. #4 OR #5 735,065 STROKE
#07. 'randomized controlled trial'/de OR 'controlled clinical trial'/de 931,378
#08. random*:ti,ab,kw 1,899,243
#09. 'randomization'/de 96,240
#10. placebo:ti,ab,kw 356,113
#11. ((double OR single OR doubly OR singly) NEAR/2 (blind OR blinded OR blindly)):ti,ab,kw 270,078
#12. 'double blind procedure'/de 204,485
#13. (controlled NEAR/7 (study OR design OR trial)):ti,ab,kw 442,116
#14. 'parallel group$':ti,ab 30,886
#15. crossover:ti,ab OR 'cross over':ti,ab 121,130
#16. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group$ OR intervention$ OR patient$ OR subject$ OR participant$)):ti,ab 398,901
#17. (open NEAR/2 label):ti,ab 103,792
#18. quasirandom*:ti,ab,kw OR 'quasi random*':ti,ab,kw OR randomi*:ti,ab,kw OR randomly:ti,ab,kw 1,545,108
#19. (control* NEAR/2 (group$ OR random*)):ti,ab,kw 1,260,658
#20. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 3,229,687
#21. ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de) AND ('human'/de OR 'normal human'/de OR 'human cell'/de) 25,866,370
#22. 'animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de 33,557,211
#23. #22 NOT #21 7,690,841
#24. #20 NOT #23 2,775,444 RANDOMIZED CONTROLLED TRIALS
#25. 'newborn'/de OR 'prematurity'/de OR 'newborn intensive care'/de OR 'newborn care'/de OR 'gestational age'/de 854,761
#26. babe:ti,ab,kw OR babes:ti,ab,kw OR baby*:ti,ab,kw OR babies:ti,ab,kw OR ‘gestational age$’:ti,ab,kw OR infant$:ti,ab,kw OR infantile:ti,ab,kw OR infancy:ti,ab,kw OR 'low birth weight':ti,ab,kw OR 'low birthweight':ti,ab,kw OR neonat*:ti,ab,kw OR 'neo‐nat*':ti,ab,kw OR newborn*:ti,ab,kw OR ‘new born$’:ti,ab,kw OR 'newly born':ti,ab,kw OR premature:ti,ab,kw OR 'pre mature':ti,ab,kw OR 'pre matures':ti,ab,kw OR prematures:ti,ab,kw OR prematurity:ti,ab,kw OR 'pre maturity':ti,ab,kw OR preterm:ti,ab,kw OR preterms:ti,ab,kw OR ‘pre term$’:ti,ab,kw OR preemie:ti,ab,kw OR preemies:ti,ab,kw OR premies:ti,ab,kw OR premie:ti,ab,kw OR vlbw:ti,ab,kw OR vlbwi:ti,ab,kw OR 'vlbw i':ti,ab,kw OR vlbws:ti,ab,kw OR lbw:ti,ab,kw OR lbwi:ti,ab,kw OR lbws:ti,ab,kw OR elbw:ti,ab,kw OR elbwi:ti,ab,kw OR elbws:ti,ab,kw OR nicu:ti,ab,kw OR nicus:ti,ab,kw 1,290,641
#27. #25 OR #26 1,606,784 NEONATES
#28. #3 AND #6 AND #24 AND #27 120 INTERVENTIONS, STROKE, RCT AND NEONATES
Number of records from literature databases 236
Search strategies in clinical trial registries
ClinicalTrials.gov (US National Library of Medicine)
Date of search: 24 February 2023
Condition or disease: stroke OR hemorrhagic stroke OR periventricular venous infarction OR cerebral sinovenous thrombosis OR AIS OR NAIS OR PAIS OR APPIS OR CSVT OR PPHS
Other terms: premature OR prematurity OR preterms OR preterm OR “very low birth” OR “low birth weight” OR newborn OR newborns OR neonate OR neonates OR infant OR infants
Intervention/treatment: stem cells OR mesenchymal cells OR mononuclear cells OR progenitor cells OR cord blood OR regenerative cells OR stromal cells OR Wharton jelly cells OR telopode OR telocyte OR interstitial cajal‐like cells OR fetal blood 4 records
International Clinical Trials Registries Platform Search Portal, ICTRP (World Health Organization)
Date of search: 24 February 2023
Advanced search Condition: stroke OR hemorrhagic stroke OR periventricular venous infarction OR cerebral sinovenous thrombosis OR AIS OR NAIS OR PAIS OR APPIS OR CSVT OR PPHS Intervention: stem cells OR mesenchymal cells OR mononuclear cells OR progenitor cells OR cord blood OR regenerative cells OR stromal cells OR Wharton jelly cells OR telopode OR telocyte OR interstitial cajal‐like cells OR fetal blood Filters: clinical trials in children 0 records
Number of records from trial registries 4 records
Total number from literature databases and trial registries 240
Number of duplicates automatically removed in EndNote 19
Appendix 2. Cochrane risk of bias tool (RoB 1)
We planned to use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological certainty of the trials. For each trial, we planned to seek information regarding the method of randomization, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We planned to assess each criterion as being at either low, high, or unclear risk of bias. Had we included any trials, two review authors would have independently assessed each study and resolved any disagreements through discussion. We planned to add this information to the Characteristics of included studies table. If needed, we would have explored the impact of the level of bias by undertaking sensitivity analyses.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we planned to categorize the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we planned to categorize the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
or unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we planned to categorize the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we planned to categorize the methods used to blind outcome assessment. Blinding was to be assessed separately for different outcomes or classes of outcomes. We planned to categorize the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we planned to describe the completeness of data including attrition and exclusions from the analysis. We planned to note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We planned to categorize the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
or unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we planned to describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we planned to compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we planned to contact study authors to gain access to the study protocol. We planned to assess the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
or unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we planned to describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We planned to each study as being at:
low risk;
high risk;
unclear risk.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baak 2022 | Not a randomized trial. Phase 1 trial (10 infants enrolled) conducted in the Netherlands. Term neonates with a diagnosis of PAIS were treated with a single intranasal application of bone marrow‐derived MSCs |
| NCT02460484 | Not a randomized trial. |
| NCT03352310 | Including newborn infants with conditions other than stroke (i.e. cerebral ischemia and anemia). |
MSC: mesenchymal stem/stromal cells; PAIS: perinatal arterial ischemic stroke.
Differences between protocol and review
We made no changes to the published protocol (Bruschettini 2023).
Contributions of authors
MB: developed and contributed to writing and editing the review, made an intellectual contribution to the review, advised on the review, approved the final version of the review prior to submission, and is the guarantor of the review. AB: developed and contributed to writing and editing the review, made an intellectual contribution to the review, advised on the review, and approved the final version of the review prior to submission. OR: conceived the review question, developed and contributed to writing and editing the review, made an intellectual contribution to the review, advised on the review, and approved the final version of the review prior to submission.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB and OR are employed by this organization.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden
Cochrane Sweden is supported by Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland.
Declarations of interest
MB: is an Associate Editor for Cochrane Neonatal. However, he had no involvement in the editorial processing of this review. AB: has no conflicts of interest to declare. OR: has no conflicts of interest to declare.
New
References
References to studies excluded from this review
Baak 2022 {published data only}
- Baak LM, Wagenaar N, Aa NE, Groenendaal F, Dudink J, Tataranno ML, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurology 2022;21(6):528-36. [DOI: 10.1016/S1474-4422(22)00117-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT03356821. Perinatal arterial stroke treated with stromal cells intranasally (PASSIoN) [Adult mesenchymal stromal cells to regenerate the neonatal brain: the PASSIoN trial (Perinatal Arterial Stroke Treated With Stromal Cells IntraNasally)]. ClinicalTrials.gov/show/NCT03356821 (first received 29 November 2017).
NCT02460484 {published data only}
- NCT02460484. Safety of autologous human umbilical cord blood treatment for perinatal arterial ischemic stroke [Safety of autologous human umbilical cord blood treatment for perinatal]. ClinicalTrials.gov/show/NCT02460484 (first received 2 June 2015).
