Abstract
In medicine, barbiturates are a class of depressive medications used as hypnotics, anticonvulsants, and anxiolytics. For the treatment of specific forms of epilepsy and seizures in young children in underdeveloped countries, the World Health Organization recommends phenobarbital (PBAR), a barbiturate drug. This review describes the fabrication and characterization of a paper-based analytical apparatus for phenobarbital detection that is straightforward, affordable, portable, and disposable. All of the solid-state ion-selective electrodes (ISEs) for PBAR as well as a Ag/AgCl reference electrode were constructed and optimized on a nonconductive paper substrate. Using carbon nanotube ink, the sensors were made to function as an ion-to-electron transducer and to make the paper conductive. A suitable polymeric membrane is drop-cast onto the surface of the carbon ink orifice. The pyrido-tetrapeptide and pyrido-hexapeptide derivatives, which were recently synthesized, functioned as distinct ionophores in the PBAR-membrane sensor, enabling its detection. With a detection limit of 5.0 × 10–7 M, the manufactured analytical device demonstrated a Nernstian response to PBAR anions in 50 mM phosphate buffer, pH 8.5, over a linear range of 1.0 × 10–6 to 1.0 × 10–3 M. The PBAR-based sensors showed quick (less than 5 s) response times for PBAR ion detection. The modified separate solution method was utilized to evaluate the selectivity pattern of these novel ionophores with respect to PBAR ions in comparison to other common anions. The analytical instrument that was exhibited on paper had good precision both within and between days. The suggested technology assisted in the detection of trace amounts of PBAR in real pharmaceutical samples. A comparison was made between the data acquired using the HPLC reference method and the information obtained by the recommended potentiometric approach. The described paper-based analytical device may be a good choice for point-of-care PBAR determination because it is cheap and easy to find and can self-pump (especially when combined with potentiometric detection).
1. Introduction
The earliest anticonvulsant medication, phenobarbital (5-ethyl-5-phenylbarbituric acid), is frequently used to treat anxiety, sleeplessness, and seizures.1 It can be helpful in treating tonic and partial clonic seizures, but its principal adverse effects—sedation and hypnosis—can lead to overdose symptoms such as shallow breathing, drowsiness, reduced urine, and fainting spells.2 Additionally, it is combined with opioids to give sedation, particularly for newborns with very low birth weight who are receiving mechanical ventilation.3 For clinical applications, phenobarbital concentration monitoring is required since an overdose is a significant medical emergency.4 Phenobarbital’s pharmacokinetics are highly interindividual variables, necessitating therapeutic medication monitoring. For the safe treatment and effectiveness of phenobarbital in NICU (newborn intensive care unit) patients, close monitoring is required for preterm infants, and a good dose guideline for oral administration is needed. According to certain data, the percentage of preterm infants within the therapeutic range was much lower than that of term newborns (52.9%).
Therefore, it is essential to develop straightforward, affordable, rapid, and reliable procedures that can accurately detect phenobarbitals in serum samples. Even though several analytical techniques, such as optical biosensors,5 capillary electrophoresis,6 spectrofluorometery,7 chemiluminescence,8 GC- MS,9 and HPLC-UV,10 have already been suggested to identify phenobarbital in biological samples and pharmaceutical formulations, these techniques are difficult and require expensive equipment. Even if each method discussed has advantages and disadvantages of its own, finding a straightforward methodology, enhancing specificity and sensitivity, and offering a reliable sensor for drug monitoring in biological matrices have always attracted a lot of attention in clinical applications.
Nanomaterials with superior physical, chemical, and mechanical properties are emerging, and this opens new opportunities for employing them as sensors for drug monitoring. As a result, electrochemical methods are frequently used for the analysis of food and pharmaceutical analytes due to their simplicity, low reagent toxicity, relative low cost, reliability, and low detection limit when compared to other analytical techniques.11−19 Because they can be used for a variety of affordable and simple analyses, the development of electrochemical sensors based on modified electrodes has been taken into consideration. The development of sensitive and focused techniques based on nanomaterials is another of electrochemistry’s most alluring disciplines. The analytical performance of the electrochemical sensors has recently been improved by the introduction of several nanomaterials, including metal nitride nanoparticles, metal oxide nanoparticles, noble metal nanoparticles, and carbon-based nanomaterials.20−22
In several application areas such as clinical diagnosis, food quality control, and environmental monitoring, simple, inexpensive, portable, and disposable analytical equipment is needed. A novel alternative technology is paper-based sensors. The key benefits of employing paper as a sensing platform are its special qualities that allow passive liquid transfer and its compatibility with chemicals and biochemicals. The fabrication processes and analysis procedures can be adjusted to meet the needs of the end-user depending on the primary objective to be accomplished with paper-based sensors. Because of its versatility, abundance, and low cost, paper has recently attracted significant interest as a viable material for sensors and devices in analytical and clinical chemistry.23−28 These analytical tools can be combined in a way that is adaptable, transportable, disposable, and simple to use. Diagnostic tools made of paper started to appear in the early 20th century29,30 after the development of paper chromatography.
