Abstract
Cortical amyloid deposition is one of the hallmark biomarkers of Alzheimer’s disease (AD). However, given how cost- and time-intensive amyloid imaging can be, there is a continued need for a low-cost, non-invasive, and accessible enrichment strategy to pre-screen individuals for their likelihood of amyloid prior to imaging. Previous work supports the use of coordinated limb movement as a potential screening tool, even after controlling for cognitive and daily function. Thirty-six patients diagnosed with amnestic mild cognitive impairment over the age of 65 underwent 18F-Flutemetamol amyloid-positron emission tomography (PET) imaging and then completed a timed motor task involving upper limb coordination. This task takes ∼5 minutes to administer and score. Multivariate linear regression and receiver operator characteristic analyses showed that including motor task performance improved model prediction of amyloid burden. Results support the rationale for including functional upper extremity motor assessment as a cost- and time-effective means to screen participants for amyloid deposition.
Keywords: motor, upper-extremity, amyloid deposition, positron emission tomography, mild cognitive impairment
Significance Statement
There is a need for a low-cost, non-invasive, and accessible way to pre-screen asymptomatic individuals for their likelihood of β-amyloid neuritic plaque density prior to positron emission tomography (PET) imaging, particularly for clinical trials that target amyloid. Results from this study suggest that pre-screening individuals with this easy-to-administer motor task could reduce a trial’s total cost for amyloid imaging by ∼36%, offering a highly feasible enrichment strategy for more efficient trial recruitment.
Introduction
Cortical amyloid deposition is one of the hallmark biomarkers of Alzheimer’s disease (AD) and its progression.1,2 Thus, numerous large-scale clinical trials in preclinical AD have focused on therapies aimed at clearing beta-amyloid neuritic plaques to slow disease progression. However, recruiting and enrolling asymptomatic individuals who are amyloid positive is time-consuming, since only ∼30% of cognitively intact individuals have elevated levels of amyloid. 3 This means that 2 out of every 3 individuals who undergo amyloid positron emission tomography (PET) as part of the screening process for clinical trial recruitment will not be eligible for enrollment. Furthermore, amyloid imaging is expensive, exposes individuals to radiation, and can only be completed select sites with the necessary technology and expertise. Thus, there is a need for a low-cost, non-invasive, and accessible way to pre-screen asymptomatic individuals for their likelihood of β-amyloid neuritic plaque density prior to PET imaging.
Although complex movements involving multilimb coordination have been associated with disease severity,4-6 recent work has also demonstrated that such movement may be sensitive to disease progression 7 when assessed with a timed motor task. To minimize cost and assessment time and improve portability, we developed an upper extremity motor task that i) does not require any hardware or software; ii) can differentiate between cognitively intact and cognitively impaired individuals 8 better than other simple motor tasks (ie, grip strength, see Ref. 9); and iii) is feasible for amnestic mild cognitive impairment (MCI) cohorts.7,10 This is in contrast to other assessments of complex movement that require demanding technology (eg, movement sensors, 6 motion capture technology, 5 electromyography, 4 or transcranial magnetic stimulation 11 ) or do not show strong prognostic effects at baseline (eg, 12 ). Given the relative advantages of this timed motor task and its prediction of functional decline in MCI, we hypothesized that task performance would be related to the extent of amyloid plaque deposition and would improve the classification of amyloid positivity in individuals with amnestic MCI, above, and beyond baseline cognitive and activities of daily living.
Materials and Methods
Participants
Thirty-six participants with amnestic MCI from a larger clinical trial sample (ClinicalTrials.gov Identifier: NCT02301546; recruitment status: Completed) participated (mean ± SD age = 73.25 ± 5.5 years; 13 women; 16.81 ± 3.0 years of education; 97% white). Inclusion criteria were 65 years old or older, had a collateral source available to answer questions about thinking abilities and daily activities, had access and the ability to use a computer and the internet, spoke English, and demonstrated that they had single- or multi-domain amnestic MCI. MCI was categorized as: 1) concern of a change in cognition from the participants or a knowledgeable informant; 2) impairment in memory (and other cognitive domains), with at least one cognitive test score in a domain being 1.5 standard deviations below an estimate of premorbid intellect; and 3) independence of daily functioning. 13 Exclusion criteria were history of major neurological (eg, stroke, Parkinson’s disease) or psychiatric illnesses (eg, schizophrenia, bipolar disorder) or substance abuse, current major depression (>7 on the 15-item Geriatric Depression Scale), or cognitive impairment suggestive of dementia. This study was approved by the University of Utah Institutional Review Board, in accordance with the World Medical Association Declaration of Helsinki. All participants provided informed consent as self or by proxy prior to enrollment.
