Abstract

Lithium-ion batteries (LIBs) are accounted as promising power tools, applicable in a wide range of energy-based equipment, from portable devices to electric vehicles. Meanwhile, approaching a cost-effective, environmentally friendly, and safe LIB array has remained sluggish yet. In this regard, cellulose, as a nontoxic natural renewable polymer, has provided a stable and cohesive electrode structure with excellent mechanical stability and reduced electrode cracking or delamination during cycling. Additionally, the porous configuration of the cellulose allows for efficient and faster ion transport as a separator component. Miniaturizing cellulose and its derivatives have revealed more fabulous characteristics for the anode, cathode, and separator resulting from the increased surface-to-volume ratio and superior porosity, as well as their thin and lightweight architectures. The focal point of this review outlines the challenges relating to the extraction and electrospinning of cellulose-based nanofibers. Additionally, the efforts to employ these membranes as the LIBs’ components are elucidated. Correspondingly, despite the great performance of cellulose-based LIB structures, a research gap is sensed in this era, possibly due to the difficulties in processing the electrospun cellulose fibers. Hence, this review can provide a source of recent advancements and innovations in cellulose-based electrospun LIBs for researchers who aim to develop versatile battery structures using green materials, worthwhile, and eco-friendly processing techniques.
1. Introduction
Energy consumption has been turned into a major critical concern in various health, environment, and economic fields. According to the released data by the International Energy Agency, it is estimated that worldwide energy consumption will gradually increase by 25% from 2016 to 2040. Energy storage devices have been introduced as key equipment to reduce fossil fuels.1 Nowadays, the lithium secondary battery (LIB) development has been considered by numerous researchers and industries to progress the usage of a wide range of devices from portable electric equipment to hybrid/electric vehicles. Accordingly, the focus on the fabrication of optimized battery structures via maximizing energy density, life cycles, and safety has received wide attention in recent decades. Besides the significance of the electrochemical performance and other physical and chemical features, it is vital to apply environmentally friendly materials and fabrication processes. Additionally, the recycling ability of the final battery structure and the scalability of the synthesis procedure are important parameters that should be attained in the battery industry.2−4
Li+ ion batteries could be prepared by attaching one or more electrochemical cells depending on the required energy density. Each cell consists of an anode, a cathode, and a separator membrane immersed in a liquid electrolyte. During the charging cycle, Li+ ions migrate to the anode side via the separator, interacting with the electrons that have passed the external circuit. Meanwhile, the reverse mechanism occurs during the discharging procedure to fill the cathode reservoir with the Li+ ions. In the commercial stage, graphite and LiCoO2 electrodes are, respectively, employed as anode and cathode structures.5,6 In addition, polypropylene or polyethylene fibrous membranes are applied as the separator element (see Figure 1a). Therefore, polymeric materials act as one of the main substances in the battery structures. Despite the fact that batteries are applied as an environmentally friendly electrochemical device, the polymeric-based components in such structures are mostly synthesized from petroleum. The application of these substances causes biohazards and global warming, motivating researchers to utilize sustainable materials. In this era, cellulose and its derivatives, as water-processable polymers, have been reported as progressing green battery components called paper batteries.7−9 In accordance with the cellulose chemical structure, the β-d-glucopyranose linear repeating unit is linked through β-1,4 glycosidic bonds covalently. Additionally, there are a large number of hydrogen bonds in intra- and intermolecular structures of cellulose, forming various cellulose structures. Flexible surface chemistry, low dimensional changes, as well as high elasticity and anisotropy are well-known cellulose characteristics, leading to the progression and development of energy application devices.10 Cellulose-based materials have shown great potential for fabricating stable and cohesive electrode configurations with boosted mechanical characteristics. They can suppress electrode cracking and delamination during cycling procedures. Cellulose-based separators could also provide versatile and faster ion transport between the electrodes.11
Figure 1.
(a) Structure of commercialized LIBs from the perspective of the applied materials and their challenges. Reproduced with permission from ref (18). Copyright 2020 Elsevier. (b) Schematic illustration of electrospinning apparatus. Reproduced with permission from ref (19). Copyright 2021 Elsevier. (c) VOSviewer bibliometric map of LIBs based on electrospun cellulosic components, and (d) number of Scopus indexed published papers with the keywords of nanostructures, electrospun nanofibers, and electrospun cellulose in LIBs.
The architecture of the battery structures acts as an influential parameter on the final characteristics of the fabricated batteries. On the basis of the literature, the synthesis of the materials on the nanoscale can enhance the battery performance. Downsizing the materials causes an increment in the surface-to-volume ratio, improving the ideal properties in each LIB component.12 Nanostructures can be synthesized through various forms, in which nanofibrous membranes have received tremendous attention due to their highly porous and interconnected structures, as well as the presence of tiny pores. The nanofibrous networks could be produced via a diverse range of methods, including dry spinning, solution blow spinning, hybrid dry-jet wet spinning, electrospinning, and so forth. Between the mentioned fabrication procedures, electrospinning has the capability to produce homogeneous cellulose-based fibers with customized features, which is schematically shown in Figure 1b.13−17 The visualizing scientific landscape of the electrospun nanofibers applicable in LIB structures is depicted in Figure 1c, showing their potential for fabricating various battery components. According to the Scopus resource data, the published research on the nanostructure battery elements has dramatically risen from 2013 to 2022. In addition, 1098 attempts have been conducted on the nanofibrous LIB components in this period. It is noteworthy that among these studies, 7% of the research cases have concentrated on applying the electrospun cellulose or its derivatives in the LIBs (Figure 1d).
Although there are few studies on the usage of electrospun cellulose and its derivatives in LIBs, an increasing trend has been observed, confirming the growing attention to the application of green materials. Regarding the beneficial features of the cellulose-based materials, along with their nanofibrous configurations, the following sections are overviewed in this paper to shed light on green and eco-friendly battery preparation in the near future: extracting and electrospinning of the cellulose and its derivatives and the efforts toward synthesizing electrospun cellulose-based anode, cathode, and separator.
2. Cellulose Resources and Derivatives
The investigation of renewable resources originating from biopolymer-based materials is a dynamic field of study that has caught more attention in industries and scientific research. Cellulose is an organic polysaccharide, which could be employed as an environmentally friendly and biocompatible alternative to replacing the oil-based product.20,21 According to its merits, cellulose is known as a promising candidate for the fabrication of LIB elements. As an example, it could be applied as a binder material in the electrode fabrication process. It helps hold the active materials and conductive additives together, forming a stable and cohesive electrode structure. Additionally, it can act as a film-forming material, ensuring uniform and smooth coatings on the electrode surface. Also, excellent mechanical properties of cellulose, such as high tensile strength and flexibility can enhance the mechanical stability and integrity of the electrode assembly, reducing the chances of electrode cracking or delamination during cycling. This improved mechanical stability contributes to the long-term performance and cycle life of the battery. Moreover, cellulose is a porous material with a high surface area, which allows for efficient ion transport within the battery. It is capable of integrating the diffusion of lithium ions between the electrolyte and the electrode, promoting faster charge and discharge rates. It also has the ability to retain and hold the electrolyte, preventing its loss and improving the overall electrolyte utilization in the battery. Furthermore, it is a naturally derived and nontoxic material, making it a safer choice for battery applications. It can help mitigate safety concerns associated with the use of conventional synthetic binders or additives. The thermal stability of cellulose can contribute to the overall thermal management of the battery, reducing the risk of thermal runaway or fire hazards. Despite remarkable features, some intrinsic characteristics, such as insolubility in most common organic solvents and hydrophilic nature, have restricted its applications.22 In this regard, several attempts have been conducted to modify cellulose structure via enzymatic or microbiological methods.23−25
Cellulose can be extracted from wood-based and nonwood-based resources. Wood has been considered the most widespread cellulose basis for industries.26 However, lately, other nonwood resources, including agricultural waste, fibers (cotton, flax, jute, etc.), marine animals, algae, and bacteria, are getting more attention.27−29 Agricultural waste is one of the nonwood-based sources that hold more than 85% of cellulose, hemicellulose, and lignin.30 This massive inexpensive waste can be used to prepare valuable products; for instance, about 100 million dry tons of dry corn stover can be collected annually in the United States.31 Xu et al.31 extracted cellulose from agricultural waste corn stover and reported more than 93% purity. In another attempt, Nang An et al.32 investigated cellulose extraction from Vietnamese agricultural wastes, including coconut husk fibers, rice husk, and Nypa fruticans trunks. In accordance with this study, highly crystalline extracted cellulose with complete lignin removal and improved thermal stability was obtained that could be applied in several potential applications.