In this work, we fabricated a new potentiometric sensor based on newly synthesized macrocyclic pyrido-tetrapeptide (ionophore I) and pyrido-hexapeptide (ionophore II) derivatives as unique ionophores for the detection of phenobarbital. The sensors were modified with CNT ink to make the paper conductive and serve as an ion-to-electron transducing material. The performance characteristics of the fabricated sensors in terms of sensitivity, response range, selectivity, and detection limit were evaluated. For measuring phenobarbital (PBAR) in pharmaceutical formulations and biological fluids, the paper-based analytical device displays a good potential response with high potential stability, durability, sensitivity, and selectivity.
2. Experimental Section
2.1. Apparatus
Using a pH/mV meter (PXSJ-216 INESA, Scientific Instrument Co., Ltd., Shanghai, China), all potentiometric measurements were performed at 25 °C. The developed paper-based reference electrode was optimized and compared using a double-junction Ag/AgCl/KCl 3/1 M CH3COOLi reference electrode (Metrohm AG 6.0726.100). The Agilent 1100 Series HPLC instrument was used for the chromatographic analysis. It has an isocratic pump (G1310A), a Rheodyne manual injector with a 20 μL loop, a G1314A variable wavelength detector (VWD) with a standard flow cell (10 mm path length, 14 μL volume, and 40 bar maximum pressure), and a G2220AA 2D-Value Solution ChemStation as a data system interface.
2.2. Chemicals and Reagents
Tetradodecylammonium tetrakis(4-chlorophenyl)borate (ETH500), 2-nitrophenyl octyl ether (o-NPOE, purity >99%), sodium phenobarbital (PBAR), fluorinated alkylsilane (CF3(CF2)7-CH2CH2SiCl3, CF10), high molecular weight polyvinyl chloride (PVC), sodium barbiton (BAR), sodium pentobarbital (PTBAR), tetrahydrofuran (THF), and poly vinyl butyral (PVB) were all purchased from Sigma-Aldrich (St. Louis, Missouri, MO, USA). Ag/AgCl ink (E2414) was purchased from Ercon (Wareham, MA). Conductive-carbon ink was purchased from Bohui New Materials Tech. Co., Ltd. (Jiangsu, China). Milli-Q PLUS deionized water (18.2 MΩ/cm) (Millipore Corporation, Bedford, MA, USA) was used for all solutions prepared.
A 50 mM phosphate buffer solution (pH 8.5) was used as a working buffer solution. A 10–2 M stock sodium of phenobarbital solution was prepared after dissolving its corresponding amount in the previously mentioned buffer solution in a 50 mL volumetric measuring flask to maintain the pH of the solution at 8.5. The working standard solutions (10–8 to 10–3 M) were prepared from the stock solution prior to the measurements.
For liquid chromatographic measurements,31 the separation column (size: l = 0.25 m, Ø = 4.6 mm) was filled with a stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm). The mobile phase is a mixture of acetate buffer, pH 4.5, and methanol (60:40 V/V). The flow rate was 1 mL/min, and the injection volume was 20 μL. The measurement was carried out at 254 nm using a UV detector.
2.3. Synthesis of ionophores
According to procedures described in the literature,32 the macrocyclic pyrido-pentapeptide derivatives were prepared, characterized, and used as artificial ionophores for PBAR membrane-based sensors. The synthesis pathway is illustrated in Figure 1. In brief, N,N-bis-[1-carboxy-2-(benzyl)]-2,6-(diamino-carbonyl)-pyridine was created by first combining the methyl ester of the l-amino acid with the acid chloride of dipicolinic acid. The product was next subjected to a reaction involving l-amino acid methyl ester hydrochloride, ethyl chloroformate, and dichloromethane, which results in the corresponding tetrapeptide pyridine methyl ester derivatives. Nα-Dipicolinoyl-bis[l-Phe-l-ILe] acid (ionophore I) was produced by hydrolysis with methanolic sodium hydroxide. To obtain the appropriate cyclic hexapeptide ester, cyclization was performed using l-lysine methyl ester. The equivalent cyclic hexapeptide acid (cyclo-(Nα-dipicolinoyl)-bis[l-Phe-l-ILe]-l-Lys-OH) (ionophore II) was then produced by hydrolyzing this molecule with methanolic sodium hydroxide.
Figure 1.

Representation for PBAR ionophore synthesis.
2.4. Design and Construction of the Electrochemical Platform
A chromatographic filter paper was employed as the supporting substrate to build the electrochemical platform. To make the paper hydrophobic, it was placed in a Petri dish with 20 mL of CF10. All capillary tubes in the paper substrate were blocked by a uniform coating of CF10 after the solvent was evaporated at 800 °C for 30 min in a drying chamber.33 After applying carbon nanotube ink (CNT) to the hydrophobic paper substrate, it was baked for 20 min to finish drying. With a measured resistance of roughly 300 Ω/sq, the paper was now conductive. The paper was then covered with a plastic mask that was 0.3 mm thick, leaving a window (2.0 mm) through which the ion-sensing membrane was dropped. The PBAR-sensing membrane was made by dissolving 3.0 mg of ionophore I or II, 1.0 mg of tetradodecylammonium tetrakis(4-chlorophenyl)borate (ETH500), 30.5 mg of polyvinyl chloride (PVC), and 64.5 wt % of 2-nitrophenyl octyl ether (NPOE) in 1.5 mL of THF. To create the solid-state reference electrode, the hydrophobic paper was painted with Ag/AgCl ink, allowed to dry, and then covered with a plastic mask, leaving a window with a 2 mm width, 28 mg of NaCl, 28.0 mg of AgNO3, and 44.0 mg of PVB that were dissolved in 1 mL of methanol to create the reference membrane.34 Both the PBAR-sensing membrane and the reference membrane (20 μL) were drop-cast on their respective electrodes, 5 μL at a time. By sandwiching the two electrodes and creating a hollow with a 50 mL volume out of 3 mm-thick neoprene rubber, the tiny cell was constructed. The conductive ends of the working and reference electrodes were then used to link the paper-based potentiometric device to the mV/pH meter. In Figure 2, a simple diagram to assemble the miniature cell was presented.