NCT03352310 {published data only}
- NCT03352310. Feasibility and safety of umbilical cord blood transfusion in the treatment of neonatal cerebral ischemia and anemia [Umbilical cord blood mononuclear cell bank in Hong Kong and treatment of neonatal cerebral ischemia and anemia - part IV clinical trial]. ClinicalTrials.gov/show/NCT03352310 (first received 24 November 2017).
Additional references
Ahn 2016
- Ahn SY, Chang YS, Park WS. Stem cells for neonatal brain disorders. Neonatology 2016;109(4):377-83. [DOI: 10.1159/000444905] [PMID: ] [DOI] [PubMed] [Google Scholar]
Archambault 2017
- Archambault J, Moreira A, McDaniel D, Winter L, Sun L, Hornsby P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: a systematic review and meta-analysis of preclinical studies. PLOS One 2017;12(12):e0189895. [DOI: 10.1371/journal.pone.0189895] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustine 2017
- Augustine S, Avey MT, Harrison B, Locke T, Ghannad M, Moher D, et al. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Translational Medicine 2017;6(12):2079-93. [DOI: 10.1002/sctm.17-0126] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustine 2020
- Augustine S, Cheng W, Avey MT, Chan ML, Lingappa SM, Hutton B, et al. Are all stem cells equal? Systematic review, evidence map, and meta-analyses of preclinical stem cell-based therapies for bronchopulmonary dysplasia. Stem Cells Translational Medicine 2020;9(2):158-68. [DOI: 10.1002/sctm.19-0193] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkholt 2013
- Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies – bridging scientific observations and regulatory viewpoints. Cytotherapy 2013;15(7):753-9. [DOI: 10.1016/j.jcyt.2013.03.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Batsali 2013
- Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Current Stem Cell Research & Therapy 2013;8(2):144-55. [DOI: 10.2174/1574888x11308020005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development-II. San Antonio, Texas: Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, TX: Harcourt Assessment, 2006. [Google Scholar]
Bolk 2022
- Bolk J, Simatou E, Söderling J, Thorell LB, Persson M, Sundelin H. Association of perinatal and childhood ischemic stroke with attention-deficit/hyperactivity disorder. JAMA Network Open 2022;5(4):e228884. [DOI: 10.1001/jamanetworkopen.2022.8884] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prevention Science 2013;14(2):134-43. [DOI: 10.1007/s11121-013-0377-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Braccioli 2017
- Braccioli L, Heijnen CJ, Coffer PJ, Nijboer CH. Delayed administration of neural stem cells after hypoxia-ischemia reduces sensorimotor deficits, cerebral lesion size, and neuroinflammation in neonatal mice. Pediatric Research 2017;81(1):127-35. [DOI: 10.1038/pr.2016.172] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bruschettini 2020
- Bruschettini M, Romantsik O, Moreira A, Ley D, Thébaud B. Stem cell-based interventions for the prevention of morbidity and mortality following hypoxic-ischaemic encephalopathy in newborn infants. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013202. [DOI: 10.1002/14651858.CD013202.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015
- Chen LX, Ma SM, Zhang P, Fan ZC, Xiong M, Cheng GQ, et al. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PLOS One 2015;10(3):e0115997. [DOI: 10.1371/journal.pone.0115997] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016
- Chen L, Zhang G, Gu Y, Guo X. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Scientific Reports 2016;6(1):32291. [DOI: 10.1038/srep32291] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2021
- Chen J, Chen Y, Du X, Liu G, Fei X, Peng JR, et al. Integrative studies of human cord blood derived mononuclear cells and umbilical cord derived mesenchyme stem cells in ameliorating bronchopulmonary dysplasia. Frontiers in Cell and Developmental Biology 2021;9:679866. [DOI: 10.3389/fcell.2021.679866] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane EPOC Group 2017
- Cochrane Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors.