Different forms of cellulose have been extracted from various sources so far, including microcrystalline cellulose (MCC),33 microfibrillated cellulose (MFC),34 cellulose nanofibers,35 cellulose nanocrystals (CNC),36 and bacterial cellulose (BC).37 Nevertheless, interest in nanocellulose materials (CNF, CNC, and BC) has recently increased dramatically due to their special chemical and physical properties. Among them, cellulose nanofibers and CNC can be obtained using top-down approaches, while BC is synthesized by a bottom-up process.
Cellulose nanofiber possesses high mechanical strength, thermal stability, functionalization possibility, and small thermal expansion.38 Correspondingly, it has been regarded as a latent matrix for numerous applications, such as drug delivery, energy storage, optoelectronic conversion, filtration, and many more.39−41 In accordance with the literature, cellulose nanofiber could be obtained through chemical, mechanical, oxidation, and biological methods. The chemical approach is based on acid digestion in which cellulose nanofibers are obtained by destroying amorphous areas of fibers.42 In the case of the mechanical way, a high rpm mill is used to grind wood pulps, and a high-intensity ultrasonic step helps to isolate nanofibers.43 Using an oxidizing agent, such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), leads to better defibrillation through the mechanical method and, therefore, higher profit for cellulose nanofibers.44 In the case of the biological procedure, a cellulolytic enzyme is applied to break the fiber structure. Meanwhile, it would be better to couple it with other methods, i.e., chemical or mechanical, due to its time-consuming process.38 After extraction of cellulose nanofibers through different approaches, a high-temperature acid treatment could result in the production of CNC. Magnificent physicochemical properties and high surface area make CNC a supreme candidate for vast applications of water purification, membrane, nanocomposite, electronic, and biomedical.45
BC is special, highly crystalline, and pure natural cellulose without lignin and hemicellulose, produced by some microorganisms like Acetobacter xylinum.46 It is assumed that the bacteria produce cellulose as a defensive mechanism to protect themselves from UV and severe chemical conditions.47 BC properties can be controlled in situ by using production conditions. It is widely accepted as a functional material for different applications, and its chemical structure is quite similar to plant cellulose.47
The cellulose chemical structure can be modified, providing tuned functionality for the desired application. Cellulose hydroxyl groups can be used for different degrees of substitution to obtain cellulose derivatives.48,49 These cellulose derivatives are mainly divided into cellulose esters and cellulose ethers. Cellulose acetate, xanthate, sulfate, phosphate, and phthalate are the most common cellulose-ester derivatives, while methyl, ethyl, carboxymethyl, hydroxyethyl, hydroxypropyl, sulfonyl, and cyanoethyl cellulose are the most known cellulose-ether derivatives.50 Among cellulose esters, cellulose acetate (CA) is the most popular. It is synthesized by adding acetic anhydride, glacial acetic acid, and sulfuric acid to cellulose. In contrast, methylcellulose (MC) is the most commercially related cellulose-ether.51 MC is synthesized by the substitution of hydroxyl groups with methoxy groups. Although MC is water-soluble, it is insoluble in most organic solvents.50Figure 2a–c shows the chemical formula of the native cellulose and its derivatives.
Figure 2.
General information regarding cellulose and its derivatives. (a) Various cellulose resources and (b) chemical structure of cellulose and its derivatives, including cellulose xanthate (CX), carboxymethyl cellulose (CMC), methylcellulose (MC, R could be H or CH3), cellulose acetate (CA), cellulose carbamate (CC, R could be H or CONH2), and hydroxyethyl cellulose (HEC), (c) hierarchical structure of the cellulose from the meter to the nanometer scale. Reproduced with permission from ref (52). Copyright 2020 MDPI. (d) Solid loading, as well as fiber diameters of the electrospun cellulose and its derivatives. Reproduced with permission from ref (19). Copyright 2021 Elsevier.
In recent decades, remarkable progress has been observed in battery performance through using nanoscience. Fabricating nanostructural battery elements have offered unique properties due to their small size, high surface area, and improved electrochemical reactivity. Nanostructured materials, such as nanowires, nanoparticles, or nanotubes, can be used as active materials in both the positive (cathode) and negative (anode) electrodes. These nanostructured electrodes provide shorter diffusion paths for lithium ions, facilitating faster charging and discharging rates. The solid electrolyte interface (SEI) layer forms on the electrode surface as a result of electrolyte decomposition during battery cycling. By incorporating nanostructured materials, such as nanocomposites or nanocoatings, the SEI layer can be engineered to be more stable and protective, reducing side reactions and improving battery cycle life. Additionally, nanostructured current collectors, such as nanoporous metals or nanofiber-based networks, can enhance the overall electrode performance by improving electron transport and reducing resistance.
Among various methods of downsizing polymers, electrospinning is a technique used to produce nanofibers by applying an electric field to a polymer solution or melt. Electrospun nanofibers can be used to fabricate separator membranes with enhanced properties for Li-ion batteries. These nanofiber-based separators offer improved mechanical strength, thermal stability, and electrolyte absorption, contributing to enhanced battery safety and performance. Electrospinning can also be employed to create three-dimensional (3D) electrode architectures by depositing active materials onto conductive nanofiber networks. These 3D electrode structures provide high surface area, efficient ion transport, and improved electrode–electrolyte contact, resulting in enhanced energy storage capabilities. Electrospinning enables the fabrication of flexible and stretchable battery components, such as electrodes and separators. These flexible Li-ion batteries are promising for wearable electronics, smart textiles, and other applications requiring conformable power sources. The next section represents the electrospinning of cellulose and its derivatives as a robust technology to produce cellulose-based nanomaterials.
3. Cellulose Electrospinning Methods and Challenges
Cellulose nanofibers could be synthesized through various procedures, including mechanical, chemical, combined, and electrospinning. In the mechanical methodology class, cellulose nanofibers can be fabricated via high-pressure homogenization or grinding technologies. Additionally, acid hydrolysis and enzymatic hydrolysis are the potential chemical solutions for generating cellulose nanostructures.53Table 1 provides the method used in each procedure, along with the reported advantages and disadvantages. Accordingly, the electrospinning procedure ensures the fabrication of more uniform fibers with less damage through a feasible procedure.54
Table 1. Summarization of Various Processes Employed to Fabricate Cellulose Nanofibers.