Figure 2.
Drawing representation of the fabricated paper-based analytical device.
To construct the glassy-carbon (GC)-based sensors, a GC disk electrode (4 mm I.D.) was polished with 0.3 mm γ-Al2O3, sonicated with ethanol and deionized water alternately, and then dried under a N2 stream. At the distal end of the GC substrate, a piece of the PVC tube (1 cm long, 5 mm in diameter, and 8 mm in outer diameter) was inserted. Above the GC disk, 10 μL of CNT ink was coated. The electrodes were dried, washed in deionized water, and then dried in a N2 gas stream. The identical composition as described above was drop-cast in a 100 μL volume of the membrane cocktail on top of the CNT layer. The membrane was then allowed to dry until it took on a consistent shape and had strong adherence to the GC substrate.
2.5. Analytical Applications
The recovery of PBAR from a spiked urine sample was assessed to evaluate the applicability of the proposed sensors. In a 25 mL beaker, 9 mL of the phosphate buffer and 1 mL of the urine sample were mixed. The diluted urine solution was mixed with various aliquots of the standard PBAR solution. An aliquot of the sample was put into the potentiometric cell, and after the equilibrium response was attained, the potential was recorded. Using the previously created calibration plot, the spiked PBAR quantity was computed.
On actual samples of commercial pharmaceutical formulations, the applicability of the paper-based sensors that were presented was evaluated. From the local market, the products were supplements (Doloran inf. 150 mg, Pharco Co., Egypt; Vegaskine ped. 150 mg, Alex Co., Egypt), tablets (Migrainil 10 mg, Nile Co., Egypt; Sominal 15 mg, Alex Co., Egypt), syrups (Minophylline 0.066%, Memphis Pharm. Co., Egypt; epicophylline phenobarbitone 3.3 mg, Eipico Co., Egypt), and ampuls (Sominal 40 mg/mL, Alex Co., Egypt). The electrochemical cell was inserted into the solution after an accurately measured aliquot (10 μL) of the syrup or suspension sample was transferred into a 50 mL volumetric flask and mixed with 1 mL of 0.1 M NaOH. The solution was then completed to the mark with phosphate buffer solution to adjust the pH of the solution to pH 8.5. The potential was then measured once equilibrium was reached, and by using the calibration that was built, the quantity of PBAR was determined.
3. Results and Discussion
3.1. Sensor Production with Paper
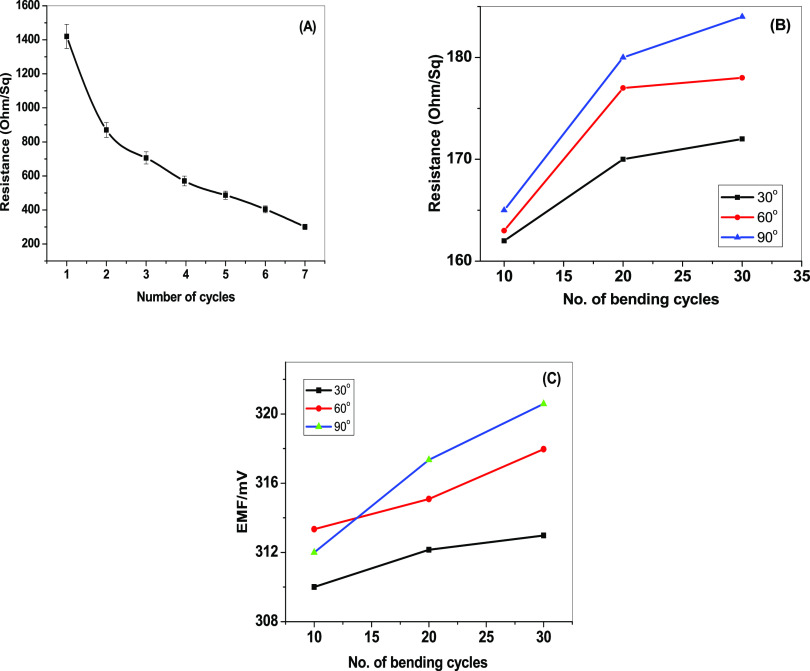
As shown in Figure 2, it illustrated the paper-based sensor’s design and the manufacturing process. CF10 was applied to the paper surface to increase its hydrophobicity and to eliminate the water layer effect. To make the paper a conductor substrate, a layer of carbon nanotube ink (CNT) was introduced to the paper. This CNT layer acts also as an ion-to-electron transducer. The electrical resistance of the conducting paper was measured as a function of the number of spraying cycles, as shown in Figure 3a. The resistivity of the paper reduces with an increase in spraying cycles. After seven spraying cycles, the resistance remains constant and reaches 145 Ω.