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Di Lullo 2017
- Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nature Reviews Neuroscience 2017;18(10):573-84. [DOI: 10.1038/nrn.2017.107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dominici 2006
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315-7. [DOI: 10.1080/14653240600855905] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunbar 2018
- Dunbar M, Kirton A. Perinatal stroke: mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet. Child & Adolescent Health 2018;2(9):666-76. [DOI: 10.1016/S2352-4642(18)30173-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunbar 2019
- Dunbar M, Kirton A. Perinatal stroke. Seminars in Pediatric Neurology 2019;32:100767. [DOI: 10.1016/j.spen.2019.08.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunbar 2020
- Dunbar M, Mineyko A, Hill M, Hodge J, Floer A, Kirton A. Population based birth prevalence of disease-specific perinatal stroke. Pediatrics 2020;146(5):e2020013201. [DOI: 10.1542/peds.2020-013201] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eckert 2015
- Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Translational Medicine 2015;4(7):841-51. [DOI: 10.5966/sctm.2014-0184] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Kadiry 2021
- El-Kadiry AE, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Frontiers in Medicine 2021;8:756029. [DOI: 10.3389/fmed.2021.756029] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2010
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Annals of Neurology 2010;67(4):488-97. [DOI: 10.1002/ana.21919] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferriero 2019
- Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, et al, American Heart Association Stroke Council and Council on Cardiovascular and Stroke Nursing. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke 2019;50(3):e51-e96. [DOI: 10.1161/STR.0000000000000183] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gebler 2012
- Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends in Molecular Medicine 2012;18(2):128-34. [DOI: 10.1016/j.molmed.2011.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed 27 February 2023). Available at gradepro.org.
Grandvuillemin 2017
- Grandvuillemin I, Garrigue P, Ramdani A, Boubred F, Simeoni U, Dignat-George F, et al. Long-term recovery after endothelial colony-forming cells or human umbilical cord blood cells administration in a rat model of neonatal hypoxic-ischemic encephalopathy. Stem Cells Translational Medicine 2017;6(11):1987-96. [DOI: 10.1002/sctm.17-0074] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The abilities of babies: a study of mental measurement. London: University of London Press, 1954. [Google Scholar]
Hagberg 2014
- Hagberg H, Mallard C, Rousset CI, Thornton C. Mitochondria: hub of injury responses in the developing brain. Lancet Neurology 2014;13(2):217-32. [DOI: 10.1016/S1474-4422(13)70261-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hare 2017
- Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. Journal of the American College of Cardiology 2017;69(5):526-37. [DOI: 10.1016/j.jacc.2016.11.009] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2020
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/V6.1.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M J, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook/archive/v6.3.
Huang 2018
- Huang H, Qian K, Han X, Li X, Zheng Y, Chen Z, et al. Intraparenchymal neural stem/progenitor cell transplantation for ischemic stroke animals: a meta-analysis and systematic review. Stem Cells International 2018;2018:4826407. [DOI: 10.1155/2018/4826407] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iyer 2008
- Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opinion on Biological Therapy 2008;8(5):569-81. [DOI: 10.1517/14712598.8.5.569] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2013
- Jensen MB, Yan H, Krishnaney-Davison R, Al Sawaf A, Zhang SC. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. Journal of Stroke and Cerebrovascular Diseases 2013;22(4):304-8. [DOI: 10.1016/j.jstrokecerebrovasdis.2011.09.008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2015