| method | performance | advantages | disadvantages |
|---|---|---|---|
| high-pressure homogenization mechanical method | cellulose fibers are subjected to high-pressure treatment through a narrow gap or nozzle; the high shear forces and cavitation phenomena break down the cellulose fibers into nanofibers | high shear forces; simple process; scalability; and preservation of fiber structure | energy-intensive; limited control over fiber length; potential fiber damage; and pressure limitations |
| grinding/disintegration mechanical method | cellulose fibers are mechanically treated by methods such as grinding, microfluidization, or cryocrushing; these techniques rupture the fiber structure, resulting in the production of CNFs | simple and affordable; size control; preservation of crystalline structure; and high aspect ratio | equipment limitation; fiber damage; broad fiber length distribution; and energy consumption |
| acid hydrolysis chemical method | cellulose fibers are treated with strong acids, such as sulfuric acid or hydrochloric acid, for a specific period; acid hydrolysis selectively removes amorphous cellulose regions, leading to the production of nanofibers | high yield; control over fiber size; high aspect ratio; and tunable properties | environmental impact; energy and time-consuming; potential loss of crystallinity; and batch-to-batch variation |
| enzymatic hydrolysis chemical method | enzymes like cellulase or hemicellulase are utilized to break down cellulose fibers into nanoscale fibrils; this method is more environmentally friendly compared to acid hydrolysis but typically demands longer reaction times | environmentally friendly; selectivity; mild reaction condition; and reduced fiber damage | longer reaction time; cost and enzyme availability; batch-to-batch variation; and substrate compatibility |
| TEMPO-mediated oxidation combined process | cellulose pulp is modified with a 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) and then mechanically processed; the TEMPO oxidation facilitates the disintegration of cellulose fibers into individual nanofibers during mechanical treatment | selective oxidation; high yield and efficiency; enhanced surface charge; and scalability | additional processing step; safety regulations; and potential fiber damage |
| electrospinning | cellulose nanofibers can also be produced through electrospinning, where a cellulose solution is electrostatically spun to form ultrafine fibers; the fibers can then be collected on a collector to obtain cellulose nanofibers | high aspect ratio; meso- to nanoscale control; tunable porosity and structure; and large-scale production | solution preparation and stability and process sensitivity |
Electrospinning has been established as a highly versatile technique for the fabrication of submicron fibers. Various solvent solutions and melt forms of different polymers have been successfully applied to prepare related nonwoven mats. During the electrospinning procedure, a high voltage is applied to charge the polymer droplet at the tip of the needle. Upon overcoming the surface tension, a fine polymer jet flies from the needle toward the grounded collector, and solvent evaporation takes place through this distance.55 Besides many influential parameters affecting this technique, the volatility of the chosen solvent is a key factor. In other words, a highly volatile solvent could lead to quick solvent evaporation and therefore clog the needle. In contrast, a solvent with low volatility results in sticking the fabricated fibers together on the collector and forming a uniform film.56 Besides, it has been proved that the morphology of the resulting fibers is governed by a set of different variables, such as solution composition, process settings, and ambient conditions.57
Similar to various synthetic and natural-based polymers, cellulose-based electrospun nanofibers have also been introduced in various studies for a wide range of applications, including water treatment,58 biomaterials,59 sensors,60 and electro-conductive material,61 batteries, and many more. Although cellulose is insoluble in the most common solvents, the electrospinning of cellulose using some recently developed solvents like N-methylmorpholine-N-oxide (NMMO), lithium chloride (LiCl) salt/dimethylacetamide (DMAc), and ionic liquids (ILs) have been performed so far.62 It is worth noting that direct electrospinning of cellulose has always been challenging. Electrospinning of more readily cellulose derivatives and subsequent conversion to cellulose is another indirect route to fabricate cellulose electrospun fibers.56 Various attempts have been made so far to fabricate the cellulose through the electrospinning procedure, as a raw material or as derivatives, such as acetate, carboxymethyl cellulose, hydroxypropyl cellulose, methylcellulose, and hydroxypropyl methylcellulose. The polymer concentration and the final fiber diameter obtained through the electrospinning of cellulose and the mentioned derivatives are shown in Figure 2d. As is apparent, the polymer concentration was varied in the range from 0.1 to 55 wt %, leading to the formation of fibers with diameters from nano- to microscales.19
3.1. Cellulose Electrospun Nanofibers via a Direct Method
NMMO is a nonvolatile solvent with the ability to dissolve cellulose by breaking the intermolecular interaction between cellulose chains. Direct electrospinning of cellulose using NMMO/water tertiary system is inspired by industrial Layocell production. However, the remaining NMMO within the fibers on the grounded collector changes the fibrous form to a uniform film.56 Intending to address this issue, Kim et al.63 applied shielded heating units to maintain the temperature of the syringe and needle at 70–110 °C. Figure 3a depicts the schematic illustration of the employed procedure. Moreover, a commercial three-dimensional cellulose filter was applied to the collector to adsorb the excess solvent from as-spun fibers, and also a coagulation bath of water with a controlled temperature of 10 °C was subjected right after the rotating collector. The results demonstrated that the applied setup could successfully maintain the fibrous structure of collected fibers and cellulose electrospun nanofibers with a diameter of 250–750 nm and approximate crystallinity of 40–60% (Figure 3b,c). The authors claimed that increasing the spinneret temperature decreases the fibers’ diameter by reducing the spinning dope viscosity. Besides, the cooling coagulation bath leads to retaining the crystallinity of cellulose.
Figure 3.
Fabrication of cellulose nanofibers through a direct method. (a) Schematic illustration of the electrospinning device, (b) SEM image of cellulose nanofiber at room temperature, and (c) SEM image of cellulose nanofiber at a heated condition. Reproduced with permission from ref (63). Copyright 2006 Elsevier. Production of cellulose nanofibers via an indirect method. (d) Electrospun system used for indirect cellulose nanofiber synthesis and (e) SEM image of tree-like cellulose nanofiber. Reproduced with permission from ref (64). Copyright 2018 Elsevier.
Another well-known solvent system for direct cellulose electrospinning is dissolving a nonvolatile lithium chloride (LiCl) salt in dimethylacetamide (DMAc). This solvent system provides sufficient volatility for the electrospinning process. Nevertheless, a post-treatment is necessary to remove LiCl salt from the electrospun fibers. It is reported that the presence of LiCl in the solvent system is essential to dissolving cellulose by bridging the electrostatic interaction between polymer chains and DMAc.65 Kim et al.63 compared the cellulose electrospun nanofibers from NMMO/water and LiCl/DMAc solvent systems. They reported that applying a coagulation bath after the collector is required to stabilize fibers’ form in both solvent systems. The X-ray diffraction studies indicated that cellulose fibers prepared using LiCl/DMAc are mostly amorphous.
ILs are organic salts containing cations and anions in liquid form at low temperatures. Having unique properties of nonvolatility, nonflammability, and chemical/thermal stability, ILs have been widely used as green solvents.22 However, there are still some concerns about ionic liquids like recyclability, purification, and high cost.19 ILs may be combined with some organic or inorganic compounds such as tetrabutylphosphonium chloride and sodium chloride.19 Studies have demonstrated that some hydrophilic ILs can be used to dissolve cellulose to fabricate electrospun fibers.66 1,3-Diallyl-2-ethyl imidazolium acetate, 1-allyl-3-methylimidazolium chloride, 1-butyl-2,3-dimethylimidazolium chloride, and 1-butyl-3-methylimidazolium chloride are some conventional ILs that have been investigated for cellulose dissolution.67−69 However, as ILs are low-volatile melted salts, using a coagulation bath to collect the fibers as well as remove IL is necessary. The applied voltage for electrospinning of ILs-based solvents is higher than conventional electrospinning, so it ranges between 15 and 35 kV.19 Viswanathan et al.70 fabricated cellulose and cellulose-heparin composite electrospun fibers using 1-butyl-3-methylimidazolium chloride as IL solvent. The concentration of the prepared solution and applied voltage were reported as 10% (w/w) and 15 kV, respectively. This research group utilized an ethanol bath to collect and consequently extract IL from the fibers. The diameter of the resulting fibers was reported in the range of microns to nanometers.
3.2. Cellulose Electrospun Nanofibers via the Indirect Method
Unlike the poor solubility of cellulose, its derivatives possess significantly improved solubility in some conventional organic and inorganic solvents. Cellulose acetate (CA) is one of the best starting materials to achieve cellulose nanofibers through electrospinning. Acetic acid, acetone/DMAc, and acetone/DMF/water are some solvent systems that have been applied in several studies.71−73 It is reported that using a binary and the ternary solvent system can help to control the evaporation rate in the air gap.73 The resulting CA nanofibers can be easily converted to cellulose by a deacetylation reaction. Zhang et al.64 fabricated CA nanofibers using acetic acid as a solvent. The deacetylation process was performed by 0.5 M NaOH/ethanol at 25 °C for 24 h, and the resulting cellulose nanofibers were characterized. Figure 3d,e illustrates the schematic of the employed setup for the fabrication of CA-based nanofibers and the SEM image of the prepared nanofibers, respectively. They investigated the morphological structure and air filtration performance and reported more than 98% efficiency for both CA and cellulose nanofibers. To reduce the deacetylation process time, Ahmed et al.74 applied ultrasonic energy. They dissolved CA in acetone/DMF and prepared CA nanofibers by the electrospinning method. Through the deacetylation step, nanofibers were treated with NaOH/ethanol solution under ultrasonic energy for different periods. Their result demonstrated complete deacetylation within 1 h, and no morphological damage was detected.
Other cellulose derivatives are also soluble in suitable solvents for electrospinning, and several studies have reported the fabrication of electrospun nanofibers of these materials for different applications. Silva et al.75 explored the electrospinning of hydroxypropyl methylcellulose (HPMC) with different molecular weights. They used ethanol/water as the solvent system and studied the rule of solution parameters on fiber production. They concluded that low-molecular-weight HPMC results in rodlike and round particles, while high-molecular-weight HPMC causes bead-free fibers at a concentration of 1.5% (w/v) and beaded-fibers at 1% (w/v). Wu et al.76 investigated the effect of the THF/DMAc solvent system of ethyl cellulose on the resulting electrospun nanofibers morphology. The authors claimed that using a multicomponent solvent system leads to a narrower diameter distribution of fibers compared to a single-component solvent. Moreover, the small tubercle fibers’ surface was evident when the multicomponent solvent system was applied.