Figure 3.
Relationship between conductivity and the quantity of CNT prints (A) as well as the effects of cycles of bending at various bending angles on resistance and EMF in panels (B) and (C), respectively.
The paper was repeatedly bent at several angles of bending (i.e., 30, 60, and 90°) to test the mechanical flexibility of the paper-based sensor. Resistance and electromotive force (EMF) drift were both 27 Ω and 6.5 mV, respectively (Figure 3b,c). These results showed that the paper-based sensor developed displayed good mechanical flexibility and high conductivity.
3.2. Potentiometric Characteristics of the Paper-Based Sensor
After evaluating the potential response at various PBAR concentrations (1.0 × 10–8 to 1.0 × 10–2 M), the performance parameters of the revealed PBAR paper-based sensors (paper/CNT/PBAR-ISE) were assessed. Figure 4 displays the calibration plot and time-trace of the demonstrated (paper/CNT/PBAR-ISE I) and (paper/CNT/PBAR-ISE II) sensors based on ionophores I and II, respectively. The presented paper-based sensors measured linear ranges of 4.0 × 10–6 to 1.0 × 10–3 and 1.0 × 10–6 to 1.0 × 10–3 M with Nernstian slopes of −64.0 ± 1.1 (R2 = 0.9993) and −63.6 ± 1.3 (R2 = 0.9971) mV/decade for ISE I and ISE II, respectively. Using the IUPAC guidelines,35 the detection limits for both sensors were determined to be 2.0 × 10–6 and 5.0 × 10–7 M, respectively.
Figure 4.
Potentiometric response and time-trace for paper/PBAR-based sensors.
PBAR-ISEs based on a glassy-carbon (GC-ISE) support were constructed as well for electrode optimization and comparison purposes, and results were compared with those obtained by the paper-based analytical device. The GC/PBAR-ISEs based on ionophore I (ISE III) and ionophore II (ISE IV) exhibited a Nernstian response with slopes of −61.9 ± 0.9 and −56.6 ± 0.3 mV/decade (30 mM phosphate buffer, pH 8.5) over linear ranges of 1.0 × 10–7 to 1.0 × 10–3 and 6.0 × 10–8 to 1.0 × 10–3 M with limits of detection of 5.3 × 10–8 and 3.1 × 10–8 M, respectively. The results obtained are very comparable to those of the paper-based analytical devices that were previously provided. Figure 5 displays the calibration plots for GC/PBAR-ISEs based on ionophores I (ISE III) and II (ISE IV). This demonstrates that there are no appreciable variations in terms of slope sensitivity and linearity range between the provided paper-based analytical devices and the solid-state GC/PBAR-ISEs.
Figure 5.
Potentiometric response and time-trace for GC/PBAR-based sensors.
Figures 4 and 5 illustrate, respectively, the time-trace responses of solid-state GC/PBAR-ISEs based on ionophores I and II as well as paper-based analytical devices. The sensors’ steady-state potential response time was 5 s, which is acceptable and appropriate for their usage in decentralized analysis. Table 1 provides a summary of the performance analytical attributes for all of the ISEs that have been presented.
Table 1. Potentiometric Characteristics of Phenobarbital Sensors.
| parameter | sensor I | sensor II | sensor III | sensor IV |
|---|---|---|---|---|
| slope (mv/decade) | –64.0 ± 1.1 | –63.6 ± 1.3 | –61.9 ± 0.9 | –56.6 ± 0.3 |
| correlation coefficient (r2) | 0.9993 | 0.9971 | 0.9990 | 0.9999 |
| linear range (M) | 4.0 × 10–6 to 1.0 × 10–3 | 1.0 × 10–6 to 1.0 × 10–3 | 1.0 × 10–7 to 1.0 × 10–3 | 6.0 × 10–8 to 1.0 × 10–3 |
| detection limit (M) | 2.0 × 10–6 | 5.0 × 10–7 | 5.3 × 10–8 | 3.1 × 10–8 |
| working pH range (pH) | 7.5–10.5 | 7.5–10.5 | 7.5–10.5 | 7.5–10.5 |
| response time (s) | <5 | <5 | <5 | <5 |
| accuracy (%) | 99.1 | 98.8 | 99.2 | 98.9 |
| trueness (%) | 98.7 | 99.1 | 98.8 | 99.2 |
| bias (%) | 0.8 | 0.7 | 0.8 | 0.9 |
| intraday precision (%) | 0.9 | 1.1 | 0.9 | 1.1 |
| interday precision (%) | 0.6 | 0.6 | 0.7 | 0.8 |
3.3. Intra- and interday Precision Assessments
For the paper-based analytical devices that were shown, intraday and interday precisions were assessed. Phenobarbital was detected in an internal quality control sample at 1.0 μg/mL (n = 6). For sensors I and II, the relative standard deviations were discovered to be 0.9 and 1.1, respectively. By adding a known amount of PBAR (0.5 μg/mL), the method’s accuracy was also assessed, and they were discovered to be 99.1 and 98.8% for sensors I and II, respectively.