- Ji G, Liu M, Zhao XF, Liu XY, Guo QL, Guan ZF, et al. NF-κB signaling is involved in the effects of intranasally engrafted human neural stem cells on neurofunctional improvements in neonatal rat hypoxic–ischemic encephalopathy. CNS Neuroscience and Therapeutics 2015;21(12):926-35. [DOI: 10.1111/cns.12441] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawai 2010
- Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. Journal of Cerebral Blood Flow and Metabolism 2010;30(8):1487-93. [DOI: 10.1038/jcbfm.2010.32] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2018
- Kim TK, Park D, Ban YH, Cha Y, An ES, Choi J, et al. Improvement by human oligodendrocyte progenitor cells of neurobehavioral disorders in an experimental model of neonatal periventricular leukomalacia. Cell Transplant 2018;27(7):1168-77. [DOI: 10.1177/0963689718781330] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirton 2013
- Kirton A, Deveber G. Life after perinatal stroke. Stroke 2013;44(11):3265-71. [DOI: 10.1161/STROKEAHA.113.000739] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kirton 2021
- Kirton A, Metzler MJ, Craig BT, Hilderley A, Dunbar M, Giuffre A, et al. Perinatal stroke: mapping and modulating developmental plasticity. Nature Reviews Neurology 2021;17(7):415-32. [DOI: 10.1038/s41582-021-00503-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lalu 2012
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al, Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLOS One 2012;7(10):e47559. [DOI: 10.1371/journal.pone.0047559] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2018
- Lau VW, Platt SR, Grace HE, Baker EW, West FD. Human iNPC therapy leads to improvement in functional neurologic outcomes in a pig ischemic stroke model. Brain and Behaviour 2018;8(5):e00972. [DOI: 10.1002/brb3.972] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laugesaar 2007
- Laugesaar R, Kolk A, Tomberg T, Metsvaht T, Lintrop M, Varendi H, et al. Acutely and retrospectively diagnosed perinatal stroke: a population-based study. Stroke 2007;38(8):2234-40. [DOI: 10.1161/STROKEAHA.107.483743] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lehnerer 2022
- Lehnerer V, Roidl A, Romantsik O, Guzman R, Wellmann S, Bruschettini M. Mesenchymal stem cell therapy in perinatal arterial ischemic stroke: systematic review of preclinical studies. Pediatric Research 2022 Jul 29 [Epub ahead of print]. [DOI: 10.1038/s41390-022-02208-3] [PMID: ] [DOI] [PMC free article] [PubMed]
Li 2002
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat. Neurology 2002;59(4):514. [DOI: 10.1212/wnl.59.4.514] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2015
- Li YF, Ren LN, Guo G, Cannella LA, Chernaya V, Samuel S, et al. Endothelial progenitor cells in ischemic stroke: an exploration from hypothesis to therapy. Journal of Hematology Oncology 2015;8:33. [DOI: 10.1186/s13045-015-0130-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017a
- Li C, Miao JK, Xu Y, Hua YY, Ma Q, Zhou LL, et al. Prenatal, perinatal and neonatal risk factors for perinatal arterial ischaemic stroke: a systematic review and meta-analysis. European Journal of Neurology 2017;24(8):1006-15. [DOI: 10.1111/ene.13337] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2017b
- Li CJ, Chen PK, Sun LY, Pang CY. Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxidative Medicine and Cellular Longevity 2017;2017:8510805. [DOI: 10.1155/2017/8510805] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liau 2020
- Liau LL, Al-Masawa ME, Koh B, Looi QH, Foo JB, Lee SH, et al. The potential of mesenchymal stromal cell as therapy in neonatal diseases. Frontiers in Pediatrics 2020;8:591693. [DOI: 10.3389/fped.2020.591693] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014
- Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvascular Research 2014;92:10-8. [DOI: 10.1016/j.mvr.2014.01.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mailo 2021
- Mailo JA, Srivastava R, Jacob FD, Kirton A. What do we know about perinatal stroke? A review of current practices, outcomes, and future directions. Pediatric Stroke 2021;1:26-62. [pediatricstrokejournal.com/what-do-we-know-about-perinatal-stroke/] [Google Scholar]
McDonald 2018
- McDonald CA, Penny TR, Paton MC, Sutherland AE, Nekkanti L, Yawno T, et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. Journal of Neuroinflammation 2018;15(1):47. [DOI: 10.1186/s12974-018-1089-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGowan 2016a