4. Electrospun Cellulose-Based Anode Structures
An anode is a polarized electrical device in the battery structure that directly influences the battery capacity and performance via its morphology and the employed content since they are responsible for storing and releasing Li+ ions during the charging and discharging procedure.77 A variety of synthetic and natural-based materials have been introduced as anode materials so far. Among a wide range of substances, alloying, silicon, and carbon-based materials have received large attention. The choice of anode material is crucial in determining the performance and characteristics of LIBs. In most commercialized LIBs, graphite has been applied as an anode for years, while low capacity, as well as poor safety, have motivated researchers and manufacturers to substitute it with alloying- and silicone-based structures. This category of anode electrodes is normally synthesized based on aluminum, silver, magnesium, tin, and antimony elements, providing 2 to 10 times superior capacity compared with the common structures. In contrast, volume expansion during lithium intercalation causes capacity loss in the first cycle and irreversible capacity fading during subsequent cycling procedures. Additionally, researchers have focused on silicon-based anodes to overcome the mentioned obstacles through providing superior gravimetric and volumetric capacity and low cost. Meanwhile, the high-volume fluctuations cause several critical issues, including unsteady and irregular electrical delamination, ineffective electron transfer, particle cracking, and many more. As a result, the LIB’s development has significantly become restricted using these anode materials.78−80
Carbon-based substances are currently applied in most commercialized battery structures, resulting from their outstanding electronic conductivity, affordable cost, and widespread availability. In addition, carbon structures offer a desirable hierarchical configuration for the insertion of Li+ ions during the charging procedure. Nevertheless, carbon structures have also revealed similar challenges to the graphite anode, suggesting further evaluations in this era. The formation of dendrite structures during cycling on the carbon-based structures is the main issue dealing with these materials, hindering their practical usage. Carbonaceous-based anodes can be evaluated from two points of view: (a) morphology and structure and (b) the initial resource material.81,82 On the basis of the literature, the microstructure, morphology, and crystal regions of the carbonaceous materials affect the litigation/delithiation process and the final battery capacity. Accordingly, recent efforts have been dedicated to the fabrication of carbon-based anodes in the form of nanoscale arrays, which can be synthesized into zero-, one-, two-, and three-dimensional nanostructures. Downsizing of the materials into the nanoscale causes approaching the electrocatalytic ability increment, shorter diffusion distances, higher range of electroactive sites, fast charging, and longer life span. One-dimensional carbon structures, known as carbon nanofibers (CNFs), result in a higher capability for storing and energy conversion compared with other nanoscale configurations and the standard graphite-based anodes due to their interconnected structures. In addition, they can transport Li+ ions via short radial directions, improving the capability rate and efficiency.83−85
CNFs could be prepared from synthetic and natural polymer resources. Polyvinylpyrrolidone (PVP), polyacrylonitrile (PAN), and poly(vinyl alcohol) (PVA) are common synthetic sources, while cellulose and lignin are well-known natural ones. The comparative investigations on the anode efficiencies according to their precursors have represented cellulose as one of the most efficient resources because of its outstanding structural and textural features. Cellulose-based CNFs are capable of providing a high charging capacity (535 mA h–1 g–1). In addition, they attest to the green and cost-effective production of anode materials from abundant and renewable resources. Moreover, cellulose-based materials could be synthesized in different forms, resulting from their great designability and versatility.86 Furthermore, these materials have shown high surface area, proper electrical conductivity, enhanced mechanical stability, unique flexibility, and compatibility with lithium.86−88
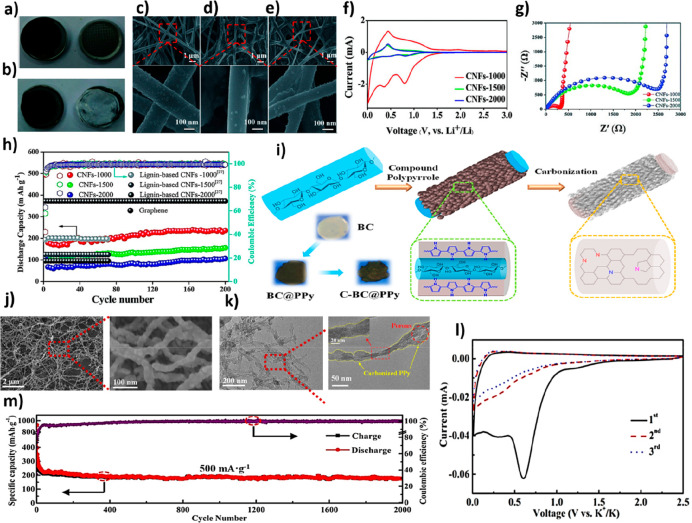
Cellulose-based carbon materials could be carbonized without a need for a catalytic supporter, under an inert atmosphere and at high temperatures (above 2000 °C) to gain an amorphous structure. They also lead to the formation of nongraphitized and disordered materials and cause high specific capacity due to the high surface area. On the other hand, global warming has motivated researchers to concentrate on deriving the precursor materials from green and environmentally friendly sources. Since the 1990s, biomass awareness has become the modern chemistry basis, promising nonharmful features of chemical procedures and products in a global vision, containing technological, scientific, and ethical concerns. In 2018, Tao et al.89 liquefied walnut shells at 150 °C for 2 h using phenol, followed by a resinification stage for the mixture of the liquefied walnut shell, sodium hydroxide, and formaldehyde. Then, they added PVA solution with a weight concentration of 12% to provide a proper electrospinning solution. The prepared electrospun fibers were then carbonized at three different temperatures of 1000, 1500, and 2000 °C under an argon condition, named CNFs-1000, CNFs-1500, and CNFs-2000. Figure 4a,b shows images of the carbonized CNFs-1000 before and after the cycling procedure. In addition, FESEM images of the various prepared fibers are depicted in Figure 4c–e. On the basis of the results, an increase in the temperature caused a reduction in the diameter of CNFs from 170 to 110 nm, possibly due to the release of more molecules at higher temperatures. Also, a slight difference was observed between the fiber diameters before and after the calcination procedure, corroborating a superior yield of carbon compared with other biomass-based precursors. In addition, the homogeneity and geometry of the electrospun fibers remained unaltered after 200 cycles. Figure 4f also depicts the CV curves of the obtained fibers. On the basis of the obtained plot, the redox peaks at 0.5 V were attributed to the insertion of the Li+ ions into the nanopores of the fabricated fibers. Among the prepared electrospun fibers, the CNFs-1000 fibers exhibited the lowest charge transfer resistance, possibly due to the desirable pore volumes and more graphitelike crystallite layers (Figure 4g). In various current densities from 30 to 2000 mA g–1, the CNFs-1000 anode structure represented the highest electrochemical performance with stable cycling after returning to the 30 mA g–1 (Figure 4h). Moreover, these fibers presented a stable discharge capacity of 239.6 mAh g–1 after the 200-cycling process, while other structures only maintained 122 and 99 mAh g–1 due to the same reason discussed above. It is worth noting that the decrease in capacity during the first ten cycles could be assigned to the SEI formation. Meanwhile, the capacity improvement after the 10th cycle could be related to the creation of a gelatinous layer on the CNFs, providing additional capacity.
Figure 4.
Characteristics of the walnut shell-based anode materials. Images of the CNFs-1000 (a) before and (b) after 3 cycles and (c–e) FESEM images of the prepared fibers at different carbonization temperatures after 200 galvanostatic charge–discharge cycling procedures. Electrochemical performance of the walnut-derived CNFs. (f) CV curves at a scan rate of 0.5 mV s–1, (g) impedance spectra, (h) cyclic performance, and Columbic efficiency at 100 mA g–1 current rate. Reproduced with permission from ref (89). Copyright 2018 Royal Society of Chemistry. Properties of the cellulose nanofibers embedded with polypyrrole. (i) Schematic illustration of the synthesis procedure, (j) SEM, (k) TEM, (l) CV curves, and (m) specific capacity over 2000 cycles at a current density of 500 mA g–1. Reproduced from ref (90). Copyright 2023 American Chemical Society.
In 2023, Liu et al.90 developed a bacterial cellulose-based CNF applicable as an anode structure of potassium ion batteries. To this end, polypyrrole was added to the bacterial cellulose, followed by a carbonization procedure, which is schematically described in Figure 4i. SEM and TEM images of the deployed structure are shown in Figure 4j,k, confirming the formation of a 3D network configuration with a diameter ranging from 20 to 40 nm. CV curves of the provided architecture are displayed in Figure 4l, confirming the insertion of potassium by the appearance of a peak in 0.01 V, as well as the potassium extraction via the peak at 0.25 V. Cycle stability of the highlighted structure at a high current density of 500 mA g–1 in 2000 cycles was also investigated (see Figure 4m). Accordingly, the reversible specific capacity of 176 mAh g–1 was observed due to the synergetic effect achieved by the composition of bacterial cellulose and polypyrrole, as well as N-doping sites of the polypyrrole content.
