Abstract
Background
In Germany, a total of 38 547 heart valve procedures were performed in 2022. With a growing number of patients undergoing the surgical and interventional implantation of heart valves, the incidence of prosthetic endocarditis is also rising.
Methods
We summarize the current state of the prophylaxis, diagnosis, and treatment of prosthetic endocarditis in a selective review of the literature.
Results
Prosthetic endocarditis accounts for 10–30% of all cases of endocarditis. As its echocardiographic and microbiologic findings are often less specific than those of native endocarditis, its diagnosis now increasingly relies on alternative imaging modalities such as F-18-FDG PET-CT. Anti-infective and surgical treatment are made more difficult by biofilm formation on the prosthetic valve and the frequent formation of perivalvular abscesses.
Conclusion
Increased awareness of this clinical entity in the outpatient setting will promote the earlier initiation of appropriate diagnostic studies. Proper diagnostic evaluation is an essential prerequisite for the early detection and timely treatment of prosthetic endocarditis, with the goal of preventing progressive destruction and thus improving the outcome. Preventive and educative measures should be intensified, and certified, multidisciplinary endocarditis teams should be established. Antibiotic prophylaxis is now given much more restrictively than in earlier years; the risk of infection must be weighed against the potential development of both individual and collective resistance to antibiotic drugs.
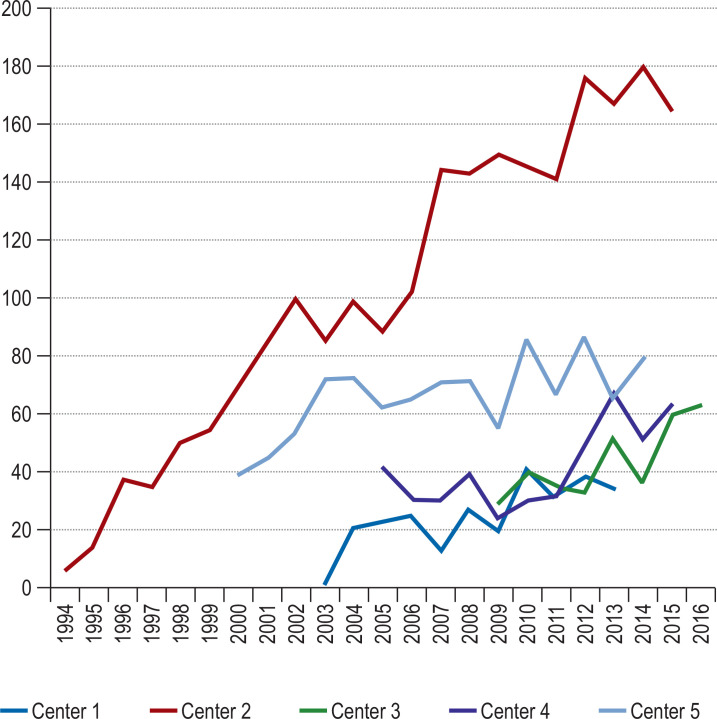
With the increasing frequency of artificial heart valve implantation, either by open surgery or as a percutaneous intervention, there has been a corresponding rise in the number of cases of prosthetic endocarditis, as well as of native endocarditis, in a patient population that is becoming steadily older while carrying an increasing burden of comorbidity (eFigure). In 2022, 38 547 heart valve procedures were performed in Germany alone (biological/mechanical valve replacement or valve reconstruction), a marked rise from 22 243 such procedures in 2008 (e1). Prosthetic endocarditis is a serious complication of valve replacement, arising in 1–6% of patients with prosthetic valves and accounting for 10–30% of all cases of infective endocarditis (1–3).
eFigure.
The number of cases of endocarditis per year in 5 German cardiac surgery centers from 1994 to 2016.
Endocarditis is diagnosed on the basis of the modified Duke criteria: the presence of two major criteria, one major criterion and three minor criteria, or five minor criteria is considered diagnostic (Box 1). The modified Duke criteria may not yield a timely diagnosis of prosthetic endocarditis, as they lead to the diagnosis in only 60% of cases (e2). This is mainly because the clinical presentation is often atypical, blood culture results are harder to interpret, and echocardiographic findings are more often nonspecific than in native endocarditis (Box 2) (4, 5).
Box 1a. Major diagnostic criteria for infective endocarditis.
-
positive blood cultures
-
typical microorganisms from two separate blood cultures
viridans streptococci, Streptococcus gallolyticus (Streptococcus bovis), HACEK group, Staphylococcus aureus; or
acquired enterococci, in the absence of a primary focus elsewhere; or
-
typical microorganisms in persistently positive blood cultures:
≥ 2 positive blood cultures from blood samples collected > 12 hours apart; or
all 3 or a majority of ≥ 4 separate blood cultures (with collection interval of ≥ 1 hour between first and last sample); or
single positive blood culture for Coxiella burnetii or phase IgG antibody titer > 1 : 800
-
-
positive imaging
-
transthoracic/transesophageal echocardiography with demonstration of:
vegetation
abscess, pseudoaneurysm, intracardiac fistula
valve perforation or aneurysm
new partial dehiscence of a prosthetic valve
abnormal activity in the vicinity of a prosthetic heart valve on 18-F FDG PET/CT (only if the prosthesis was implanted more than 3 months previously) or on SPECT/CT with radiolabeled leukocytes
demonstration of paravalvular lesions on cardiac CT
-
CT, computed tomography; F-18-FDG-PET; positron emission tomography with tintravenously administered solution containing weakly radioactively labeled F-18-fluorodeoxyglucose
Box 2. Warning signs of infective endocarditis.
fever, chills, night sweats, fatigue, anorexia, weight loss / → often nonspecific symptoms
respiratory complaints, arthralgia/myalgia, anemia
concomitant new-onset heart murmur
-
rash
bluish-red lentil-sized nodules on fingers and toes (Osler‘s nodes)
small, painless, reddish hemorrhages, a few millimeters in size, on the palms and soles (Janeway lesions)
nail-bed hemorrhages (splinter lesions)
neurologic abnormalities (septic embolism?)
hematuria
Suspect endocarditis in case of:
preceding dental treatment
poor dental status
presence of a prosthetic heart valve or congenital heart defect
preceding infection with inflammatory signs or pus
history of endocarditis
intravenous drug abuse
immunodeficiency
Ambulatory measures
-
physical examination:
cardiac auscultation: a new heart murmur may be heard
look for hemorrhages/nodules on skin and mucous membranes
multiple blood cultures (at least 2 pairs)
blood drawing for: ESR, CRP, leukocytes, hemoglobin (anemia?), BUN/creatinine (rule out end-organ damage)
echocardiography, especially transesophageal, to detect vegetations, valve destruction, or perivalvular abscesses
Prosthetic endocarditis.
With the increasing frequency of artificial heart valve implantation, there has been a corresponding rise in the number of cases of prosthetic endocarditis.
Rise in cardiac valve procedures.