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 Guideline Statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGowan 2016b
- McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&E). Ottawa: CADTH, 2016. [DOI] [PubMed] [Google Scholar]
Mitsialis 2016
- Mitsialis SA, Kourembanas S. Stem cell-based therapies for the newborn lung and brain: possibilities and challenges. Seminars Perinatology 2016;40(3):138-51. [DOI: 10.1053/j.semperi.2015.12.002] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moharir 2010
- Moharir MD, Shroff M, Stephens D, Pontigon AM, Chan A, MacGregor D, et al. Anticoagulants in pediatric cerebral sinovenous thrombosis: a safety and outcome study. Annals of Neurology 2010;67(5):590-9. [DOI: 10.1002/ana.21936] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moreira 2019
- Moreira A, Alayli Y, Balgi S, Winter C, Kahlenberg S, Mustafa S, et al. Upcycling umbilical cords: bridging regenerative medicine with neonatology. Journal of Maternal-Fetal & Neonatal Medicine 2019;32(8):1378-87. [DOI: 10.1080/14767058.2017.1405387] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2021
- Nair S, Rocha-Ferreira E, Fleiss B, Nijboer CH, Gressens P, Mallard C, et al. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: role of mitochondria, inflammation, and reactive oxygen species. Journal of Neurochemistry 2021;158(1):59-73. [DOI: 10.1111/jnc.15267] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01962233
- Umbilical cord derived mesenchymal stem cells therapy in hypoxic ischemic encephalopathy [Safety and efficacy investigation of patients with hypoxic ischemic encephalopathy by transplantation of umbilical cord derived mesenchymal stem cells]. clinicaltrials.gov/ct2/show/NCT01962233 (first received 14 October 2013). [CENTRAL: CN-02023314]
NCT02854579
- NCT02854579. Neural progenitor cell and paracrine factors to treat hypoxic ischemic encephalopathy [Safety and efficacy study of neural progenitor cell transplantation and paracrine factors from human mesenchymal stem cells to treat newborn with hypoxic‐ischemic encephalopathy]. clinicaltrials.gov/ct2/show/NCT02854579 (first received 3 August 2016). [CENTRAL: CN-01592246]
NCT02890953
- NCT02890953. Efficacy and safety of pneumostem® for IVH in premature infants (phase 2a) [Efficacy and safety evaluation of pneumostem® versus a control group for treatment of IVH in premature infants (phase 2a)]. clinicaltrials.gov/ct2/show/NCT02890953 (first received 7 September 2016). [CENTRAL: CN-01580101]
NCT03635450
- NCT03635450. Study of hCT-MSC in newborn infants with moderate or severe HIE. clinicaltrials.gov/ct2/show/NCT03635450 (first received 17 August 2018).
Negoro 2019
- Negoro T, Okura H, Maehata M, Hayashi S, Yoshida S, Takada N, et al. Trends in clinical trials for stroke by cell therapy: data mining ClinicalTrials.gov and the ICTRP portal site. NPJ Regenerative Medicine 2019;4(20):1-10. [DOI: 10.1038/s41536-019-0082-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2004
- Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurology 2004;3(3):150-8. [DOI: 10.1016/S1474-4422(04)00679-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oh 2020
- Oh SH, Jeong YW, Choi W, Noh JE, Lee S, Kim HS, et al. Multimodal therapeutic effects of neural precursor cells derived from human-induced pluripotent stem cells through episomal plasmid-based reprogramming in a rodent model of ischemic stroke. Stem Cells International 2020;2020:4061516.eCollection. [DOI: 10.1155/2020/4061516] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oki 2012
- Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 2012;30(6):1120-33. [DOI: 10.1002/stem.1104] [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2017
- Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Mesenchymal stem cells: the magic cure for intraventricular hemorrhage? Cell Transplantation 2017;26(3):439-48. [DOI: 10.3727/096368916X694193] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parolini 2014
- Parolini O, De D, Rodrigues M, Caruso M. Placental stem/progenitor cells: isolation and characterization. In: Perinatal Stem Cells. New York (NY): Springer, 2014:141-57. [Google Scholar]
Pierro 2017
- Pierro M, Thébaud B, Soll R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD011932. [DOI: 10.1002/14651858.CD011932.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pimentel‐Coelho 2012
- Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LM, Mendez-Otero R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatric Research 2012;71(4Pt2):464-73. [DOI: 10.1038/pr.2011.59] [PMID: ] [DOI] [PubMed] [Google Scholar]
Podesta 2015
- Podesta M, Bruschettini M, Cossu C, Sabatini F, Dagnino M, Romantsik O, et al. Preterm cord blood contains a higher proportion of immature hematopoietic progenitors compared to term sample. PLOS One 2015;10(9):e0138680. [DOI: 10.1371/journal.pone.0138680] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raju 2007
- Raju TN, Nelson KB, Ferriero D, Lynch JK, NICHD-NINDS Perinatal Stroke Workshop Participants. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007;120(3):609-16. [DOI: 10.1542/peds.2007-0336] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Romantsik 2023
- Romantsik O, Moreira A, Thébaud B, Ådén U, Ley D, Bruschettini M. Stem cell‐based interventions for the prevention and treatment of intraventricular haemorrhage and encephalopathy of prematurity in preterm infants. Cochrane Database of Systematic Reviews 2023, Issue 2. Art. No: CD013201. [DOI: 10.1002/14651858.CD013201.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2022
- Roy B, Walker K, Morgan C, Finch-Edmondson M, Galea C, Epi M, et al. Epidemiology and pathogenesis of stroke in preterm infants: a systematic review. Journal of Neonatal-Perinatal Medicine 2022;15(1):11-8. [DOI: 10.3233/NPM-200597] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanberg 2014
- Sanberg PR, Eve DJ, Borlongan CV. Umbilical cord blood cells in the repair of central nervous system diseases. In: Atala A, Murphy S, editors(s). Perinatal Stem Cells. New York (NY): Springer, 2014:269-87. [DOI: 10.1007/978- 1-4939-1118-9_25] [Google Scholar]
Sato 2018
- Sato Y, Ueda K, Kondo T, Hattori T, Mikrogeorgiou A, Sugiyama Y, et al. Administration of bone marrow-derived mononuclear cells contributed to the reduction of hypoxic-ischemic brain injury in neonatal rats. Frontiers in Neurology 2018;9:987. [DOI: 10.3389/fneur.2018.00987] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Serrenho 2021
- Serrenho I, Rosado M, Dinis A, Cardoso CM, Grãos M, Manadas B, et al. Stem cell therapy for neonatal hypoxic-ischemic encephalopathy: a systematic review of preclinical studies. International Journal of Molecular Sciences 2021;22(6):3142. [DOI: 10.3390/ijms22063142] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2018
- Shin JE, Jung K, Kim M, Hwang K, Lee H, Kim IS, et al. Brain and spinal cord injury repair by implantation of human neural progenitor cells seeded onto polymer scaffolds. Experimental & Molecular Medicine 2018;50(4):1-18. [DOI: 10.1038/s12276-018-0054-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2012
- Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells 2012;30(4):785-96. [DOI: 10.1002/stem.1024] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2021
- Smith MJ, Paton MC, Fahey MC, Jenkin G, Miller SL, Finch-Edmondson M, et al. Neural stem cell treatment for perinatal brain injury: a systematic review and meta-analysis of preclinical studies. Stem Cells Translational Medicine 2021;10(12):1621-36. [DOI: 10.1002/sctm.21-0243] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorg 2020
- Sorg AL, Kries R, Klemme M, Gerstl L, Weinberger R, Beyerlein A, et al. Risk factors for perinatal arterial ischaemic stroke: a large case-control study. Developmental Medicine and Child Neurology 2020;62(4):513-20. [DOI: 10.1111/dmcn.14347] [PMID: ] [DOI] [PubMed] [Google Scholar]
Srivastava 2018
- Srivastava M, Ahlawat N, Srivastava A. Amniotic fluid stem cells: a new era in regenerative medicine. Journal of Obstetrics and Gynaecology India 2018;68(1):15-9. [DOI: 10.1007/s13224-017-1034-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srivastava 2021
- Srivastava R, Kirton A. Perinatal stroke: a practical approach to diagnosis and management. NeoReviews 2021;22(3):e163-76. [DOI: 10.1542/neo.22-3-e163] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sundelin 2021
- Sundelin HE, Tomson T, Zelano J, Söderling J, Bang P, Ludvigsson JF. Pediatric ischemic stroke and epilepsy: a nationwide cohort study. Stroke 2021;52(11):3532-40. [DOI: 10.1161/STROKEAHA.121.034796] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thébaud 2021