Timed Motor Task
A full visual description of the timed motor task can be viewed on Open Science Framework (https://osf.io/phs57/wiki/Functional_reaching_task/; see also Supplementary Movie 1), and its methods have been published previously.7-10 To summarize, participants use a standard plastic spoon to acquire 2 raw kidney beans at a time from a central cup (all cups 9.5 cm diameter and 5.8 cm deep) to 1 of 3 distal cups arranged at a radius of 16 cm at −40°, 0°, and 40° relative to the central cup. All cups were the same size. Participants were tested using their nondominant hand and started by moving to the cup ipsilateral (same side) of the hand used. They then returned to the central cup to acquire 2 more beans at a time to transport to the middle cup, then the contralateral cup, and then repeated this sequence 4 more times for a total of 15 out-and-back movements. Task performance was measured as trial time (in seconds), that is, how long it took to complete 15 movements, such that lower values indicate better performance. Movement errors, such as dropping beans mid-reach, were recorded; however, only 1 error (.1% of all reaches) was made in this dataset. Participants first completed 3 trials for practice and task familiarization.
Amyloid-Positron Emission Tomography Imaging
Participants received 18F-Flutemetamol imaging as described previously. 14 18F-Flutemetamol was produced under PET current Good Manufacturing Practice (cGMP) standards and the studies were conducted under an approved Federal Drug Administration Investigational New Drug application. Imaging was performed 90 minutes after the injection of 185 mBq (5 mCi) of 18F-Flutemetamol. Emission imaging time was approximately 20 minutes. A GE Discovery PET/CT 710 (GE Health care) was used in this study. This PET/CT scanner has a full width at half-maximum spatial resolution of 5.0 mm and excellent performance characteristics.15,16 18F-Flutemetamol uptake was analyzed using a regional semi-quantitative technique.17,18 Regional (prefrontal, anterior cingulate, precuneus/posterior cingulate, parietal, mesial temporal, lateral temporal, occipital, sensorimotor, cerebellar gray matter, and whole cerebellum) standardized uptake value ratios (SUVRs) were generated automatically and normalized to the pons. Based on the regional values, a composite (composite SUVR) of the cerebral cortex was generated automatically and normalized to the pons using the CortexID Suite software. 19 This software uses a threshold z score of 2.0 to indicate abnormally increased regional amyloid burden that corresponds to a composite SUVR of .59 when normalized to the pons, providing a 99.4% concordance with visual assessment. 17 For 18F-Flutemetamol amyloid imaging, there is no specific age-related “normal” level of binding in the CortexID Suite database to assess age-matched normality. Thus, the study images were compared to the intrinsic software database control group as a whole to calculate the z-scores compared to clinically negative amyloid scans.
Measures of Cognitive and Daily Functioning
As part of the clinical trial, participants underwent extensive neuropsychological assessment at baseline; however, only the Delayed Memory Index from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS 20 ) was examined here. All subtests were administered and scored as defined in the manual, and normative data from RBANS manual was used to calculate the index score, which are presented as age-corrected standard score (M = 100, SD = 15) with higher scores indicating better cognition. Mean ± SD RBANS Delayed Memory Index scores for this sample were 74.42 ± 21.01, consistent with their diagnosis. Baseline activities of daily living (ADL) function was measured using the self-report portion of the 18-item Alzheimer’s Disease Cooperative Study-Activities of Daily Living scale adapted for MCI (ADCS-ADL-MCI). 21 Possible scores on this scale range from 0 to 57, with higher scores indicating better daily functioning. Mean ± SD ADCS-ADL-MCI scores were 46.08 ± 3.82, again consistent with their diagnosis.
Statistical Analysis
Multiple linear regression was conducted to predict 18F-Flutemetamol pons normalized composite SUVRs using participants’ motor task performance (ie, trial time) as a predictor while controlling for age, gender, years of education, RBANS Delayed Memory Index score, and ADCS-ADL-MCI-18 score. Assumptions for regression were inspected visually using Q–Q plots, and all analyses were performed in R (v3.5.1). Statistical models with and without motor task performance as a dependent variable were compared by analysis of variance to determine if the contribution of motor task performance to prediction accuracy was statistically significant.