In 2022, 38 547 heart valve procedures were performed in Germany alone (biological/mechanical valve replacement or valve reconstruction), a marked rise from 22 243 such procedures in 2008
In patients undergoing cardiac surgery for prosthetic endocarditis, the reported mortality rate is still high (19–50%) (6). A large-scale multicenter study revealed, however, that the patients’ comorbidities affected their long-term survival more than the prosthetic endocarditis itself. After adjustment for comorbidities, the mortality from prosthetic valve endocarditis at 1 year was 39%, which is comparable to the mortality from native valve endocarditis (7). Potential predictors of a severe course are debated in the literature. These may include a variety of comorbid illnesses, the site of the affected valve, the microbial pathogen (especially Staphylococcus aureus), and infective endocarditis on a prosthetic valve (7, 8, e3).
Learning objectives
This article should enable readers to:
know the recommendations for antibiotic prophylaxis and essential preventive measures
understand the principles of microbiological and imaging diagnostics and the challenges and complications of surgical treatment
make a proper choice of rational empirical and targeted antimicrobial treatment.
Mortality of prosthetic endocarditis.
In patients undergoing cardiac surgery for prosthetic endocarditis, the reported mortality rate is still high (19–50%)
Prevention and prophylaxis.
Antibiotic prophylaxis should be restricted to the patients at highest risk, i.e., those who have undergone valve replacement, who have had a previous bout of endocarditis, or who bear certain congenital heart defects [Box 3] and are about to have a high-risk dental procedure.
Box 3.
Antibiotic prophylaxis before high-risk dental procedures is recommended for patients with any of the following:
a prosthetic heart valve, including transcatheter valve implantation (TAVI), or a valve that has been reconstructed with prosthetic material (rings or clips)
a left ventricular assist device (LVAD)
previous, recurrent or recurring infective endocarditis
-
any of the following congenital heart defects:
an uncorrected cyanotic congenital heart defect, including palliative shunts and conduits
a fully corrected congenital heart defect with prosthetic material up to 6 months after surgical or interventional correction
a corrected congenital heart defect with a residual shunt or valvular insufficiency
the surgical or catheter-assisted implantation of a pulmonary artery valve or conduit, e.g., a Melody valve or Contegra conduit
status post heart transplantation with subsequent valvular heart disease
No recommendation for antibiotic prophylaxis:
implantable electronic devices such as a pacemaker, ICD, CRT or event recorder
devices used to close septal defects, when complete occlusion has been achieved
peripheral vascular grafts and patches, including those used for hemodialysis
coronary or vascular stents
ventriculo-atrial cerebrospinal fluid shunts
vena cava filters
pledgets
CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator
Prevention/Prophylaxis
The U.S. and European guideline recommendations for antibiotic prophylaxis in persons at risk for infective endocarditis were substantially changed between 2007 and 2009, because the benefit of antibiotic prophylaxis had been demonstrated only in retrospective studies without any evidence from controlled trials (e4). Ever since this paradigm shift, the recommendation for the antibiotic prophylaxis has been restricted to patients at highest risk, i.e., those who have undergone valve replacement, who have had a previous history of infective endocarditis, or who bear certain congenital heart defects [Box 3] and are about to have a high-risk dental procedure (manipulation of the gingiva or periapical dental region [Box 4] (9). Antibiotic prophylaxis is not recommended before any other diagnostic or therapeutic intervention (e.g., not before bronchoscopy, gastrointestinal endoscopy, cystoscopy, or transesophageal echocardiography) if there is no evidence of infection. The recommendation was made musch more restrictive because the risk of infection must be weighed against the individual and collective development of antibiotic resistance (5).
Box 4.
High-risk dental procedures that can lead to bacteremia and for which antibiotic prophylaxis is recommended only in high-risk patients:
manipulation of the gingiva
manipulation of the periapical region
perforation of the oral mucosa
biopsy procedures
placement of orthodontic bands
intraligamentary anesthesia
Antibiotic prophylaxis is not recommended in the following situations:
local anesthetic injection into healthy tissue,except for intraligamentary anesthesia
dental radiographs
placement or adjustment of prosthetic or orthodontic anchoring elements
placement of orthodontic braces
suture removal
lip trauma
trauma to the oral mucosa
physiological deciduous tooth loss
Strict oral hygiene
Good oral hygiene and regular dental checkups are even more important than antibiotic prophylaxis (e5). The following preventive measures should ideally be followed not only by high-risk patients but also by the general population to lower the risk of bacteremia (5, 10):
Brush teeth at least twice daily with a fluoride toothpaste so as to remove as much of the biofilm as possible.
If food debris and biofilm cannot be adequately removed by brushing alone, us additional dental hygiene aids (dental floss, interdental brushes) as well.
Limit the consumption of sugary foods and beverages.
Have dental check-ups annually, or twice a year if at high risk.
Eliminate any foci of bacterial infection.
Do not self-medicate with antibiotics.
Follow sterile precautions meticulously in all high-risk procedures.
Avoid piercing and tattooing.
Avoid intravenous catheters and invasive procedures if possible.
Use peripheral in preference to central venous catheters.
Medical personnel should be trained in the early recognition of clinical signs of infective endocarditis.
The observed rises in staphylococcal and health-care-system-associated endocarditis underscore the need for such preventive measures (e6).
Procedure for other types of intervention.
Antibiotic prophylaxis is not recommended before any other diagnostic or therapeutic intervention (e.g., not before bronchoscopy, gastrointestinal endoscopy, cystoscopy, or transesophageal echocardiography) if there is no evidence of infection.
Strict oral hygiene.
Good oral hygiene and regular dental checkups are even more important than antibiotic prophylaxis.
The interdisciplinary endocarditis team
The management of patients with infective endocarditis by multidisciplinary teams consisting of experts in infectious disease, microbiology, radiology, cardiology, and cardiac surgery is recommended in the European and American guidelines without exception (5, 11, e7, e8). Patients at high risk (those with worsening heart failure, perivalvular abscess, or large emboli) should be transferred early to a specialized center with a cardiac surgery department (12). In a French study, the introduction of a protocol for diagnostic evaluation, antibiotic and surgical treatment, and subsequent follow-up lowered 1-year mortality from 18.5% to 8.2% (12, 13).
Diagnostic evaluation
Diagnostic criteria
Since 2000, the modified Duke criteria have been the standard means of diagnostic classification of infective endocarditis (e9). They incorporate relevant echocardiographic, microbiologic, and clinical findings to estimate the probability of infective endocarditis. Although the modified Duke criteria are widely used in clinical practice, they detect only 80% of cases of native endocarditis and only 60% of cases of prosthetic endocarditis (e2). This is mainly due to the much lower sensitivity and specificity of transesophageal echocardiography in prosthetic endocarditis compared with native endocarditis (78–91% vs. 91–96%, and 57–75% vs. 67–88%, respectively) (14).
Microbiological testing
Empiric therapy should be preceded by appropriate diagnostic testing. At least three pairs of blood cultures should be taken at 30-minute intervals before starting. In case of culture-negative endocarditis despite the taking of adequate blood cultures, serologic testing is recommended, particularly for Coxiella burnetti, Bartonella, mycoplasma, and Legionella (5, 14). If the patient undergoes surgery, valve material should be sent for culture and for molecular diagnosis with the polymerase chain reaction as a further means of identifying pathogens that can then be the target of treatment (e10).