Two main strategies have also been applied in several attempts to boost the electrochemical performance in carbon-based anodes, including the fabrication of porous structures and the development of composite structures through combining carbon materials with alloy- and metal-type substances.91 To figure out the effect of porosity on the resulting performance, Wang et al.8 employed KOH activation to produce porous carbon nanofibers from bacterial cellulose precursors. The fabricated structure could deliver a high discharge capacity of 857.6 mAh g–1 after 100 cycles and retain the capacity of about 325 mAh g–1 after 4000 cycles. On the other hand, conjoining the cellulose-based anode nanofibers with metal-based components has also been declared an influential method. For example, Li and Huang92 deposited silver nanoparticles and titania on the carbon nanofibers derived from the cellulose with varied concentrations of 0.5, 1.0, 1.5, and 2 M silver nitrate solutions to obtain samples A, B, C, and D respectively. Figure 5a schematically shows the fabrication procedure employed for the production of the anode structures. Correspondingly, sample Ag-NP/titania/carbon-D illustrated a high specific capacity of 540 mAh g–1 in the initial charge/discharge cycle (Figure 5b). In addition, this sample showed a superior rate performance in comparison with the other designed anode structures, due to the highest loading density (Figure 5c). The obtained results clearly showed the effect of the silver nanoparticles on the enhancement of the conductivity, superior specific surface area, and more feasible electron transportation. Similar attempts have also been reported to benefit the advantages of both cellulose-based carbon nanofibers and Fe3O4,93,94 SnO2,95 Sn,96 Si,97,98 and so on. Wang and Huang99 developed a ternary nanocomposite based on carbon nanofibers loaded by Ag and SnO2 nanoparticles, which is shown in Figure 5d. The galvanostatic discharge/charge cycling performance of the designed anode structure at the current density of 100 mA g–1 is provided in Figure 5e. On the basis of the results, loading the highest amount of Ag nanoparticles caused approaching the highest capacity retention of 700 mAg h–1 after 120 cycles. Additionally, this sample exhibited superior specific capacities at various current rates, resulting from the improved cycling stability and electrochemical performance provided by the presence of carbon structure and Ag nanoparticles, respectively (see Figure 5f). Notably, the nanofibrous appearance after the cycling procedure was similar to the structure before cycling, showing the great structural durability benefitting from the presence of Ag nanoparticles, which could reinforce the mechanical strength and inhibit the agglomeration of SnO2 nanocrystals. A similar trend was also carried out by this group through substituting Cu nanoparticles with Ag ones.100
Figure 5.
Properties of the Ag-NP/titania/cellulose-based carbon anode structure. (a) Schematic illustration of the method used for the fabrication, (b) cycling performance of the prepared samples at a current density of 100 mA g–1, and (c) charge–discharge profile of the samples at different current rates. Reproduced with permission from ref (92). Copyright 2015 Royal Society of Chemistry. Characterization of CNF loaded by SnO2 and Ag nanoparticles. (e) specific capacity at 100 mA g–1 over 120 cycles, and (f) specific capacity at various current densities. Reproduced with permission from ref (99). Copyright 2019 Elsevier. Features of the bacterial cellulose/Fe2O3/Fe3M/CNT anode structure: (g) synthesis process, (h) SEM (top) and TEM (bottom) illustrations, (i) CV curves, (j) voltage–time behavior, and (k) specific capacity. Reproduced with permission from ref (101). Copyright 2023 Elsevier.
In 2023, Ma et al.101 produced a composite, containing bacterial cellulose/Fe2O3/Fe3M/CNT for potassium and sodium ion batteries. The synthesis procedure is schematically illustrated in Figure 5g. SEM images of the prepared anode materials showed the creation of a jungle-like CNT network on the bacterial cellulose surface with the average diameter and length of 0.35 and 9 μm, respectively, which could enhance the electron transport and cyclability (see Figure 5h). The TEM illustrations displayed average thickness 6.4 nm of carbon on the nanoparticles in the composite and the lattice fringe formation of Fe nanoparticles (see Figure 5i). CV curves of the bacterial cellulose/Fe2O3/Fe3M/CNT in various voltage rates of 0.1 to 0.9 mV s–1 are provided in Figure 5j, showing an increment in the depotentiation peak by the rise in the voltage rate. The voltage versus time is also depicted in Figure 5k, attesting to the K+ ion diffusion coefficient as a result of rising in the nanotube carbon on the bacterial cellulose surface. The long-term cyclability at 400 mA h–1 of the designed composite is shown in Figure 5l, showing the 280.4 mAg h–1 specific capacity after 1000 cycles, which demonstrates the positive effect of the provided highly stable 3D network structure. With regard to similar mechanisms in the lithium, sodium, and potassium batteries, the proposed structure in the mentioned effort could also be deployed in Li+ ion batteries.
Besides cellulose resources, cellulose derivatives such as cellulose acetate, hydroxyethyl cellulose, methylcellulose, and carboxy methyl cellulose have also been employed as anode-based materials. In one research study, Han et al.102 engineered a carbon nanofiber structure derived from cellulose acetate doped with Fe3O4 nanoparticles. During the synthesis stages, CA/Fe(acac)3 was first electrospun, and then deacetylation and carbonization processes were conducted to produce a fine Fe3O4@CNFs structure. The prepared anode showed high discharge capacities of 773 and 596 mAh g–1 at 1 and 2 A g–1. Qiu et al.103 also prepared a nanofibrous composite anode membrane based on carboxy methyl cellulose, which demonstrated a discharge capacity of 226.4 mAh g–1 at the first cycle. Table 2 summarizes other findings obtained toward substituting the graphite with the cellulose- and cellulose-derivative-based electrospun fibers.
Table 2. CNF-Based Anode Structures Obtained from Cellulose and Its Derivatives.
| nanofibrous anode content | natural-based component | electrochemical performance | main results | ref |
|---|---|---|---|---|
| microwaved rGO/CNF (MrGO-CNF) | wood-derived cellulose | 176 mAh g–1 after 200 cycles at 600 mA g–1 | initial capacity of 558 mAh g–1 was obtained for the MrGO-CNF, while the commercial hard carbon revealed the first cycle capacity of 375 mAh g–1; in addition, the hard carbon faded down to 70 mAh g–1 after 100 cycles; however, MrGO-CNF exhibited a capacity of 360 mAh g–1 | Shi et al.104 |
| CNF/Fe3O4/Fe | bacterial cellulose | 472 mAh g–1 after 500 cycles at 1000 mA g–1 | high electrochemical stability of the designed anode could be linked with the synergetic effects of several parameters, including: (a) presence of nanopores, as well as proper bioactivity, thermal stability, and mechanical strength obtained from sulfuric acid-hydrolyzed CNC fibers, and (b) great adhering of Fe3O4/Fe nanoparticles to the calcinated fibers due to proper fibrous morphology | Zhang et al.105 |
| C-CNF/CNT/Li4Ti5O12 | wood-derived cellulose | 143 mAh g–1 after 1000 cycles at 0.5 C | LTO/C-CNF/CNT and LTO/CNF/CNT structures were obtained and compared in this attempt; accordingly, LTO/C-CNF/CNT represented higher capacity, cycle stability, and columbic efficiency due to better transportation behavior, higher electrical conductivity, and lower Li-ion resistance, as well as a faster lithium diffusion coefficient | Cao et al.106 |
| CNF/SnO2 | bacterial cellulose | 600 mAh g–1 after 100 cycles at 100 mA g–1 | obtained electrochemical performance could be linked with several beneficial factors, including (a) a large number of hierarchical pores in the structure, which are effective for Li+ ion storage, (b) high surface area which leads to adsorption of the Li+ ions, along with their fast transferring, (c) reduction of the transport length due to the formation of nanosized fibers, and (d) creation of a continuous pathway for electron transport by the fabrication of interconnected carbon network | Wang et al.8 |
| cCNF/Si | wood-derived cellulose | 808 mAh g–1 after 500 cycles at 2000 mA g–1 | compared with bare silicon anode, cCNF/Si exhibited an excellent rate performance, resulting from a broad interconnection between the Si nanoparticles and the carbon structure; this valuable point could improve the electric conductivity, easing the motion of Li+ ions; additionally, high electric contact area between Si nanoparticles and the carbon network allows the supply of the electrons; it also should be noted that the Si volume expansion was suppressed by the cCNF cover on the Si nanoparticles; more densely packed Si nanoparticles by the cCNF coating also could provide superior volumetric capacity for the cCNF/Si anode | Kim et al.97 |
| CNF/Si | waste rice straw-derived cellulose | 640 mAh g–1 after 100 cycles | it is shown that the CNF/Si material could lead to a higher cycle performance, compared with the pristine Si anode; this is linked with the detachment of active materials in the pristine Si, which is inhibited in the CNF/Si structure due to the provided hydrogen bonds; notably, a decay in the electrochemical performance was observed by excessing the CNF content, which could be attributed to the NCF agglomeration, creation of a nonconductive CNF layer on the Si anode, and interfering with the Li-ion transfer on the surface of silicon | Wu et al.107 |