3.4. Effect of pH on the Potentiometric Response
Using two PBAR concentrations (1.0 × 10–4 and 1.0 × 10–3 M) at different pH values (from pH 4 to 11), the potential stability of the demonstrated sensors throughout a range of pH values was examined. For all PBAR membrane-based sensors, they revealed a consistent potential response over the pH range of 7.5–10.5. This shows that the sensors can detect PBAR in its anionic form. Due to the formation of the nonsensed neutral phenobarbital (pKa = 7.3), a potential drift below pH 7.0 was noticed.36 Therefore, 30 mM phosphate buffer at pH 8.5 was used for all measurements.
3.5. Selectivity Study
The modified separate solution method (MSSM) proposed by Bakker37 was used for selectivity evaluation. Successive calibration curves with increasing concentrations of the interfering ions and the last calibration were carried out with PBAR ions. The extrapolated potentials of each curve at a 1 M concentration were entered into the SSM equation to determine the potentiometric selectivity values. In Table 2, the log KpotPBAR,J values were displayed. It was noticed that ISE I has greater selectivity for PBAR ions than for barbital, valsartan, and Cl– and NH4+ ions than ISE II. The selectivity behavior over Na+, K+, urea, pentobarbital, and glucose was substantially the same for both ionophores. ISE II demonstrated more selectivity for PBAR ions than for creatinine and oxalate ions.
Table 2. Selectivity Coefficients for Phenobarbital Membrane-Based Sensors.
| log KpotPBAR,J ± SDaa |
||
|---|---|---|
| interfering ion, J | ISE I | ISE II |
| barbital | –1.5 ± 0.1 | –1.1 ± 0.2 |
| pentobarbital | –1.7 ± 0.1 | –1.5 ± 0.2 |
| valsartan | –3.7 ± 0.2 | –3.3 ± 0.1 |
| oxalate | –4.0 ± 0.2 | –4.4 ± 0.2 |
| chloride | –5.1 ± 0.1 | –4.7 ± 0.3 |
| creatinine | –3.8 ± 0.3 | –4.3 ± 0.1 |
| urea | –4.9 ± 0.2 | –4.9 ± 0.1 |
| glucose | –5.5 ± 0.3 | –5.4 ± 0.3 |
| NH4+ | –4.3 ± 0.1 | –4.0 ± 0.1 |
| Na+ | –5.6 ± 0.1 | –5.6 ± 0.2 |
| K+ | –5.4 ± 0.1 | –5.4 ± 0.1 |
SD = standard deviation (n = 4).
3.6. Measurements of Phenobarbital Recovery in Spiked Urine Samples
It is crucial to monitor the presence of phenobarbital in a biological fluid as complex as human urine because it may aid in the quick identification of an overdose patient. Therefore, after dilution with phosphate buffer (30 mM, pH 8.5) in a ratio of 1:5, various concentrations of PBAR were spiked into a sample of human urine. The potentiometric measurements were carried out by using the described sensors. Despite the presence of several species in the human urine sample (such as Na+, K+, creatinine, and urea), the presented sensors demonstrated an outstanding recovery of PBAR, as shown in Table 3. The existence of these species did not exhibit any interfering effects during the measurements. This confirms the robustness, selectivity, and effective use of the suggested sensors.
Table 3. Recovery Values for Phenobarbital Determination in Spiked Urine Samples.
| ISE
I |
ISE
II |
||||
|---|---|---|---|---|---|
| sample no. | spiked, μM | found, μMa | recovery, % | found, μMa | recovery, % |
| 1 | 5.0 | 4.91 ± 0.2 | 98.2 | 4.88 ± 0.1 | 97.6 |
| 2 | 10.0 | 9.87 ± 0.1 | 98.7 | 10.17 ± 0.1 | 101.7 |
| 3 | 20.0 | 20.12 ± 0.1 | 100.6 | 19.62 ± 0.3 | 98.1 |
Average of three measurements (n = 3).
3.7. Assessment of Phenobarbital in Pharmaceutical Products
For the determination of PBAR in various pharmaceutical formulations containing phenobarbital, the proposed potentiometric approach was also introduced. Different pharmaceutical products containing phenobarbital were collected from a local market. The products were supplements (Doloran inf. 150 mg, Pharco Co., Egypt; Vegaskine ped. 150 mg, Alex Co., Egypt), tablets (Migrainil 10 mg, Nile Co., Egypt; Sominal 15 mg, Alex Co., Egypt), syrups (Minophylline 0.066%, Memphis Pharm. Co., Egypt; epicophylline phenobarbitone 3.3 mg, Eipico Co., Egypt), and ampuls (Sominal 40 mg/mL, Alex Co., Egypt). The acquired results were contrasted with the conventional HPLC approach, and Table 4 displays the results. The collected data supported the viability of employing the proposed sensors for routine PBAR assessment in pharmaceutical formulations.