Cardiac imaging diagnostics: echocardiography
In the current guidelines, transesophageal echocardiography (TEE) is considered the basic method for diagnosing prosthetic endocarditis (5). Echocardiographic evidence of vegetations is much rarer in prosthetic endocarditis; discrete thickenings and irregularities can be found on the normally smooth contour of the suture ring, but these are much more difficult to detect echocardiographically. Perivalvular complications are especially common in early prosthetic endocarditis because the space around the valve annulus is usually the primary site of infection (2, 15). The diagnostic value of TEE is generally high, but nonetheless limited by artifacts caused by the prosthetic material, and it may not detect complications of prosthetic endocarditis such as abscesses or pseudoaneurysms. According to the guidelines, if endocarditis is still suspected after a negative TEE, the examination should be repeated in three to seven days or else performed with a different imaging modality (5). Newer multimodality imaging methods such as 18F-fluorodeoxyglucose positron emission tomography (F-18-FDG/PET-CT) and leukocyte scintigraphy can provide additional help beyond the standard investigation and increase the diagnostic yield, particularly in cases that are difficult to diagnose; they have therefore been included in the modified diagnostic criteria of the current 2015 ESC guidelines, as listed in Box 1 (14, 16, 17, e11, e12).
Duke criteria.
The modified Duke criteria incorporate relevant echocardiographic, microbiologic, and clinical findings to estimate the probability of endocarditis.
Transesophageal echocardiography.
In the current guidelines, transesophageal echocardiography (TEE) is considered the basic method for diagnosing prosthetic endocarditis
Cardiac and extracardiac imaging diagnostics: nuclear medicine procedures
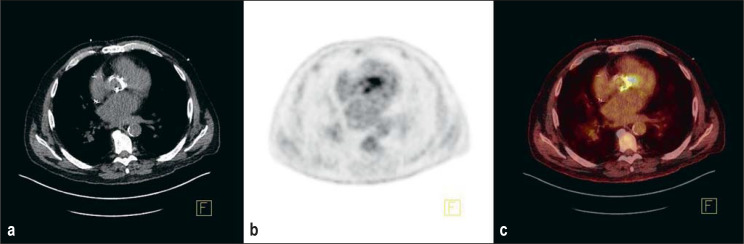
The available nuclear medicine procedures include F-18 FDG PET/CT and leukocyte SPECT/CT. Fluorodeoxyglucose (FDG) is a glucose analog labeled with F-18. It is absorbed into cells like glucose, but it is not metabolized and remains in the cell. F-18 FDG PET/CT can thus reveal inflammatory lesions as sites of increased metabolism in activated inflammatory cells. As the injected radiopharmaceutical is distributed throughout the body, a standard PET/CT also includes images of the body trunk, and thus disseminated septic lesions or extracardiac foci can be detected in addition to the cardiac focus. In the early postimplantation phase, the findings are often nonspecific, because physiologic repair processes are not reliably distinguishable from infection (18). According to the guidelines, therefore, PET/CT should not be performed any sooner than three months after the valve implantation (e13). An illustrative finding of florid endocarditis is shown in Figure 1.
Figure 1.
Florid endocarditis nine months after aortic valve replacement: intense F-18-FDG enhancement in the area of the annulus, extending medially. a) computed tomography, b) positron emission tomography, c) fused PET and CT (Institute of Radiology, Nuclear Medicine and Molecular Imaging, Heart and Diabetes Center of North Rhine-Westphalia).
The advantages of supplementary testing with nuclear medical methods include a marked reduction of unconfirmed suspected cases of endocarditis (19, 20, e14) in favor of confirmed diagnoses, as well as the detection of septic emboli and thromboembolic infarcts (5, e15).
Cardiac and extracardiac imaging: nuclear medical methods.
The available nuclear medicine procedures include F-18 FDG PET/CT and leukocyte SPECT/CT.
Extracardiac imaging:
Cerebrovascular complications are among the more common complications of infective endocarditis, arising in 20–40% of patients. Ischemic stroke is the most common type (70%); hemorrhagic stroke is much less common (15%).
Extracardiac diagnostics
Cerebrovascular complications are among the more common complications of infective endocarditis, arising in 20–40% of patients. Ischemic stroke is the most common type (70%); hemorrhagic stroke is much less common (15%). Infectious aneurysms, abscesses, and meningoencephalitis are rare (5% each). Routine brain imaging reveals silent cerebral emboli in up to 70% of patients with infective endocarditis (21). Systemic abdominal emboli, e.g., to the spleen, may be clinically silent as well. Helpful tools for the detection of such complications include systematic abdominal and cerebral noninvasive imaging with contrast ultrasonography, computed tomography, PET/CT, and/or magnetic resonance imaging (22, e16).
Pathogen profile and anti-infective therapy
The typical pathogen profile varies depending on the pathogenesis of the infection. Early prosthetic endocarditis is caused by direct intraoperative contamination or by hematogenous spread to the valve in the first days to weeks after surgery. Infective endocarditis in the period 2–12 months after surgery is secondary to late nosocomial infection or other infections acquired outside the hospital.
Artificial valve endocarditis in the first two months after surgery is most often caused by Staphylococcus aureus, followed by coagulase-negative Staphylococcus and, much less commonly, Gram-negative bacteria and Candida species. From 2 to 12 months after surgery, streptococci, Staphylococcus aureus and coagulase-negative staphylococci are the most common pathogens, followed by enterococci (1).
Late prosthetic endocarditis (more than a year after surgical intervention) arises secondarily to other acquired infections. The spectrum of pathogens is similar to that of native valve endocarditis, with streptococci and Staphylococcus aureus being most common, followed by enterococci and coagulase-negative staphylococci. Both early and late prosthetic endocarditis can be culture-negative. Rarer causes of prosthetic endocarditis include nontuberculous mycobacteria (Mycobacterium chimera) and enteroviruses (e17, e18).
Anti-infective therapy
General principles
The goal of antibiotic therapy is to eradicate the bacteria; this is made more difficult by a high bacterial density in the vegetations and by bacteremia, which often leads to further colonization. In prosthetic valve infections, the foreign material is also covered by a biofilm, i.e., a thin layer of mucus in which microorganisms are present in an organized arrangement; the attenuated metabolism in the biofilm renders the bacteria in it less vulnerable to attack by antibiotics. There is thus a need for long-term anti-infective therapy with high serum levels.