| CNF/rGO | wood-derived cellulose | 340 mAh g–1 after 200 cycles at 100 mA g–1 | MrGO-CNF anode material exhibited superior electrochemical performance, compared with the commercial hard carbon in 100 cycles; initial capacity of 558 mAh g–1 was obtained for the employed anode, while the hard carbon showed a capacity of 375 mAh g–1 in the first cycle; after 100 cycles, the capacity of the designed anode and hard carbon dropped to 70 and 360 mAh g–1, respectively; capacity was kept approximately constant for up to 200 cycles in the modified anode structure; better performance observed could be referred to the higher electrical conductivity of this configuration | Shi et al.104 |
| CNF/SnO2 | bacterial cellulose | 600 mAh g–1 after 100 cycles at 100 mA g–1 | pyrolyzed bacterial cellulose embedded with SnO2 and Ge displayed a capacity of 600 and 500 mA g–1 with an efficiency of 98.5% after 100 cycles; developed hybrid materials represented superior specific capacity and good cyclic stability compared with pure carbon nanomaterials derived from bacterial cellulose, which could be a result of the structural advantages of dispersed metallic active nanoparticles in the prepared interconnected conductive CNFs; in fact, interconnected CNF structures facilitate the transportation of the electrons and lithium ions | Illa et al.108 |
| C-CNFs/Co9S8 | wood-derived cellulose | 700 mAh g–1 after 100 cycles at 500 mA g–1 | employed materials in the anode structure could enhance the electrochemical performance, as well as storing the Li ions, resulting from several synergizing effects, including preventing the Co9S8 agglomeration by the presence of CNFs, formation of smaller Co9S8 particles due to their growth limit by CNFs, and facilitation of the electron transfer by the CNF conductive skeleton | Guo et al.109 |
| CNF/rGO/p-Ti3C2Tx MXene | wood-derived cellulose | 280 mAh g–1 after 1000 cycles at 100 mA g–1 | better cyclability was obtained using this designed hybrid material because of increasing the number of active sites for the adsorption and storing of the ions by the presence of nanopores on the Ti3C2Tx sheets; also, the created TiO2 in the sonication step could effectively provide more channels for the insertion and extraction of the sodium ions at the interface of the Ti3C2Tx-TiO2 | Zhang et al.110 |
| CNF | wood- and crab-derived cellulose/chitosan | 399 mAh g–1 after 300 cycles at 30 mA g–1 | obtained data from this evaluation were compared with the data declared for the cellulosic materials extracted from walnut shells, sisal fiber, bean shells, rice straws, and olive stones; accordingly, the modified structure via chitosan bio-N-dopping could promote the electrochemical behavior of the prepared carbonaceous structure | Wang et al.111 |
In summary, a great need has been declared for the investigation of novel anode materials in recent years to overcome the low capacity of the common graphite anode. So, it has been widely suggested in various studies to apply to alloy-, silicon-, and carbon-based components. In most reported anode structures, poor conductivity has hindered the final practical usages, in which the addition of carbon materials could be a valuable method. Besides the inherent features of carbon-based materials, they could be easily extracted from green resources, such as cellulose and its derivatives, which provide a safer, cost-effective, and eco-friendly fabrication method.
5. Electrospun Cellulose-Based Fibers As Cathode Structures
Another crucial component in the LIBs is the cathode electrode. During the discharging procedure, Li+ ions migrate toward the cathode electrode and react with the electrons moved back from the anode material. In commercial LIBs, the capacity of the anode electrode is 1 order of magnitude larger than the cathode one. Accordingly, developing the LIBs has been restricted, resulting in the capacity mismatch between the employed electrodes. Toward approaching an ideal and high-capacity battery configuration, there should be a large difference between the energy levels of the negative and positive electrodes. Therefore, the cathode energy is desirable to be as low as possible, implying the need for cathode arrays with higher oxidation state stabilization. In contrast, the anode energy should lie as high as possible. On the basis of the theory mentioned, various efforts have been devoted to designing a cathode structure with higher specific capacity and rate capability. In this era, researchers have focused on the modification of morphology and microstructure of the applied materials as well as their electrochemical features.112
Cathode structures are mainly categorized into two classes based on their energy-storing mechanisms, including intercalation- and conversion types. In the intercalation-based cathodes, the applied structure acts as a host for reversible insertion and extraction of the Li+ ions during cycling procedures. The intercalation cathodes can also be classified into three groups transition metal oxides (TMs), chalcogenides, and polyanion compounds, in which TMs have received more attention due to superior energy storage capacity along with an outstanding operating voltage. Meanwhile, the cathode conversion type experiences breaking of the chemical bonds during the charging cycle and so recombining in the discharging process. Metal halides are well-known conversion-based cathodes, suffering from poor electron conductivity as well as high volume expansion which has restricted their practical usage. In the case of TM structures, LiCoO2 and LiNiCoMnO2 have been widely applied in the commercial market. Nevertheless, the Co element is expensive and owns limited resources, motivating the researchers to substitute it with other valuable chemical elements and compounds. Correspondingly, LiFePO4 has been extensively evaluated due to its low toxicity features, high safety performance, and great stability in high temperatures. Of note, this structure has revealed a low diffusion coefficient and poor electric conductivity, which is highly essential for developing electric and hybrid vehicles. Downsizing the LiFePO4 particle size, the introduction of conductive materials and polymers into the structure, and cationic doping have been the main strategies applied so far to compensate for the drawbacks declared for the LiFePO4 structure.113,114
To the best of our knowledge, there are a few attempts considering the enhancement of the chemical performance in the cathode materials using environmentally friendly techniques. In 2011, Li et al.115 added carboxymethyl cellulose as a binder into the Li[Li0.2Mn0.56Ni0.16Co0.08]O2. Carboxymethyl cellulose is a fluorine-free binder that can be easily dissolved in water. The resulting homogeneous electrode could reveal a high discharge capacity of 255.4 mAh g–1 at 0.2 C and a very high voltage of 4.8 V, due to providing better structural stability. In another attempt, Zolin et al.116 introduced a natural microfibrillated cellulose, carboxymethyl cellulose, as a binder into the LiFePO4 cathode structure to obtain a discharge capacity of 165 mAh g–1 at 0.1 C through providing a proper mechanical integrity, as well as a fabulous reversible cycling stability. In another attempt carried out in 2020, Park et al.117 extracted CNFs from the cellulose and combined them with LiFePO4 and GO to prepare the LFP/G/cCNF cathode structure. In accordance with the obtained results, the rGO was well-attached to the LFP/cCNF, causing a reduction in contact and internal resistances. In fact, the presence of CNF in the structure led to preventing □-□ stacking of GO sheets and attesting the uniform attachment of the LFP nanoparticles to the GO sheets. Also, the LFP/G/cCNF cathode structure represented higher performances at various current densities, especially at C-rates higher than 5 C. Moreover, the resulted LFP/G/cCNF material illustrated an outstanding cycling stability over 500 cycles. The observed trends could be due to the presence of CNF in the structure, which boosted the electron conductivity and acted as a bridge between LiFePO4 and GO via making hydrogen bindings. Wang et al.118 prepared a MXene film embedded with cellulosic CNF and compared it with the Cu anode. The structure of the evaluated electrochemical cell is depicted in Figure 6a. Correspondingly, similar charge/discharge profiles were obtained for both prepared cells, showing a reversible capacity of 151 mAh g–1 at 0.2 C after 100 cycles for this novel anode structure. Noteworthy, high stability of 95.5% was observed for the MXene@CNF array, while the Cu anode lost 10% of the efficiency after the cycling process (see Figure 6b,c). In fact, uneven deposition of the Li atoms, and the aggregation of dead Li metals on the Li foil, caused a rapid decrease in the discharge capacity. Yoo et al.119 examined the properties of an environmentally friendly cathode/separator combined structure based on pillar[5]quinone (P5Q) and CNF. The results implied great entangling of the CNF in the P5Q configuration. The Li stripping/plating procedure was also evaluated to determine the stability of the cell, illustrating the superior stability of the CNF separator compared with the polyolefin-based membrane. This could be due to a highly porous structure and the presence of nanopores in the CNF, leading to a high affinity to electrolyte uptake and uniform flux of the Li+ ions. Furthermore, a homogeneous SEI layer could be grown on the surface of the electrode in this case.
Figure 6.
Cathode structure based on MXene film filled with cellulose-based CNFs. (a) Schematic illustration of the prepared electrochemical cell, (b) voltage vs capacity, and (c) charge/discharge cycling at 0.2 C. Reproduced with permission from ref (118). Copyright 2020 Elsevier. Features of combined pillar[5]quinone/CNF cathode-separator structure; CNF/LFP cathode material. (d) Schematic illustration of the synthesis process, (e) TEM image, (f) elemental mapping, and (g) specific capacity. Reproduced with permission from ref (120). Copyright 2023 Elsevier.