- Thébaud B, Lalu M, Renesme L, Katwyk S, Presseau J, Thavorn K, et al. Benefits and obstacles to cell therapy in neonates: the INCuBAToR (Innovative Neonatal Cellular Therapy for Bronchopulmonary Dysplasia: Accelerating Translation of Research). Stem Cells Translational Medicine 2021;10(7):968-75. [DOI: 10.1002/sctm.20-0508] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toda 2001
- Toda H, Takahashi J, Iwakami N, Kimura T, Hoki S, Mozumi-Kitamura K, et al. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neuroscience Letters 2001;316(1):9-12. [DOI: 10.1016/s0304-3940(01)02331-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Trounson 2015
- Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 2015;17(1):11-22. [DOI: 10.1016/j.stem.2015.06.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsuji 2014
- Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Sato Y, Tsuda H, et al. Effects of intravenous administration of umbilical cord blood CD34+ cells in a mouse model of neonatal stroke. Neuroscience 2014;263:148-58. [DOI: 10.1016/j.neuroscience.2014.01.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vahidy 2019
- Vahidy FS, Haque ME, Rahbar MH, Zhu H, Rowan P, Aisiku IP, et al. Intravenous bone marrow mononuclear cells for acute ischemic stroke: safety, feasibility, and effect size from a phase I clinical trial. Stem Cells 2019;37(11):1481-91. [DOI: 10.1002/stem.3080] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Velthoven 2010
- Velthoven CT, Kavelaars A, Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. Journal of Neuroscience 2010;30(28):9603. [DOI: 10.1523/JNEUROSCI.1835-10.2010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Velthoven 2011
- Velthoven CT, Kavelaars A, Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain, Behavior, and Immunity 2011;25(7):1342-8. [DOI: 10.1016/j.bbi.2011.03.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Velthoven 2012
- Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatric Research 2012;71(2):474-81. [DOI: 10.1038/pr.2011.64] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verina 2013
- Verina T, Fatemi A, Johnston MV, Comi AM. Pluripotent possibilities: human umbilical cord blood cell treatment after neonatal brain injury. Pediatric Neurology 2013;48(5):346-54. [DOI: 10.1016/j.pediatrneurol.2012.10.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Volarevic 2018
- Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. International Journal of Medical Sciences 2018;15(1):36-45. [DOI: 10.7150/ijms.21666] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagenaar 2018
- Wagenaar N, Theije CG, Vries LS, Groenendaal F, Benders MJ, Nijboer CH. Promoting neuroregeneration after perinatal arterial ischemic stroke: neurotrophic factors and mesenchymal stem cells. Pediatric Research 2018;83(1-2):372-84. [DOI: ] [PMID: 10.1038/pr.2017.243] [DOI] [PubMed] [Google Scholar]
Wang 2021
- Wang X, Zang J, Yang Y, Lu S, Guan Q, Ye D, et al. Transplanted human oligodendrocyte progenitor cells restore neurobehavioral deficits in a rat model of preterm white matter injury. Frontiers in Neurology 2021;12:749244. [DOI: 10.3389/fneur.2021.749244] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2013
- Wu CC, Chen YC, Chang YC, Wang LW, Lin YC, Chiang YL, et al. Human umbilical vein endothelial cells protect against hypoxic-ischemic damage in neonatal brain via stromal cell-derived factor 1/C-X-C chemokine receptor type 4. Stroke 2013;44(5):1402-9. [DOI: 10.1161/STROKEAHA.111.000719] [PMID: ] [DOI] [PubMed] [Google Scholar]
Xia 2020
- Xia Y, Ling X, Hu G, Zhu Q, Zhang J, Li Q, et al. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Research and Therapy 2020;11(1):313. [DOI: 10.1186/s13287-020-01834-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2022
- Zhou L, McDonald C, Yawno T, Jenkin G, Miller S, Malhotra A. Umbilical cord blood and cord tissue-derived cell therapies for neonatal morbidities: current status and future challenges. Stem Cells Translational Medicine 2022;1 1(2):135-45. [DOI: 10.1093/stcltm/szab024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bruschettini 2023
- Bruschettini M, Badura A, Romantsik O. Stem cell‐based interventions for the treatment of stroke in newborn infants. Cochrane Database of Systematic Reviews 2023, Issue 2. Art. No: CD015582. [DOI: 10.1002/14651858.CD015582] [DOI] [PMC free article] [PubMed] [Google Scholar]