Empirical therapy
The ESC recommends two different antibiotic regimens for prosthesis infections depending on the time of occurrence, because of the different pathogen spectra (eTable) (5):
eTable. Selected therapeutic regimens for empiric therapy and common pathogens of prosthetic valve endocarditis – modified from Habib (5)*.
| Pathogen | Comments | Therapy | Dose |
| Unknown | |||
| expected pathogens: S.aureus coagulase-negative staphylococci streptococci enterococci occ. Gram-negative and fungi TAVI: enterococci S. aureus coagulase-negative staphylococci |
early, < 12 months after valve implantation early and late, nosocomial or health care system-associated infections |
vancomycin*1 + rifampicin*2 + gentamicin*3 |
30-(60) mg/kg/d in 2 doses 900 mg/d in 2 doses 3 mg/kg in 1 dose |
| expere pathogens: S. aureus, strreptococci, enterococci |
late ≥ 12 months after valve implantation (analogous to native valve) | ampicillin + flucloxacillin + gentamicin*3 |
12 g/d in 4–6 doses 12 g/d in 4–6 doses 3 mg/kg/d in 1 dose |
| Staphylococci | |||
| S. aureus or coagulase-negative staphylococci (methicillin-sensitive) | flucloxacillin + rifampicin*2 + gentamicin*3 (2 weeks) |
12 g/d in 4–6 doses 900 mg/d in 2 doses 3 mg/kg in 1 dose |
|
| S. aureus or coagulase-negative staphylococci (methicillin-sensitive) | vancomycin*1 + rifampicin*2 + gentamicin*3 (2 weeks) |
30–(60) mg/kg/d in 2 doses 900 mg/d in 2 doses 3 mg/kg in 1 dose |
|
| Streptococci | |||
| < 0.125 mg/l (sensitive) | penicillin G | 24 million IU/d in 4–6 doses | |
| MIC 0.125–2 mg/L (intermediate sensitivity) | penicillin G + gentamicin*3 (2 weeks) |
24 million IU/d in 4–6 doses 3 mg/kg/d in 1 dose |
|
| Enterococci or resistant streptococci | |||
| ampicillin + gentamicin*3 (2–6 weeks) |
16 g/d in 4–6 doses 3 mg/kg/d in 1 dose |
||
| Candida spp.*4, *5 | |||
| immediate surgery | liposomal amphotericin B or caspofungin | 5 mg/kg/d in 1 dose 70–(150) mg/d in 1 dose |
|
* see also (https://infektiopedia.de/wiki/Endokarditis – last access 21.10.2022)
Duration of treatment: 6 weeks unless otherwise noted
1 Level determination: trough 15–20 mg/L
2 Dose according to American guidelines (e7) – high dose of 1 200 mg controversial (e32), Note: check interactions – see „decide wisely“ recommendation (e33)
3 Level determination: trough < 1 mg/L
4 Optimal duration of treatment unclear; in some cases, lifelong suppressive therapy with fluconazole 400 mg/d if sensitive (e34, e35)
5 Antifungal therapy, lip. Amphotericin B 5 mg/kg, or, alternatively, caspofungin 70/50 mg (up to 150 mg in U.S. recommendations); later step-down to fluconazole 400–800 mg if sensitive, in some cases lifelong (e34, e35)
MIC, minimal inhibitory concentration
< 12 months after implantation (nosocomial or health-care-system-associated): vancomycin + rifampicin + gentamicin.
≥ 12 months after implantation (corresponding to the treatment of native endocarditis): ampicillin + flucloxacillin + gentamicin.
A detailed infectious disease assessment should also be carried out so that the treatment can be individually optimized in view of the patient’s history (e.g., urosepsis or catheter-associated bacteremia), previous therapies, pre-existing illnesses, and resistance profile.
Pathogen spectrum in the first two months.
Prosthetic valve endocarditis in the first two months after surgery is most often caused by Staphylococcus aureus, followed by coagulase-negative Staphylococcus and, much less commonly, Gram-negative bacteria and Candida species.
Pathogen spectrum after the first two months.
From 2 to 12 months after surgery, streptococci, Staphylococcus aureus, and coagulase-negative staphylococci are the most common pathogens, followed by enterococci. Late prosthetic endocarditis (more than a year after surgery) arises secondarily to other acquired infections.
Targeted therapy
Anti-infective therapy for prosthetic valve infections is given for six weeks for all pathogens (streptococci, enterococci, staphylococci, gram-negative pathogens) (5). Prosthetic valve infections with streptococci, enterococci and gram-negative pathogens are treated with the same antibiotics as native valve infections. The regimen differs only for staphylococci, with the recommended addition of rifampicin (for 6 weeks) and gentamicin (for 2 weeks) to flucloxacillin (methicillin-sensitive) or vancomycin (methicillin-resistant). It remains to be seen whether gentamicin will continue to be recommended: an observational study of S.-aureus prosthetic endocarditis revealed no survival benefit for the addition of gentamicin to cloxacillin or vancomycin plus rifampicin. Gentamicin is no longer recommended for the treatment of native valve endocarditis (e19). (e19).
Oral sequential therapy
The POET trial was a randomized landmark trial that challenged the dogma of the need for intravenous antibiotic therapy. It showed the noninferiority of oral therapy (23) with respect to a combined clinical endpoint consisting of death, recurrent bacteremia, the need for cardiac surgery, or an embolic event. Noninferiority was also shown in the subgroup after stratification into native or prosthetic valve endocarditis (23). It must be noted, however, that only a selected subgroup was included (small vegetations, stable clinical course, no abscess) with three weeks of preceding intravenous therapy, close monitoring, and a very low overall mortality (5%). In high-risk prosthetic infections, anti-infective therapy should only be switched to the oral route in exceptional cases and with close clinical monitoring, as the state of the evidence for oral therapy is not yet sufficient.
Targeted therapy.
Anti-infective therapy for prosthetic valve infections is given for six weeks for all pathogens (streptococci, enterococci, staphylococci, gram-negative pathogens).
Surgery
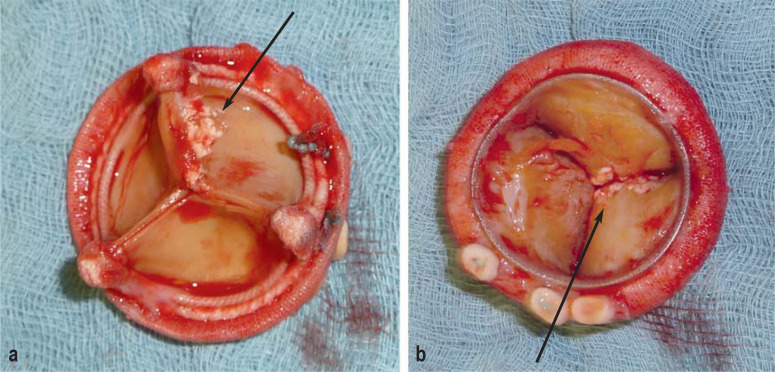
The interdisciplinary endocarditis team must weigh the indication, timing, and overall benefit of surgery against its risks for the individual patient, in the light of the clinical situation and the patient’s comorbidity. On the one hand, early surgery should prevent progressive, irreversible destruction, as well as systemic emboli (Figure 2) (24, e20); on the other hand cardiac surgery in the active phase of the disease is associated with considerable risks (5). The indications for early surgery are listed in the eBox and discussed in the following paragraphs.
Figure 2.
Explanted biological heart valve prosthesis. The black arrows mark attached vegetations.
a) View from the aorta.
b) View from the ventricle.
eBox.