To test whether motor task performance improved amyloid positivity classification (Aβ+ or Aβ−), we first developed a null model using best practices of model selection 22 that included age, sex, education, RBANS Delayed Memory Index score, and ADCS-ADL-MCI-18 score. A generalized linear model was selected since amyloid positivity follows a binomial distribution. We then generated a motor task model that included the null model plus the motor task variable. Akaike information criteria (AIC) and analysis of variance (ANOVA) using a Chi-squared distribution were used to test for model superiority (null vs task). This determined if including motor task performance as a variable improved prediction accuracy of amyloid classification without added model complexity. An AIC difference of >3 between the null and task model would indicate improved data fit by the task model. Receiver operator characteristics (ROCs) and precision recall curves were also generated to assess model specificity, sensitivity, precision, and recall with and without motor task performance.
Results
A summary of participant characteristic data is provided in Table 1. No adverse events were reported during the injection, uptake time, or imaging studies with the investigational imaging agent 18F-Flutemetamol. Mean composite of SUVRs normalized to the pons was .68 (SD = .18, range = .41–.97). Mean motor task performance was 63.88 seconds (SD = 15.66, range = 39.81–121.75). For reference, cognitively intact older adults tend to be faster (M = 58.50 seconds, data from Refs. 8,23).
Table 1.
Group Characteristics (n = 36).
| Age (years) | 73.3 (5.5) |
|---|---|
| Sex | 13 F, 23 M |
| Education (years) | 16.8 (3.0) |
| Race/Ethnicity | 97% white/100% non-Hispanic |
| RBANS Delayed Memory Index a | 74.41 (21.01) |
| ADCS-ADL-MCI-18 | 46.08 (3.82) |
Mean (SD) unless otherwise noted.
aStandard score (M = 100, SD = 15).
Abbreviations; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status.
Regression analyses revealed that motor task performance was a significant predictor of composite SUVR (β = .004; 95% CI = [.0004, .008]; P = .03), even when controlling for age (P = .17), gender (P = .1), years of education (β = .03; 95% CI = [.013, .05]; P = .002), RBANS Delayed Memory Index score (P = .34), and ADCS-ADL-MCI score (P = .25). The full model yielded an adjusted R2 = .25 [F(6,29) = 3.11; P = .022]. Comparison of regression models with and without motor task performance (R2 =.15; P = .08) through analysis of variance demonstrated that the inclusion of motor task performance significantly improved prediction (P = .03) of composite SUVR by over 65%.
Based on established thresholds, 26 of the 36 participants (72%) were classified as amyloid-positive. The best generalized linear model of the covariate data, that is, the null model, included age, sex, education, RBANS Delayed Memory Index score, and ADCS-ADL-MCI score (AIC = 44.1) as predictors of amyloid positivity classification. Adding motor task performance to the null model improved model accuracy (AIC = 41.4). ANOVA confirmed that the motor task model was more accurate than the null model (P = .03) in predicting amyloid classification.
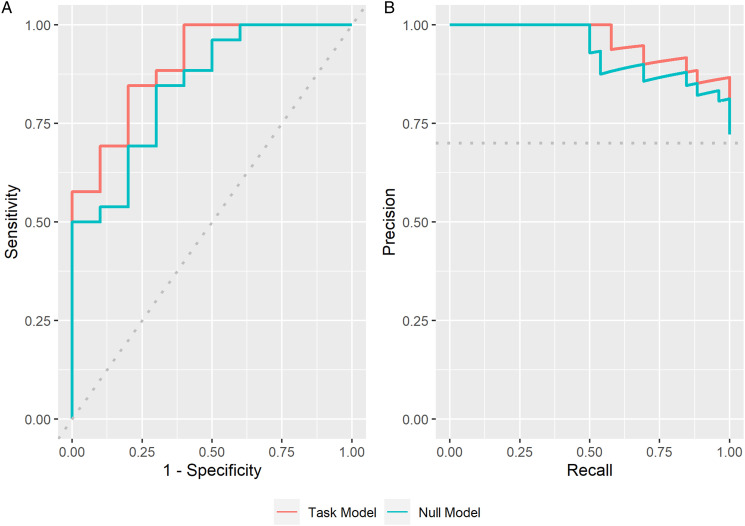
Receiver operator characteristics (ROCs) showed that the motor task model had a specificity of 60% (6/10 prediction accuracy of Aβ−), and a sensitivity of 88% (23/26 prediction accuracy of Aβ+) with an overall accuracy of 80% (29/36), compared to the null model that had a specificity of 50% (5/10 prediction accuracy of Aβ−) and a sensitivity of 93% (24/26 prediction accuracy of Aβ+) with a comparable overall accuracy of 80% (29/36). Overall, the motor task model had an AUC of 90%, compared to the null model AUC of 84% (Figure 1(A)), indicating that the motor task model is superior to the null model in classifying amyloid status.