Table 4. Determination of Phenobarbital in Different Pharmaceutical Formulation Samplesa.
| amount
found, ± SD* |
||||||
|---|---|---|---|---|---|---|
| sample | labeled amount | potentiometry | recovery, % | HPLC method | recovery, % | F-test |
| Doloran inf. (Pharco Co., Egypt) (supplement) | 150 mg | 147.9 ± 0.4 | 98.6 | 151.2 ± 0.1 | 100.8 | 4.43 |
| Vegaskine ped. (Alex Co., Egypt) (supplement) | 150 mg | 153.2 ± 0.8 | 102.1 | 149.3 ± 0.2 | 99.5 | 6.20 |
| Migrainil (Nile Co., Egypt) (tablet) | 10 mg/tablet | 9.8 ± 0.6 | 98 | 10.1 ± 0.2 | 101 | 3.46 |
| Sominal (Alex Co., Egypt) (tablet) | 15 mg/tablet | 14.3 ± 1.1 | 95.3 | 14.9 ± 0.1 | 99.3 | 3.21 |
| Minophylline (Memphis Pharm. Co., Egypt) (syrup) | 66 mg/100 mL | 64.2 ± 2.1 | 97.2 | 65.3 ± 0.6 | 98.9 | 2.45 |
| Sominal (Alex Co., Egypt) (ampul) | 40 mg/mL | 40.9 ± 0.4 | 102.2 | 39.8 ± 0.3 | 99.5 | 2.32 |
± SD* (standard deviation for an average of five measurements).
3.8. Advantages and Novelty
The proposed phenobarbital paper-based sensor has several notable improvements over some of the earlier suggested test methods in terms of sensitivity, accuracy, stability, and selectivity, according to a comparison of the general potentiometric properties of the two systems.38,39 A table of comparison for the potentiometric characteristics between the previously reported PBAR sensors and the presented sensor in this work is shown in Table 5. The current sensor consists of reference electrodes without internal filling solutions on the same paper strip as a combined tiny planar (5 × 20 mm) identification sensor. It provided benefits including reduced size, adaptability, affordability, environmental friendliness, paper-based, good stability, long-term durability, and ease of fabrication in mass production of disposable devices.
Table 5. Comparison of Previously Reported Phenobarbital Potentiometric Electrodes.
| sensory material | electrode type | slope (mV/decade) | detection limit (M) | linear range (M) | pH range (pH) | time response (s) | ref. |
|---|---|---|---|---|---|---|---|
| β-cyclodextrin | liquid polymeric electrode | –59.1 | 3.5 × 10–6 | 5.0 × 10–6 to 1.0 × 10–2 | 9–11 | 25 | (38) |
| γ-cyclodextrin | liquid polymeric electrode | –62.0 | 7.0 × 10–6 | 8.0 × 10–6 to 1.0 × 10–2 | 9–11 | 25 | |
| tetraoctylammonium/phenobarbaturate | solid contact | –59.0 | not reported | 2.0 × 10–4 to 1.0 × 10–1 | 9–11.5 | 15 | (39) |
| Ionophore I | Paper-based | –64.0 ± 1.1 | 2.0 × 10–6 | 4.0 × 10–6-1.0 × 10–3 | 7.5–10.5 | <5 | This work |
| Ionophore II | –63.6 ± 1.3 | 5.0 × 10–7 | 1.0 × 10–6-1.0 × 10–3 | ||||
| Ionophore I | GC-Solid Contact | –61.9 ± 0.9 | 5.3 × 10–8 | 1.0 × 10–7-1.0 × 10–3 | |||
| Ionophore II | –56.6 ± 0.3 | 3.1 × 10–8 | 6.0 × 10–8-1.0 × 10–3 |
4. Conclusions
Herein, a straightforward, affordable, portable, and disposable paper-based analytical device has been developed and characterized for the purpose of phenobarbital assessment in real samples. On a nonconductive paper substrate, all of the solid-state ion-selective electrodes (ISEs) for PBAR and a Ag/AgCl reference electrode were built and tested. The development of the membrane sensors for phenobarbital detection utilized newly synthesized macrocyclic pyrido-tetrapeptide (ionophore I) and pyrido-hexapeptide (ionophore II) derivatives to serve as particular ionophores. These novel ionophores showed an impressive affinity for PBAR detection in 50 mM phosphate buffer (pH 8.5) with linearity ranges of 4.0 × 10–6 to 1.0 × 10–3 and 1.0 × 10–6 to 1.0 × 10–3 M for ionophores I and II, respectively. The sensors revealed sensitivities of −64.0 ± 1.1 and −63.6 ± 1.3 mV/decade and detection limits of 2.0 × 10–6 and 5.0 × 10–7 M, respectively. With a response time of <5 s, the sensors displayed rapid PBAR ion detection. The presented potentiometric device was successfully applied for PBAR assessment in different pharmaceutical formulation samples. The obtained results were compared to those obtained from the HPLC measurements. The sensors are simple to design, are reasonably priced, are reliable, and have a short response time. Additionally, they exhibit desirable features for point-of-care analysis, such as a small sample size (50 μL) and no sample pretreatment.
As a result, this study can be viewed as a significant contribution to the field of paper-based analytical platforms in point-of-care testing, which is currently expanding.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research (IFKSURC-1-0109).
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through project no. IFKSURC-1-0109.
The authors declare no competing financial interest.