Acute heart failure
aortic or mitral valve endocarditis with severe acute heart failure and progressive hemodynamic impairment (IB)
Uncontrolled infection
locally uncontrolled infection (abscess, aneurysm, fistula, progressive vegetation) (IB)
infection with fungi or multidrug-resistant pathogens (IC)
persistent positive blood cultures despite appropriate antibiotic therapy (IIa B)
prosthetic endocarditis due to staphylococci or non-HACEK Gram-negative pathogens (IIa C)
Prevention of emboli
-
aortic or mitral valve endocarditis with vegetations > 10 mm and
embolic events under appropriate antibiotic therapy (IB) or
severe valvular involvement and low surgical risk (IIa B)
aortic or mitral valve endocarditis with isolated, very large vegetations > 30 mm (IIa B)
Surgery.
Patients with prosthetic valve endocarditis should be referred to a specialized center with a cardiac surgery department and an interdisciplinary endocarditis team.
Acute heart failure
Progressive valve destruction can cause marked acute heart failure. This is the main surgical indication in most patients with endocarditis (5, e20). The severity of acute heart failure may necessitate urgent or emergency surgery even in the presence of other risk factors (5).
Uncontrolled infection
The second most common reason for surgery is uncontrolled infection. In particular, this includes the perivalvular extension of endocarditis (abscesses, pseudoaneurysms, and fistulae), which arises in 56–100% of cases of prosthetic endocarditis (15, e7). The identified risk factors for perivalvular complications are a prosthetic valve, infection with coagulase-negative staphylococci, and aortic valve involvement (e21). Moreover, patients with perivalvular involvement have both a higher perioperative mortality and a higher risk of endocarditis recurrence (e22, e23). This is because the spread of infection beyond the valve annulus requires more extensive surgery, with radical debridement and a more comprehensive reconstruction (25).
Surgery should be considered in patients with prosthetic valve endocarditis due to certain pathogens, such as staphylococci or non-HACEK gram-negative bacteria (5, e24, e25). Fungal infection is rare, but more common in prosthetic than in native valves; in such cases, surgery within a few days is recommended (5).
Surgical treatment should also be considered if fever and positive blood cultures persist for more than 7–10 days despite appropriate antibiotic therapy (5).
The prevention of embolism
The risk of embolism-related complications is highest (20–50%) in the first few days after antibiotic therapy is begun; it is already lower (6–21%) in the ensuing two weeks (24, 26). For this reason, surgery for the prevention of embolism has the greatest benefit during the first two weeks of antibiotic therapy.
Embolic events are hard to predict, but risk factors have been identified, including vegetation size and mobility, vegetations on the mitral valve, increase or decrease in vegetations with antibiotic therapy, and certain types of pathogen, including Staphylococcus aureus (26, 27).
When deciding on early surgery for embolic prophylaxis, the presence of previous embolic events, other complications of infective endocarditis, the size and mobility of the vegetation, the type of microorganism and the duration of antibiotic therapy should be taken into account (24).
Current evidence suggests that, in patients with infective endocarditis and extensive vegetations, early treatment significantly reduces the combined endpoint of death from any cause and embolic events, in comparison to later or conventional treatment (28), and is associated with lower in-hospital and long-term mortality (29, 30). The evidence regarding the timing of surgery for infective endocarditis is weaker, consisting mainly of expert consensus. Surgery is often unfavorably delayed because of a time lapse between the diagnosis of infective endocarditis and the recognition of the surgical indication arising from a complication of infective endocarditis, as well as by delays in transferring patients from the external institutions where infective endocarditis is often diagnosed to a specialized center for cardiac surgery (31). Therefore, larger prospective clinical trials are needed to determine the optimal timing of surgery (32). The guidelines for the diagnosis and treatment of infective endocarditis are currently being revised; more specific recommendations on the timing of surgery in these complex patients can be expected.
Septic emboli.
Septic emboli arise in 20–50% of patients with prosthetic endocarditis and may be clinically silent.
Prosthetic endocarditis in the TAVI era
Over the past 15 years, transcatheter aortic valve implantation (TAVI) has revolutionized the treatment of aortic stenosis and has increased the number of patients living with prosthetic valves (e26). The number of patients with prosthetic valve endocarditis after TAVI has risen accordingly. Multiple previous studies have compared the incidence of endocarditis after surgical and interventional valve replacement (TAVI) (33–35, e27): observational analyses revealed no difference in incidence (ca. 3–25 per 1000 person-years) over a follow-up period of 5–44 months (e26).
Multiple reviews and analyses of national and international registries have yielded two main findings: patients with TAVI endocarditis have poor survival, with a mortality of 40–70% one year after diagnosis, and the infected prosthesis is surgically explanted in only 2–14% of cases, despite a clear indication in more than 80% (33, 36–38, e28). The reason for the latter finding may be that these patients underwent TAVI in the first place because of what was considered to be a high surgical risk, and were subsequently even less likely to be operated on in the presence of TAVI prosthetic endocarditis.
Many patients with infective endocarditis after TAVI have early prosthetic endocarditis (39), which, in most cases, affects not only the valve leaflets, but the stent scaffold and annulus as well, causing valve dehiscence and perivalvular abscesses (40). Possible destruction of the aortic root, ingrowth of the stent into the ascending aorta, and destruction of the aorto-mitral continuity are feared complications (40). The surgical treatment is generally highly demanding, requiring extensive debridement, repair of perivalvular abscesses or fistulae, and the implantation of a new prosthetic valve.
Enterococci are more prominent in the spectrum of pathogens in prosthetic valve endocarditis after TAVI than after surgical valve replacement. It has been suggested that vascular access for TAVI through the inguinal region may be a contributing cause. Reguiero et al. detected enterococci (25%) and S. aureus (23 %) in almost equal amounts, followed by coagulase-negative staphylococci (17%) (37).
One factor elevating the risk of prosthetic endocarditis after TAVI seems to be an increased gradient after implantation (e26, e29). Higher transvalvular gradients may cause turbulent flow and endothelial damage, which can then serve as a nidus for the development of vegetations (e30).
The limitations of diagnostic imaging, the complexity of surgical treatment, and the predisposing factors combine to make TAVI prosthetic endocarditis a serious condition. Nonetheless, even though this disease presents major medical and technical challenges, timely and radical surgery can rescue the patient from sepsis and heart failure and yield an acceptable outcome over the intermediate term and is probably the best therapeutic option for these patients (e28, 40).
Prosthetic valve endocarditis.
Prosthetic valve endocarditis is classified as either early or late because these two types have a different pathogenesis and a different spectrum of pathogens. Prosthetic valve endocarditis has a worse prognosis than native valve endocarditis, with a mortality of 19–50 %.
Conclusion and future prospects
As more and more people are living with artificial heart valves, programs for the prevention of infection need to be intensified, and methods for the diagnosis and treatment of prosthetic valve endocarditis need to be further refined in order to improve outcomes. Patients with suspected prosthetic endocarditis should be transferred as soon as possible to a specialized center with a multidisciplinary endocarditis team, as delayed transfer worsens outcomes, and surgery for prosthetic valve endocarditis is often these patients’ last chance.
Transcatheter valve implantation.
Prosthetic valve endocarditis in a valve that has been implanted percutaneously is more likely to be due to enterococci than prosthetic valve endocarditis in a surgically implanted valve.
Prevention programs.