Figure 1.
(A) Receiver operator characteristic and (B) precision recall curves for the null (blue) and motor task (orange) models for predicting the probability of amyloid positivity.
Given that the majority of participants were classified as amyloid positive, precision recall curves (PRCs) were also generated for each model. 24 Briefly, a precision recall curve determines the trade-off of a model between its true-positive rate and its positive prediction rate by varying the ratio between positive and negative cases and assessing the predictive skill of the model throughout. This can be an especially important metric when evaluating samples with a disproportionate number of positive or negative cases. 24 Here, the area under the PRC of the motor task model was 96% compared to that of the null model, which was 93% (Figure 1(B)). This further demonstrates that advantage of including motor task performance for predicting amyloid-positive cases even when the ratio between positive and negative cases may be skewed, such as in preventative clinical trials where the number of amyloid-negative cases is much higher (eg, Ref. 25).
To determine an optimal cut-off of motor task performance to predict amyloid-positive cases, a permutation test was run that varied motor task cut-off threshold across the range of performance times observed in this sample, followed by a calculation of the resulting odds ratio for amyloid positivity. The cut-off value with the highest odds ratio was determined to be the optimal threshold, which was a task performance of 68 seconds with an odds ratio of 4.76. This threshold indicates that a patient with MCI from this sample who had a motor task performance greater than 68 seconds was nearly 5 times more likely to be amyloid positive than a patient with a motor task performance below 68 seconds. Two of the 36 cases clearly demonstrate these findings (Figure 2).
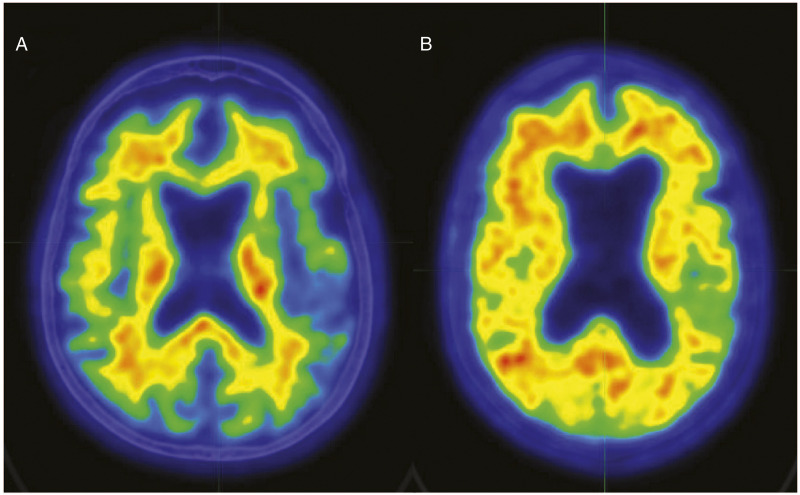
Figure 2.
Color scale (white > red > yellow > green > blue) representative 18F-Flutemetamol amyloid images for 2 participants. Participant A is an Aβ-participant (SUVR = .46); Participant B is an Aβ+ participant (SUVR = .85). Abnormal 18F-Flutemetamol uptake is indicated by loss of the normal distinct gray and white matter contrast (ie, white matter uptake greater than cortex) and areas of equal or greater uptake in the cortex in relation to white matter. Both participants were 80-year-old females with 16 years of education, and fell in the severely impaired range on memory testing (eg, first percentile for RBANS Delayed Memory Index). Participant A’s motor task performance was 62.89 seconds, while Participant B’s was 93.59 seconds. PET images follow radiological convention; both images are of CT slice 21 of 47.PET: positron emission tomography; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; SUVR: standardized uptake value ratios.