References
- Carrasco M.; Bonifacio S. L.; deVeber G.; Chau V. Early Discontinuation of phenobarbital after acute symptomatic neonatal seizures in the term newborn. Neurol.: Clin. Pract. 2023, 13, e200125 10.1212/CPJ.0000000000200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasiry Z.; Shorvon S. D. How phenobarbital revolutionized epilepsy therapy: the story of phenobarbital therapy in epilepsy in the last 100 years. Epilepsia 2012, 53, 26–39. 10.1111/epi.12026. [DOI] [PubMed] [Google Scholar]
- Anand K.; Hall R. Pharmacological therapy for analgesia and sedation in the newborn. Arch. Dis. Childhood-Fetal Neonat. Ed. 2006, 91, F448–F453. 10.1136/adc.2005.082263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W.; Ye K. N.; Kim J. T.; An S. H. Phenobarbital dosing and therapeutic drug monitoring in the neonatal intensive care unit. Yakhak Hoeji 2018, 62, 49–53. 10.17480/psk.2018.62.1.49. [DOI] [Google Scholar]
- Garzón V.; Pinacho D. G.; Bustos R. H.; G. Garzón G.; Bustamante S. Optical biosensors for therapeutic drug monitoring. Biosensors 2019, 9, 132. 10.3390/bios9040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A.; Xu X.; Stewart J. T. Determination of ephedrine, theophylline and phenobarbital in a tablet dosage form by capillary electrophoresis. J. Pharm. Biomed. Anal. 1999, 21, 1063–1067. 10.1016/S0731-7085(99)00226-5. [DOI] [PubMed] [Google Scholar]
- Shariati R.; Rezaei B.; Jamei H. R.; Ensafi A. A. Application of coated green source carbon dots with silica molecularly imprinted polymers as a fluorescence probe for selective and sensitive determination of phenobarbital. Talanta 2019, 194, 143–149. 10.1016/j.talanta.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Li X.; Niu L.; He X.; Song Z. Determination of phenobarbital in human urine and serum using flow injection chemiluminescence. Biochem. (Moscow) Suppl., Ser. B 2011, 5, 184–187. 10.1134/S1990750811020077. [DOI] [PubMed] [Google Scholar]
- Roveri F. L.; Paranhos B. A. P. B.; Yonamine M. Determination of phenobarbital in hair matrix by liquid phase microextraction (LPME) and gas chromatography–mass spectrometry (GC–MS). Forensic Sci. Int. 2016, 265, 75–80. 10.1016/j.forsciint.2015.12.033. [DOI] [PubMed] [Google Scholar]
- Amiri Pebdani A.; Dadfarnia S.; Haji Shabani A. M.; Khodadoust S.; Talebianpoor M. S. Modified dispersive liquid-phase microextraction based on sequential injection solidified floating organic drop combined with HPLC for the determination of phenobarbital and phenytoin. J. Sep. Sci. 2018, 41, 509–517. 10.1002/jssc.201701111. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh L.; Khoshroo A.; Adib K.; Rahimi-Nasrabadi M.; Ahmadi F. Determination of homocysteine using a dopamine-functionalized graphene composite. Microchem. J. 2021, 165, 106124 10.1016/j.microc.2021.106124. [DOI] [Google Scholar]
- Sobhani-Nasab A.; Hoseinpour S. M.; Rahimi-Nasrabadi M.; Pourmasoud S.; Eghbali-Arani M.; Ahmadi F. Synthesis of Fe3O4/CdWO4/carbon dots heterostructure with excellent visible light photocatalytic stability and activity for degradation of 4-nitrophenol and organic pollutant. J. Mater. Sci. Mater. Electron. 2021, 32, 26998–27013. 10.1007/s10854-021-07073-0. [DOI] [Google Scholar]
- Chupradit S.; Nasution M. K. M.; Rahman H. S.; Suksatan W.; Jalil A. T.; Abdelbasset W. K.; Bokov D.; Markov A.; Fardeeva I. N.; Widjaja G.; Shalaby M. N.; Saleh M. M.; Mustafa Y. F.; Surendar A.; Bidares R. Various types of electrochemical biosensors for leukemia detection and therapeutic approaches. Anal. Biochem. 2022, 654, 114736 10.1016/j.ab.2022.114736. [DOI] [PubMed] [Google Scholar]
- Yumashev A. V.; Rudiansyah M.; Chupradit S.; Kadhim M. M.; Jalil A. T.; Abdelbasset W. K.; Suksatan W.; Romero Parra R. M. R.; Mustafa Y. F.; Abdullaev B.; Bidares R. Optical-based biosensor for detection of oncomarker CA 125, recent progress and current status. Anal. Biochem. 2022, 655, 114750 10.1016/j.ab.2022.114750. [DOI] [PubMed] [Google Scholar]
- Abd-Rabboh H. S. M.; Kamel A. H. Mimicking a receptor for cyanide ion based on ion imprinting and its applications in potential transduction. Electroanalysis 2012, 24, 1409–1415. 10.1002/elan.201200069. [DOI] [Google Scholar]
- Abdalla N. S.; Youssef M. A.; Algarni H.; Awwad N. S.; Kamel A. H. All Solid-State Poly (Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and Rapid Detection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. 10.3390/molecules24040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmawy N. H.; Almehizia A. A.; Youssef T. A.; Amr A.E.-G.E.; Al-Omar M. A.; Kamel A. H. Novel carbon/PEDOT/PSS-based screen-printed biosensors for acetylcholine neurotransmitter and acetylcholinesterase detection in human serum. Molecules 2019, 24, 1539. 10.3390/molecules24081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafaela L.G. J.; Kamel A. H.; Sales M. G. F. FIA potentiometric system based on periodate polymeric membrane sensors for the assessment of ascorbic acid in commercial drinks. Food Chem. 2010, 120, 934–939. 10.1016/j.foodchem.2009.11.015. [DOI] [Google Scholar]