Prevention programs need to be intensified, and the diagnosis and treatment of patients with prosthetic valve endocarditis need to be improved.
Box 1b. Minor diagnostic criteria for infective endocarditis.
predisposition, e.g., a predisposing heart disease or intravenous drug abuse
fever > 38° C
vascular phenomena, including those detected only on imaging („silent events“): large arterial emboli, septic pulmonary infarcts, infectious (mycotic) aneurysms, intracranial hemorrhage, conjunctival hemorrhage, and Janeway lesions
immunological phenomena: glomerulonephritis, Osler‘s nodes, Roth spots, rheumatoid factor
microbiological evidence: positive blood culture that does not fulfill a main criterion, or serological evidence of active infection with an organism that can cause endocarditis
Further information on CME.
Participation in the CME certification program is possible only via the Internet: cme.aerzteblatt.de. This unit can be accessed until 12 October 2024. Submissions by letter, email, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de.
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed using the “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in “Meine Daten,” and consent must be given for results to be communicated. The 15-digit EFN can be found on the CME card (8027XXXXXXXXXXX)
Participation is possible at cme.aerzteblatt.de. The submission deadline is 12 October 2024.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
For which patients is antibiotic prophylaxis recommended before high-risk dental procedures?
patients with cardiac pacemakers
patients with high-grade aortic valvular stenosis
patients with grade 3 tricuspid insufficiency
patients with a biological aortic valve prosthesis
patients with mitral valve prolapse
Question 2
Patients at high risk should receive endocarditis prophylaxis before which of the following procedures?
colonoscopy
esophagogastroduodenoscopy
skin biopsy
tooth extraction
cystoscopy
Question 3
What preventive measures should be taken in patients at high risk?
antibiotic prophylaxis before the insertion of central venous catheters
dental check-ups three times a year
the elimination of foci of bacterial infection
the preferential use of central, rather than peripheral, catheters for venous access
antibiotics in case of a common cold
Question 4
When, by definition, does late prosthetic endocarditis arise?
3 months after surgery
6 months after surgery
9 months after surgery
>12 months after surgery
>24 months after surgery
Question 5
What treatment regimen is recommended in the ESC guideline for patients with a prosthetic valve infection 7 months after implantation?
vancomycin + rifampicin + gentamicin
ampicillin + flucloxacillin + gentamicin
fosmidomycin + ampicillin + flucloxacillin
chloramphenicol + fusidic acid + vancomycin
azithromycin + doxycyclin + rifampicin
Question 6
What findings are consistent with the modified Duke criteria?
abnormal activity in the vicinity of the heart vves 2 weeks after implantation
a positive ANA titer of 1/320
a single positive blood culture for Coxiella burnetii
a positive Borrelia serology
an elevated erythrocyte sedimentation rate of 100 mm/h
Question 7
Prosthetic valve endocarditis is often treated with the same antibiotics as native valve endocarditis. For which class of pathogen is the antibiotic treatment different in these two conditions?
enterococci
staphylococci
streptococci
Gram-negative pathogens
Candida spp.
Question 8
How long should antibiotics be given to treat streptococcal <prosthetic valve endocarditis?
2 weeks
4 weeks
6 weeks
8 weeks
12 weeks
Question 9
What is the most common surgical indication in prosthetic valve endocarditis?
vegetations > 10 mm
fungal infection
acute heart failure
perivalvular abscess
persistent positive blood cultures
Question 10
Which of the following is a risk factor for embolization in prosthetic valve endocarditis?
a vegetation on the mitral valve
hypotension
ST-segment elevation
E. coli bacteremia
peripheral pulmonic stenosis
Participation is only possible online: cme.aerzteblatt.de
Case Illustration.
A 72-year-old man presents to the emergency department. He has been suffering from subfebrile temperatures, chills, and exhaustion for several weeks, and he also complains of nonspecific joint pain. He reports that, two years ago, he underwent a biological aortic valve replacement because of aortic valve stenosis.
The laboratory findings include an elevated C-reactive protein level of 54 mg/L (normal: ≤ 5 mg/L) and an elevated erythrocyte sedimentation rate of 88 mm/h, with a normal white blood count.
Transesophageal echocardiography (TEE) does not reveal any vegetations on the aortic valve prosthesis or any clear evidence of perivalvular destruction. Staphylococcus aureus is detected in 3/3 serially taken blood cultures one day later. A PET/CT then reveals a small area of increased F-18-FDG uptake in the vicinity of the aortic valve ring. Prosthetic valve endocarditis is diagnosed.
The interdisciplinary board (cardiology, cardiac surgery, infectious disease, microbiology, nuclear medicine, radiology) confirms the indication for surgical valve replacement, and antibiotic treatment is initiated with with flucloxacillin 12 g/d in 3–4 divided doses (for 6 weeks) + rifampicin 900 mg/d in 2 divided doses (for 6 weeks) + gentamicin 3 mg/kg per day (for 2 weeks).
The intraoperative findings are much more pronounced than initially expected from the echocardiographic images. A perivalvular abscess is identified; radical debridement is performed, and continuity is restored with a pericardial patch. Postoperatively, the control blood cultures are immediately negative and the patient feels markedly less tired. Follow-up echocardiography demonstrates the correct position and intact function of the newly inserted aortic valve prosthesis.
On ambulatory follow-up three months later, the patient states that he feels well, and the inflammatory parameters are normal.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
NNJ has received payment for the preparation of manuscripts for Bayer, Gilead, Infectopharm, Medacta, and MSD. She has received reimbursement of travel expenses from Basilea, Correvio, Gilead, Novartis, and Pfizer.
The other authors declare that they have no conflict of interest.