In 2023, Jiang et al.120 figured out the CNF-based cathode structure loaded by LFP and metal–organic frameworks. Figure 6d shows a schematic illustration of the synthesis procedure employed to obtain the mentioned 3D network structure. Figure 6e,f represents the TEM image and elemental mapping of the attained structure, corroborating the distribution of LFP nanoparticles with 100–200 nm on the carbon-CNF conductive network. Figure 6g also displays the enhanced specific capacity of the provided cathode configuration, which could be linked with the promotion of the Li+ ion transfer by the fabricated carbon network, as well as the N-doping carbon matrix and the interconnected carbon network. The cellulose-based carbon nanofibers could integrate the number of active sites toward obtaining a better interaction with LFP and providing a feasible pathway for transferring electrons in the structure. Furthermore, an improved electrolyte/electrode interface and boosted Li+ ions’ diffusion channels were provided by using the metal–organic framework.
Overall, exploring a high-energy power and lifetime cathode structure has remained a big challenge in approaching a high-density LIB with a long cycle life. Several strategies have been proposed to compensate for the downsides of the commercialized cathode materials, including nanostructure manipulation, creating defects in the cathode configuration, doping with various chemical elements, TM substitution, etc. On the other hand, growth in the attitudes and concerns to apply green materials rather than subsequent recycling of synthetic substances has led to switching from common materials and methodologies to environmentally friendly substances and green routes.
6. Fabrication of Separators Based on Cellulose Nanofibers
A separator, a thin porous membrane, is used in the LIBs to avoid surficial contact between the anode and cathode electrodes while transmitting the Li+ ions between the electrodes in the charge/discharge cycles. With regard to its role in the battery, the separator should be resistant to the electrons to overcome battery discharging and be ionically conductive to accelerate the migration of the Li+ ions during cycling. Wettability, structural stability, chemical stability, and uniform and homogeneous thickness are other crucial properties of an ideal battery separator. Polyethylene and polypropylene nonwoven membranes have been applied as separators in commercialized LIBs due to their proper mechanical strength and chemical stability. However, these structures suffer from poor wettability and thermal shrinkage, weakening the final electrochemical performances. Polyvinylidene difluoride (PVDF) and its copolymer with polyolefin have also been introduced as a modified separator generation, while the resulting environmental concerns and inappropriate performances motivated researchers to develop green substances. Among a wide range of natural-based polymers, cellulose and its derivatives have illustrated great hydrophilicity features, as well as proper thermal, dimensional, and chemical stability.
Filtration dewatering, papermaking, phase inversion, coating, casting, freeze-drying, and electrospinning methods have been widely used to prepare cellulose-based separators so far. Among them, nanofibrous structures produced through the electrospinning procedure can excel in the pore size and porosity characteristics which both play crucial roles in the separator membrane function.121 On the basis of the literature, electrospun cellulose-based separators have revealed appropriate wettability, electron resistance, and electrolyte uptake. As an example, Gwon et al.122 synthesized bacterial cellulose nanofibrous membranes as a separator using a fermentation method, which maintained 80% of the capacity after 1000 cycles. This could correspond to the cross-linked three-dimensional network structure of the cellulose, which could provide superior porosity, crystallinity, and electrolyte wettability, along with lower resistance. In 2018, a three-layer nanofibrous structure containing Cladophora green algae-derived cellulose and a plasma-treated polyethylene was engineered using the papermaking process as a high energy density separator, demonstrating 97.5% capacity retention after 65 cycles. The observed stability of the commercial PE could be linked with the sustaining of smooth and glossy surfaces even after cycling. Meanwhile, the inhomogeneous current distribution could occur in the PE structure, resulting in the creation of a porous Li deposit and yielding more dead Li atoms. The presence of uniform and even pore distribution in the engineered structure could be responsible for the cell’s longer lifetime.123
Considering the cellulose derivatives, Huang et al.124 extracted cellulose acetate from cigarette filtration recycling and electrospun a cellulose/fluoropolymer core–shell nanofibrous structure as a battery separator. On the basis of the obtained data, the fabricated fibers represented 355% electrolyte uptake, a high ionic conductivity of 6.16 mS cm–1, and a rate capability of 75.4% after 100 cycles, possibly due to the appropriate ionic conductivity, along with the integrated Li+ ion transference number and better interfacial compatibility between the electrolyte and the designed separator. In another attempt, Wang et al.125 fabricated a multilayer electrospun separator containing cellulose acetate and PVDF nanofibers. The reason for selecting cellulose acetate as an element in the electrospun separator is stated as its feasible electrospinning process. The results revealed that the designed separator could retain 91% of the capacity after 50 cycles, which could be attributed to the superior structural durability provided by the cellulose acetate skeleton. The features of cellulose acetate-based nanofibers could be further improved through the addition of nanoparticulate fillers. As an example, a porosity of 78% and a discharge capacity of 79 mAh g–1 was reported by Boriboon et al.126 through the incorporation of 10% titania nanoparticles into the cellulose acetate nanofibers. In this attempt, more lithium-ion transference numbers could be approached because of providing more anionic interaction of titania with the employed anionic salt.
In 2023, Xie et al.127 also suggested using an electrospun layer composed of CA and PAN nanofibers. Figure 7a shows a schematic illustration of the procedure employed to fabricate this structure. Since ester compounds of CA lead to poor stability in ester-based electrolytes, alkaline hydrolysis was employed in this evaluation to transform some ester functional groups into hydroxyl ones. In accordance with the obtained results, finer fibers were fabricated through a rise in the ratio of CA due to its better compatibility with the DMF solvent and reducing the solution viscosity (see Figure 7b). The designed membranes represented 61.47% porosity and 121.4% electrolyte uptake, resulting in a high ionic conductivity of 1.148 mS cm–1. Additionally, the lithium transference number was enhanced from 0.26 (PE) to 0.44 due to providing a higher affinity to the liquid electrolyte by the presence of nitrile, ester, and hydroxyl groups. Moreover, the alkaline hydrolysis step caused the generation of more hydroxyl groups on the surface of the nanofibrous layer, causing the interaction with PF6– and so limited its movements and increased the ionic conductivity (see Figure 7c). Evaluation of cellulose/aramid,128,129 bacterial cellulose/SiO2,130 and deacetylated cellulose acetate/PVDF131 nanofibrous separators have also been conducted in this year using other techniques rather than the electrospinning procedure. Table 3 summarizes other advancements carried out toward engineering versatile cellulose-based separators.
Figure 7.
Characteristics of the PAN/cellulose acetate electrospun separator. (a) Schematic illustration of the fabrication procedure, (b) SEM image, and (c) ionic conductivity. Reproduced with permission from ref (127). Copyright 2023 Elsevier. PLLA/cellulose acetate/halloysite nanotubes gel polymer electrolyte. (d) SEM image, (e) impedance spectra, and (f) discharge capacity. Reproduced with permission from ref (145). Copyright 2016 Royal Society of Chemistry. Characterization of electrospun sulfonated bacterial cellulose loaded by polyaniline. (g) SEM illustration and (h) impedance spectra. Reproduced with permission from ref (146). Copyright 2017 Elsevier. Analysis of the PEO-based gel electrolyte embedded with bacterial cellulose nanofibers. (i) Impedance spectra and (j) specific capacity. Reproduced with permission from ref (147). Copyright 2021 Wiley.