- Amr A. E.; Kamel A. H.; Almehizia A. A.; Sayed A. Y. A.; Elsayed E. A.; Abd-Rabboh H. S. M. Paper-Based Potentiometric Sensors for Nicotine Determination in Smokers’ Sweat. ACS Omega 2021, 6, 11340–11347. 10.1021/acsomega.1c00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.; Hooman M.; Yarigarravesh M.; Algarni M.; Opulencia M. J. C.; Alsaikhan F.; Jalil A. T.; Mohamed A.; AboRasi K. M.; Rahman L.; Sarjadi M. S. Vibration analysis of size dependent micro FML cylindrical shell reinforced by CNTs based on modified couple stress theory. Arabian J. Chem. 2022, 15, 104115 10.1016/j.arabjc.2022.104115. [DOI] [Google Scholar]
- Mazloum-Ardakani M.; Eslami V.; Khoshroo A. Nickel nitride nanoparticles as efficient electrocatalyst for effective electro-oxidation of ethanol and methanol in alkaline media. Mater. Sci. Eng., B 2018, 229, 201–205. 10.1016/j.mseb.2017.12.038. [DOI] [Google Scholar]
- Mehmandoust M.; Erk N.; Karaman O.; Karimi F.; Bijad M.; Karaman C. Three-dimensional porous reduced graphene oxide decorated with carbon quantum dots and platinum nanoparticles for highly selective determination of azo dye compound tartrazine. Food Chem. Toxicol. 2021, 158, 112698 10.1016/j.fct.2021.112698. [DOI] [PubMed] [Google Scholar]
- Martinez A. W.; Phillips S. T.; Whitesides G. M.; Carrilho E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- Bracher P. J.; Gupta M.; Whitesides G. M. Patterning precipitates of reactions in paper. J. Mater. Chem. 2010, 20, 5117–5122. 10.1039/c000358a. [DOI] [Google Scholar]
- Zhao W. A.; van den Berg A. Lab on paper. Lab Chip 2008, 8, 1988–1991. 10.1039/b814043j. [DOI] [PubMed] [Google Scholar]
- Clegg D. L. Paper chromatography. Anal. Chem. 1950, 22, 48–59. 10.1021/ac60037a014. [DOI] [Google Scholar]
- Dungchai W.; Chailapakul O.; Henry C. S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. 10.1039/C0AN00406E. [DOI] [PubMed] [Google Scholar]
- Hossain S. M. Z.; Luckham R. E.; Smith A. M.; Lebert J. M.; Davies L. M.; Pelton R. H.; Filipe C. D. M.; Brennan J. D. Development of a bioactive paper sensor for detection of neurotoxins using piezoelectric inkjet printing of sol-gel-derived bioinks. Anal. Chem. 2009, 81, 5474–5483. 10.1021/ac900660p. [DOI] [PubMed] [Google Scholar]
- Yu J. H.; Ge L.; Huang J. D.; Wang S. M.; Ge S. G. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011, 11, 1286–1291. 10.1039/c0lc00524j. [DOI] [PubMed] [Google Scholar]
- Comer J. Semiquantitative specific test paper for glucose in urine. Anal. Chem. 1956, 28, 1748–1750. 10.1021/ac60119a030. [DOI] [Google Scholar]
- British Pharmacopoeia, (Ph. Eur. monograph 0522); 2013.
- Amr A. E.; Abo-Ghalia M. H.; Moustafa G. O.; Al-Omar M. A.; Nossier E. S.; Elsayed E. A. Design, synthesis and docking studies of novel macrocyclic pentapeptides as anticancer multi-targeted kinase inhibitors. Molecules 2018, 23, 2416. 10.3390/molecules23102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavan A. C.; Martinez R. V.; Subramania A. B.; Yoon H. J.; Nunes R. M. D.; Lange H.; Thuo M. M.; Whitesides G. M. Omniphobic “RF Paper” Produced by Silanization of Paper with Fluoroalkyltrichlorosilanes. Adv. Funct. Mater. 2014, 24, 60–70. 10.1002/adfm.201300780. [DOI] [Google Scholar]
- Guinovart T.; Crespo G. A.; Rius F. X.; Andrade F. J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 2014, 821, 72–80. 10.1016/j.aca.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Recommendations for nomenclature of ion-selective electrodes. Pure Appl. Chem. 1976, 48, 127. [Google Scholar]
- http://pubchem.ncbi.nlm.nih.gov.
- Bakker E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. 10.1021/ac960891m. [DOI] [Google Scholar]
- Alrablah H.; AL-Majed A.; Abounassif M.; Mostafa G. A. E. Ionophore-based potentiometric PVC membrane sensors for determination of phenobarbitone in pharmaceutical formulations. Acta Pharm. 2016, 66, 503–514. 10.1515/acph-2016-0042. [DOI] [PubMed] [Google Scholar]
- Lima J. L.; Montenegro M. C. B.; Da Silva A. R. A phenobarbital ion-selective electrode without an inner reference solution, and its application to pharmaceutical analysis. J. Pharm. Biomed. Anal. 1990, 8, 701–704. 10.1016/0731-7085(90)80106-Y. [DOI] [PubMed] [Google Scholar]