References
- 1.Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354–1361. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Thuny F, Avierinos JF. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis. 2008;50:274–281. doi: 10.1016/j.pcad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Miro JM, Ambrosioni J. Infective endocarditis: an ongoing global challenge. Eur Heart J. 2019;40:3233–3236. doi: 10.1093/eurheartj/ehz694. [DOI] [PubMed] [Google Scholar]
- 4.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet (London, England) 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 5.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The task force for the management of infective Eendocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 6.Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013;173:1495–1504. doi: 10.1001/jamainternmed.2013.8203. [DOI] [PubMed] [Google Scholar]
- 7.Weber C, Petrov G, Luehr M, et al. Surgical results for prosthetic versus native valve endocarditis: a multicenter analysis. J Thorac Cardiovasc Surg. 2021;161:609–619.e10. doi: 10.1016/j.jtcvs.2019.09.186. [DOI] [PubMed] [Google Scholar]
- 8.Luehr M, Weber C, Misfeld M, et al. Virulence of staphylococcus infection in surgically treated patients with endocarditis: a multicenter analysis. Ann Surg. 2022 Jul 8; doi: 10.1097/SLA.0000000000005448. doi: 10.1097/SLA.0000000000005448. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Naber CK, Al-Nawas B, Baumgartner H, et al. Prophylaxe der infektiösen Endokarditis. Kardiologe. 2007;1:243–250. [Google Scholar]
- 10.Cahill TJ, Dayer M, Prendergast B, Thornhill M. Do patients at risk of infective endocarditis need antibiotics before dental procedures? BMJ. 2017;358 doi: 10.1136/bmj.j3942. [DOI] [PubMed] [Google Scholar]
- 11.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 12.Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69:325–344. doi: 10.1016/j.jacc.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 13.Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169:1290–1298. doi: 10.1001/archinternmed.2009.192. [DOI] [PubMed] [Google Scholar]
- 14.Kouijzer JJP, Noordermeer DJ, van Leeuwen WJ, Verkaik NJ, Lattwein KR. Native valve, prosthetic valve, and cardiac device-related infective endocarditis: a review and update on current innovative diagnostic and therapeutic strategies. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.995508. 995508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber C, Rahmanian PB, Nitsche M, et al. Higher incidence of perivalvular abscess determines perioperative clinical outcome in patients undergoing surgery for prosthetic valve endocarditis. BMC Cardiovasc Disord. 2020;20 doi: 10.1186/s12872-020-01338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmood M, Kendi AT, Ajmal S, et al. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol. 2019;26:922–935. doi: 10.1007/s12350-017-1092-8. [DOI] [PubMed] [Google Scholar]
- 17.Primus CP, Clay TA, McCue MS, et al. (18)F-FDG PET/CT improves diagnostic certainty in native and prosthetic valve infective endocarditis over the modified Duke criteria. J Nucl Cardiol. 2022;29:2119–2128. doi: 10.1007/s12350-021-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swart LE, Scholtens AM, Tanis W, et al. 18F-fluorodeoxyglucose positron emission/computed tomography and computed tomography angiography in prosthetic heart valve endocarditis: from guidelines to clinical practice. Eur Heart J. 2018;39:3739–3749. doi: 10.1093/eurheartj/ehx784. [DOI] [PubMed] [Google Scholar]
- 19.Pizzi MN, Roque A, Fernandez-Hidalgo N, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tTomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015;132:1113–1126. doi: 10.1161/CIRCULATIONAHA.115.015316. [DOI] [PubMed] [Google Scholar]
- 20.Hohmann C, Michels G, Schmidt M, et al. Diagnostic challenges in infective endocarditis: is PET/CT the solution? Infection. 2019;47:579–587. doi: 10.1007/s15010-019-01278-6. [DOI] [PubMed] [Google Scholar]
- 21.Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation. 2016;134:1280–1292. doi: 10.1161/CIRCULATIONAHA.116.024156. [DOI] [PubMed] [Google Scholar]
- 22.Paul G, Michels G, Hohmann C, et al. Contrast-enhanced ultrasound for the detection of abdominal complications in infective endocarditis: first experience from a prospective cohort. Ultrasound Med Biol. 2020;46:2965–2971. doi: 10.1016/j.ultrasmedbio.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380:415–424. doi: 10.1056/NEJMoa1808312. [DOI] [PubMed] [Google Scholar]
- 24.Thuny F, Beurtheret S, Mancini J, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J. 2011;32:2027–2033. doi: 10.1093/eurheartj/ehp089. [DOI] [PubMed] [Google Scholar]
- 25.Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121:1141–1152. doi: 10.1161/CIRCULATIONAHA.108.773598. [DOI] [PubMed] [Google Scholar]
- 26.Vilacosta I, Graupner C, San Roman JA, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol. 2002;39:1489–1495. doi: 10.1016/s0735-1097(02)01790-4. [DOI] [PubMed] [Google Scholar]
- 27.Cabell CH, Pond KK, Peterson GE, et al. The risk of stroke and death in patients with aortic and mitral valve endocarditis. Am Heart J. 2001;142:75–80. doi: 10.1067/mhj.2001.115790. [DOI] [PubMed] [Google Scholar]
- 28.Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–2473. doi: 10.1056/NEJMoa1112843. [DOI] [PubMed] [Google Scholar]
- 29.Liang F, Song B, Liu R, Yang L, Tang H, Li Y. Optimal timing for early surgery in infective endocarditis: a meta-analysis. Interact Cardiovasc Thorac Surg. 2016;22:336–345. doi: 10.1093/icvts/ivv368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anantha Narayanan M, Mahfood Haddad T, Kalil AC, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart (British Cardiac Society) 2016;102:950–957. doi: 10.1136/heartjnl-2015-308589. [DOI] [PubMed] [Google Scholar]
- 31.Wang A, Fosbol EL. Current recommendations and uncertainties for surgical treatment of infective endocarditis: a comparison of American and European cardiovascular guidelines. Eur Heart J. 2022;43:1617–1625. doi: 10.1093/eurheartj/ehab898. [DOI] [PubMed] [Google Scholar]
- 32.Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8:630–644. doi: 10.21037/acs.2019.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolte D, Goldsweig A, Kennedy KF, et al. Comparison of incidence, predictors, and outcomes of early infective endocarditis after transcatheter aortic valve implantation versus surgical aortic valve replacement in the United States. Am J Cardiol. 2018;122:2112–2119. doi: 10.1016/j.amjcard.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama N, Laakso T, Biancari F, et al. Prosthetic valve endocarditis after transcatheter or surgical aortic valve replacement with a bioprosthesis: results from the FinnValve Registry. EuroIntervention. 2019;15:e500–e507. doi: 10.4244/EIJ-D-19-00247. [DOI] [PubMed] [Google Scholar]
- 35.Summers MR, Leon MB, Smith CR, et al. Prosthetic valve endocarditis after TAVR and SAVR: insights from the PARTNER trials. Circulation. 2019;140:1984–1994. doi: 10.1161/CIRCULATIONAHA.119.041399. [DOI] [PubMed] [Google Scholar]
- 36.Bjursten H, Rasmussen M, Nozohoor S, et al. Infective endocarditis after transcatheter aortic valve implantation: a nationwide study. Eur Heart J. 2019;40:3263–3269. doi: 10.1093/eurheartj/ehz588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regueiro A, Linke A, Latib A, et al. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in-hospital death. JAMA. 2016;316:1083–1092. doi: 10.1001/jama.2016.12347. [DOI] [PubMed] [Google Scholar]