Discussion
The purpose of this brief report was to test whether performance on a timed motor task was related to the extent of amyloid plaque deposition in individuals with amnestic MCI, and would improve the classification of amyloid positivity. Results showed that even after controlling for age, gender, education, delayed memory, and ADL function, motor task performance was still a significant predictor of composite (SUVR), with worse task performance being associated with more amyloid deposition. Furthermore, adding motor task performance as a predictor variable improved amyloid positivity classification, being able to better identify individuals with elevated amyloid than with just age, gender, education, delayed memory, and ADL function. Overall, these findings support the rationale for including functional upper extremity motor assessment as a means to better screen participants for clinical trial recruitment that requires elevated amyloid for enrollment (eg, Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease [A4]).
Although several complex upper extremity motor tasks have been shown to be sensitive to disease severity,4,6 this is among the first to show a relationship with disease biomarkers, above and beyond other measures such as memory or ADL function. While this study does not provide a clear mechanism of this relationship, it is possible that unimanual motor performance may be sensitive to amyloid deposition patterns in sensory-motor areas specifically, 26 which may track with global composite measures. It is also likely that this task, more so than grip dynamometry or finger tapping that do not have a strong visuospatial demand, recruits relevant neural structures (eg, hippocampus) that are particularly susceptible to early stages of dementia.27,28 Future research is needed, however, to further explore the underlying mechanism between complex motor tasks and both global and regional amyloid deposition.
It is acknowledged that screening for amyloid deposition is already a time- and cost-intensive process, particularly in mild cases or those who are asymptomatic. Efforts to identify Aβ+ individuals have been enriched by additional biomarkers, genetic testing, and extensive neuropsychological evaluation, which also take time and/or money, and are still not always sensitive and specific to amyloid or disease progression. We therefore highlight the fact that the motor task used in this study takes <5 minutes to administer and costs less than $10 to fabricate from household items, thereby potentially improving the likelihood of identifying individuals with amyloid accumulation with virtually no additional time or cost. It is also extremely portable, with data collection easily available in clinics and the community. In fact, using these time and cost parameters as inputs into the Biomarker Prognostic Enrichment Tool (BioPET), 29 along with published rates of amyloid positivity in cognitively intact adults, 3 it is estimated (with a power of .9) that just by pre-screening individuals with the timed motor task could reduce the total cost for amyloid scanning by ∼36%. For example, in a preventative AD clinical trial that attempts to recruit 1000 amyloid-positive subjects, this 36% could reflect millions of dollars in savings (as well as countless hours for the study personnel and patients and their families). Furthermore, the task’s extremely low price and rapid testing time compared to amyloid-PET still outweigh the estimated 1.5x increase in total individuals screened, thereby streamlining and improving the efficiency of clinical trial recruitment through additional enrichment strategies.
We acknowledge the high education levels and lack of racial/ethnic diversity within the relatively small sample, which warrant future research in larger and more diverse cohorts to better estimate the potential of motor behavior as an affordable enrichment strategy for AD clinical trials. We also acknowledge that the relative strength of the timed motor task as a predictor of amyloid was not directly compared to 1) other existing motor tasks (eg, grip dynamometry, 10 Meter Walk Test) or 2) a control group of cognitively intact individuals, but we have previously shown that the motor task presented here is likely more sensitive to disease severity than other motor assessments 9 (see also Ref. 30). As such, motor assessments have promise as cost-effective and non-invasive screening tools that would allow for enriching samples in clinical trials in AD.
Supplemental Material
Aerial View
Acknowledgments
The ADCS-ADL-MCI survey was used with permission from the NIA Alzheimer’s Disease Cooperative Study (NIA Grant AG10483).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [grant numbers R01AG045163 and K01AG047926]. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the article; or in the review or approval of the article.
Supplementary Material: Supplementary material for this article is available online.