- 38.Amat-Santos IJ, Messika-Zeitoun D, Eltchaninoff H, et al. Infective endocarditis after transcatheter aortic valve implantation: results from a large multicenter registry. Circulation. 2015;131:1566–1574. doi: 10.1161/CIRCULATIONAHA.114.014089. [DOI] [PubMed] [Google Scholar]
- 39.Fukuhara S, Brescia AA, Shiomi S, et al. Surgical explantation of transcatheter aortic bioprostheses: results and clinical implications. J Thorac Cardiovasc Surg. 2021;162:539–547.e1. doi: 10.1016/j.jtcvs.2019.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha S, Ali A, Schnackenburg P, et al. Surgery for aortic prosthetic valve endocarditis in the transcatheter era. J Clin Med. 2022;11 doi: 10.3390/jcm11123418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.DGTHG. DGHTG Leistungsstatistik. www.dgthg.de/sites/default/files/Grafiken-DGTHG-Leistungsstatistik%202021_free-access_2.pdf (alst accessed on 26 August 2023) [Google Scholar]
- E2.Silbiger JJ, Rashed E, Chen H, Wiesenfeld E, Robinson SE, Cagliostro M. Cardiac imaging for diagnosis and management of infective endocarditis. J Am Soc Echocardiogr. 2022;35:910–924. doi: 10.1016/j.echo.2022.04.007. [DOI] [PubMed] [Google Scholar]
- E3.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-prospective cohort study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2007;138:739–745. doi: 10.14219/jada.archive.2007.0262. 747-60. [DOI] [PubMed] [Google Scholar]
- E5.DGZ; DGZMK. Kariesprophylaxe bei bleibenden Zähnen - grundlegende Empfehlungen. https://register.awmf.org/assets/guidelines/083-021k_S2k_Kariesprophylaxe_2017-03.pdf (last accessed on 5 September 2023) [Google Scholar]
- E6.Vogkou CT, Vlachogiannis NI, Palaiodimos L, Kousoulis AA. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis. 2016;35:1227–1245. doi: 10.1007/s10096-016-2660-6. [DOI] [PubMed] [Google Scholar]
- E7.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- E8.Blomström-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Europace. 2020;22:515–549. doi: 10.1093/europace/euz246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- E10.Mularoni A, Mikulska M, Barbera F, et al. Molecular analysis with 16S rRNA PCR/Sanger sequencing and molecular antibiogram performed on DNA extracted from valve improve diagnosis and targeted therapy of infective endocarditis: a prospective study. Clin Infect Dis. 2023;76:e1484–e1491. doi: 10.1093/cid/ciac452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Eder MD, Upadhyaya K, Park J, et al. Multimodality imaging in the diagnosis of prosthetic valve endocarditis: a brief review. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.750573. 750573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Slart R, Glaudemans A, Gheysens O, et al. Procedural recommendations of cardiac PET/CT imaging: standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: a joint collaboration of the EACVI and the EANM. Eur J Nucl Med Mol Imaging. 2021;48:1016–1039. doi: 10.1007/s00259-020-05066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Scholtens AM, Budde RPJ, Lam M, Verberne HJ. FDG PET/CT in prosthetic heart valve endocarditis: there is no need to wait. J Nucl Cardiol. 2017;24:1540–1541. doi: 10.1007/s12350-017-0938-4. [DOI] [PubMed] [Google Scholar]
- E14.Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–2382. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- E15.Van Riet J, Hill EE, Gheysens O, et al. (18)F-FDG PET/CT for early detection of embolism and metastatic infection in patients with infective endocarditis. Eur J Nucl Med Mol Imaging. 2010;37:1189–1197. doi: 10.1007/s00259-010-1380-x. [DOI] [PubMed] [Google Scholar]
- E16.Snygg-Martin U, Gustafsson L, Rosengren L, et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. 2008;47:23–30. doi: 10.1086/588663. [DOI] [PubMed] [Google Scholar]
- E17.Chu VH, Miro JM, Hoen B, et al. Coagulase-negative staphylococcal prosthetic valve endocarditis—a contemporary update based on the International Collaboration on Endocarditis: prospective cohort study. Heart (British Cardiac Society) 2009;95:570–576. doi: 10.1136/hrt.2008.152975. [DOI] [PubMed] [Google Scholar]
- E18.Lee JH, Burner KD, Fealey ME, et al. Prosthetic valve endocarditis: clinicopathological correlates in 122 surgical specimens from 116 patients (1985-2004) Cardiovas Pathol. 2011;20:26–35. doi: 10.1016/j.carpath.2009.09.006. [DOI] [PubMed] [Google Scholar]
- E19.Ramos-Martínez A, Muñoz Serrano A, de Alarcón González A, et al. Gentamicin may have no effect on mortality of staphylococcal prosthetic valve endocarditis. J Infect Chemother. 2018;24:555–562. doi: 10.1016/j.jiac.2018.03.003. [DOI] [PubMed] [Google Scholar]
- E20.Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart (British Cardiac Society) 2005;91:571–575. doi: 10.1136/hrt.2003.032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Chan KL. Early clinical course and long-term outcome of patients with infective endocarditis complicated by perivalvular abscess. CMAJ. 2002;167:19–24. [PMC free article] [PubMed] [Google Scholar]
- E22.Hryniewiecki T, Zatorska K, Abramczuk E, et al. The usefulness of cardiac CT in the diagnosis of perivalvular complications in patients with infective endocarditis. Eur Radiol. 2019;29:4368–4376. doi: 10.1007/s00330-018-5965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Gahide G, Bommart S, Demaria R, et al. Preoperative evaluation in aortic endocarditis: findings on cardiac CT. AJR Am J Roentgenol. 2010;194:574–578. doi: 10.2214/AJR.08.2120. [DOI] [PubMed] [Google Scholar]
- E24.Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. Fungal endocarditis: evidence in the world literature, 1965-1995. Clin Infect Dis. 2001;32:50–62. doi: 10.1086/317550. [DOI] [PubMed] [Google Scholar]
- E25.Baddley JW, Benjamin DK, Jr., Patel M, et al. Candida infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27:519–529. doi: 10.1007/s10096-008-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Cahill TJ, Raby J, Jewell PD, et al. Risk of infective endocarditis after surgical and transcatheter aortic valve replacement. Heart (British Cardiac Society) 2022;108:639–647. doi: 10.1136/heartjnl-2021-320080. [DOI] [PubMed] [Google Scholar]
- E27.Butt JH, Olesen JB, Gundlund A, et al. Long-term thromboembolic risk in patients with postoperative atrial fibrillation after left-sided heart valve surgery. JAMA Cardiol. 2019;4:1139–1147. doi: 10.1001/jamacardio.2019.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Malvindi PG, Luthra S, Sarvananthan S, Zingale A, Olevano C, Ohri S. Surgical treatment of transcatheter aortic valve infective endocarditis. Neth Heart J. 2021;29:71–77. doi: 10.1007/s12471-020-01494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.McElhinney DB, Sondergaard L, Armstrong AK, et al. Endocarditis after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2018;72:2717–2728. doi: 10.1016/j.jacc.2018.09.039. [DOI] [PubMed] [Google Scholar]
- E30.Werdan K, Dietz S, Loffler B, et al. Mechanisms of infective endocarditis: pathogen-host interaction and risk states. Nat Rev Cardiol. 2014;11:35–50. doi: 10.1038/nrcardio.2013.174. [DOI] [PubMed] [Google Scholar]
- E31.Wilson WR, Gewitz M, Lockhart PB, et al. Prevention of viridans group streptococcal infective endocarditis: a scientific statement from the American Heart Association. Circulation. 2021;143:e963–e978. doi: 10.1161/CIR.0000000000000969. [DOI] [PubMed] [Google Scholar]
- E32.Plicht B, Kaasch A, Kern WV. [Infective endocarditis] Dtsch Med Wochenschr. 2011;136:2470–2473. doi: 10.1055/s-0031-1297269. [DOI] [PubMed] [Google Scholar]
- E33.Hasenfuß G, Gamstätter T, Jung N, et al. Klug entscheiden: No-Gos bei Medikamentenkombis. Dtsch Arztebl. 2021;118 A-630. [Google Scholar]
- E34.Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- E35.Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]