ORCID iD
Sydney Y. Schaefer https://orcid.org/0000-0002-6976-8419
References
- 1.Jack CR, Jr., Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 2010;133(11):3336-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016;8(6):595-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. J Am Med Assoc 2015;313(19):1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubicki A, Fautrelle L, Bourrelier J, et al. The early indicators of functional decrease in Mild Cognitive Impairment. Front Aging Neurosci 2016;8:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogojin A, Gorbet DJ, Hawkins KM, et al. Cognitive-motor integration performance is affected by sex, APOE status, and family history of dementia. J Alzheim Dis 2019;71(2):685-701. [DOI] [PubMed] [Google Scholar]
- 6.Toosizadeh N, Najafi B, Reiman EM, et al. Upper-extremity dual-task function: an innovative method to assess cognitive impairment in older adults. Front Aging Neurosci 2016;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer SY, Hooyman A, Duff K. Using a timed motor task to predict one-year functional decline in amnestic Mild Cognitive Impairment. J Alzheim Dis 2020;77(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer SY, Dibble LE, Duff K. Efficacy and feasibility of functional upper extremity task-specific training for older adults with and without cognitive impairment. Neurorehabilitation Neural Repair 2015;29(7):636–644. [DOI] [PubMed] [Google Scholar]
- 9.Hooyman A, Malek-Ahmadi M, Fauth EB, et al. Challenging the relationship of grip strength with cognitive status in older adults. Int J Geriatr Psychiatr 2021;36(3):433-442. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer SY, Duff K. Within-session and one-week practice effects on a motor task in amnestic Mild Cognitive Impairment. J Clin Exp Neuropsychol 2017;39(5):473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padovani A, Benussi A, Cantoni V, et al. Diagnosis of mild cognitive impairment due to alzheimer’s disease with transcranial magnetic stimulation. J Alzheimers Dis 2018;65(1):221–230. [DOI] [PubMed] [Google Scholar]
- 12.Zammit AR, Robitaille A, Piccinin AM, et al. Associations between aging-related changes in grip strength and cognitive function in older adults: a systematic review. J Gerontol A Biol Sci Med Sci A. 2019;74(4):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of Mild Cognitive Impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duff K, Foster NL, Dennett K, et al. Amyloid deposition and cognition in older adults: the effects of premorbid intellect. Arch Clin Neuropsychol 2013;28(7):665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunderland JJ, Christian PE. Quantitative PET/CT scanner performance characterization based upon the society of nuclear medicine and molecular imaging clinical trials network oncology clinical simulator phantom. J Nucl Med 2015;56(1):145–152. [DOI] [PubMed] [Google Scholar]
- 16.Yester M, Al-Senan R, White S. NEMA testing of GE discovery 710 PET scanner compared to a simplified protocol for routine testing of PET scanners. J Nucl Med 2014;55:2157. [Google Scholar]
- 17.Thurfjell L, Lilja J, Lundqvist R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med 2014;55(10):1623–162. [DOI] [PubMed] [Google Scholar]
- 18.Vandenberghe R, Van Laere K, Ivanoiu A, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010;68(3):319-329. [DOI] [PubMed] [Google Scholar]
- 19.Lundqvist R, Lilja J, Thomas BA, Lötjönen J, Villemagne VL, Rowe CC, et al. Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J Nucl Med 2013;54(8):1472–1478. [DOI] [PubMed] [Google Scholar]
- 20.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 21.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(Suppl 2):S33-S39. [PubMed] [Google Scholar]
- 22.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Socio Methods Res 2004;33(2):261–304. [Google Scholar]
- 23.Lingo VanGilder J, Hengge CR, Duff K, et al. Visuospatial function predicts one-week motor skill retention in cognitively intact older adults. Neurosci Lett 2018;664:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PloS One 2015;10(3):e0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014;6(228):228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raji CA, Becker JT, Tsopelas ND, et al. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer’s disease with Pittsburgh Compound B. J Neurosci Methods 2008;172(2):277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira LK, Diniz BS, Forlenza OV, et al. Neurostructural predictors of Alzheimer’s disease: a meta-analysis of VBM studies. Neurobiol Aging 2011;32(10):1733-1741. [DOI] [PubMed] [Google Scholar]
- 28.Schroeter ML, Stein T, Maslowski N, et al. Neural correlates of Alzheimer’s disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage 2009;47(4):1196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerr KF, Roth J, Zhu K, et al. Evaluating biomarkers for prognostic enrichment of clinical trials. Clin Trials 2017;14(6):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauretani F, Ruffini L, Scarlattei M, et al. Relationship between comprehensive geriatric assessment and amyloid PET in older persons with MCI. BMC Geriatr 2020;20(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aerial View