Table 3. Characteristics and Main Performances of the Electrospun Cellulose-Based Separators.
| separator content | main features | outcomes | ref |
|---|---|---|---|
| PAN/cellulose | porosity ∼ 66.5%; electrolyte uptake ∼ 238%; and σ ∼ 1.99 mS.cm–1 | the addition of 15 wt.% cellulose caused an increase in the porosity from 60 to 66.5%, due to the formation of finer fibers; in addition, the enhanced porosity caused an enhancement in the electrolyte uptake from 193 to 238%; the observed increase in the porosity and electrolyte uptake led to easier transferring of the Li+ ions and improved the Li+ ion conduction. | Dong et al.132 |
| PAN/CA/boehmite | porosity ∼ 82%; electrolyte uptake ∼ 707%; and σ ∼ 1.694 mS.cm–1 | the proposed structure showed a higher porosity rate compared with the pristine PAN, due to the fabrication of finer fibers; also, higher porosity and great wettability of the boehmite and CA caused superior electrolyte uptake, as well as greater ionic conduction. | Na Yang et al.133 |
| poly d,l-lactide/CNC | porosity ∼ 67.19%; and electrolyte uptake ∼ 1100% | high affinity of the CNC to the ions caused a significant increase in the wettability and electrolyte uptake; so, the addition of CNC caused obtaining a capacity retention of higher than 95% after 50 cycles at 1 C rate. | Laezza et al.134 |
| PEO/lignocellulose/P(VDF-TrFE) | porosity ∼ 86%; electrolyte uptake ∼ 440%; and σ ∼ 7.04 mS.cm–1 | the proposed separator exhibited 647% higher electrolyte uptake than the Celgard commercial separator, which could be assigned to the superior porosity percentage; the ion conductivity was also enhanced in the designed structure compared to that of the commercial one, linking with the reduced contact angle due to the interaction between polymer polar groups and the liquid electrolyte Li+ ions. | Bicy et al.135 |
| cellulose/carboxylated polyimide | porosity ∼ 78%; electrolyte uptake ∼ 638%; and σ ∼ 0.51 mS.cm–1 | mechanical strength was enhanced through the addition of cellulose, due to an increase in the solution viscosity and so the formation of fibers with larger diameters; the prepared structure could maintain 88% of the electrolyte after 100 min at ambient temperature, which was significantly higher than the PP (∼20%); frequent H-bong donating groups in the suggested network could provide numerous channels for feasible transferring of the Li+ ions. | Deng et al.136 |
Generally, exploring versatile separators requires strong scientific efforts, focusing on the electrical and mechanical property improvement in the LIB structures. Compatibilization between the electrodes and the separator component enables easy migration of the Li+ ions during cycling processes. Considering both efficient fabrication methods and environmentally friendly concerns, electrospinning of the cellulose and its derivatives has been suggested as a promising technique for the separator component. Notably, future research can be carried out to improve the battery performance via the addition of highly efficient additives into these electrospun membranes.
7. Electrospun Cellulose-Based Polymer Electrolytes
Solvent-free and gel polymer electrolytes have been introduced in many studies as new components of the next generation of batteries. These membranes act as both electrolytes and the separator through separating the electrodes and transferring the Li+ ions during charging/discharging cycles. The all-solid-state electrolytes are commonly synthesized through the casting of a polymer/salt solution. In accordance with the literature, PEO has been extensively reported as the best polymer matrix for such configurations due to its low glass transition temperature and highly amorphous structure.137 Lithium salts, such as LiClO4, LiBF4, and LiTFSi, are also employed in the matrix to feasible ion transferring between the electrodes. Despite the beneficial features of polymer electrolytes, poor ionic conductivity has restricted their practical applications. Correspondingly, additive loading, manipulating the structure, and fabrication techniques are the strategies that have been used to increase the electrochemical features. As an example, the electrospinning method could modify such properties via providing a highly porous structure with interconnected pores, facilitating the ion movements.138
Combining the polymers also causes enhancing the amorphous phases and increases the ionic conductivity. As a result, cellulose biopolymer has been utilized as a polymer agent in various attempts.139−141 As an example, Samad et al.142 reported the ionic conductivity of 10–4 at 100 °C for a gel polymer, composed of a PEO polymer host loaded with laboratory-graded MCC/PEO particles. According to the data obtained, the introduction of MCC/PEO reinforcement particles enhanced the mechanical features of the PEO electrolyte matrix without any adverse effect on the electrochemical performance. It is declared that the enhancement of mechanical properties could be linked to the interaction between the polymer host and the embedded particles on the submicron level. Abdulkadir et al.143 also introduced a gel polymer electrolyte synthesized through electrospinning of cellulose acetate and PVA polymers as well as K2CO3 and SiO2 as additives. Accordingly, the ionic conductivities of 3.73 × 10–3 and 7.54 × 10–3 S cm–1 were approached at room temperature and 100 °C, respectively. In different research, Bhute et al.144 designed an electrospun PVDF/cellulose acetate-based gel polymer electrolyte loaded by Ag and TiO2 nano fillers. Approaching improved conductivity could result from the addition of CA, which caused an improvement in the wettability, and embedding of the nanofillers, which inclined the PVDF amorphous regions. The obtained results exhibited the ionic conductivity of 6.8 × 10–3 S cm–1 at ambient temperature for this configuration. In an attempt, Zhu et al.145 electrospun a biodegradable PLLA/cellulose acetate gel electrolyte embedded with halloysite nanotubes. The SEM image of the synthesized fine fibers is illustrated in Figure 7d. Impedance spectra of the PLLA/cellulose acetate, PLLA/cellulose acetate/halloysite nanotubes, and Celgard 2500 are depicted in Figure 7e. On the basis of the obtained curves, the PLLA/cellulose acetate gel showed the highest resistance, resulting from some reactions between lithium electrode and active hydroxyl groups which might be due to the low acetylation degree. However, the addition of the additive caused a resistance reduction of 150 Ω. However, the larger conservation rate of the liquid electrolyte resulted in better cycling performance of the designed gel electrolyte compared with that of the Celgard 2500 (see Figure 7f).
In 2017, Yue et al.146 synthesized polyaniline on the surface of electrospun sulfonated bacterial cellulose via an oxidative polymerization mechanism. Two parameters were analyzed on the obtained features, containing immersion of bacterial cellulose in 0.02, 0.04, and 0.06 M NaIO4 as well as stirring of the solution in various 24 and 48 h reaction times. It was observed that the SP48-0.06 electrospun sample, displayed in Figure 7g, leads to the highest ionic conductivity and proper oxidative decomposition potential of 1.5 V, which could be linked with its sulfonation degree (Figure 7h). Moreover, Li et al.147 incorporated bacterial cellulose nanofiber into a PEO-based gel electrolyte to obtain a stable behavior to 4.98 V, 35.5 Ω after 10 cycles, and higher electrochemical stability (see Figure 7i,j).
Overall, various attempts have been made to substitute the cellulose-based gel polymer electrolytes in the battery structures. However, to the best of the authors’ knowledge, no report has been found so far using solvent-free electrospun cellulosic polymer electrolytes which could be a great research line in future works, since all-solid-state polymer electrolytes could result in the fabrication of a flexible, lightweight, and versatile LIB structure.
8. Conclusion and Perspective
The rapidly developing energy storage power tools require designing versatile and high-performance system components. In this era, lithium-ion batteries are counted as one of the most promising and fast-growing devices due to their high energy density and lightweight. Meanwhile, approaching a versatile and highly efficient LIB structure through a green and eco-friendly route is still challenging. Downsizing the element materials has been introduced as an influential strategy toward reaching superior efficiencies. Accordingly, electrospinning has been declared as a controllable, scalable, and affordable strategy to synthesize fine, highly porous fibrous layers containing homogeneously distributed, tiny, and interconnected pores. Electrospun fibers have gradually increased the electrochemical and stability behaviors of the battery electrodes, boosted the electrolyte uptake and ion conductivity of the separators, and enhanced the overall performance of solid-state electrolytes.
Research studies in the field of employing electrospun fibers obtained through natural resources have been quite exciting within the past few decades to decline the usage of petroleum-based synthetic polymers, reduce the environmental impacts, and so inhibit global warming. Without a doubt, cellulose and its derivatives are known as one of the most common green materials, resulting from their outstanding thermal and mechanical stability, inherent hydrophilicity, affordable cost, and so forth. Electrospun cellulose-based structures have revealed desirable characteristics as battery components, possibly because of high surface-to-volume area, porous configuration, nontoxicity, renewability, and biodegradability, which facilitates transportation and storage of the lithium ions through a homogeneous structure.
Despite the fabulous features of the electrospun cellulose-based battery components, such structures are still unable to be applied in commercial architectures, confirming further essential elucidations in this technological field. Figure 8 summarizes the current advantages, challenges, and future directions for employing cellulose and its derivatives to synthesize LIB components. Researchers are actively working on improving the energy density of cellulose-based batteries. By optimizing the structure and composition of cellulose electrodes, as well as designing novel nanofibrous architectures, the energy stored in the batteries is likely to be enhanced. This would make them more competitive with existing LIBs and enable longer-lasting power for various applications. In addition, with the increase in demand for sustainable energy solutions, there is a growing focus on developing cellulose-based LIB elements using waste biomass or agricultural byproducts to further decrease the environmental impact of battery production. This aligns with the goals of approaching a circular economy and reducing reliance on nonrenewable resources. Also, the flexibility and versatility of cellulose-based electrodes make them well-suited for integration into flexible and wearable devices. As the field of wearable electronics continues to expand, cellulose-based batteries can play a crucial role in providing lightweight and conformable power sources. This could enable the development of wearable sensors, smart textiles, and other innovative applications in healthcare, fitness, and consumer electronics. Moreover, as cellulose is abundant and can be sourced from a variety of plant materials, cellulose-based electrospun batteries have the potential for large-scale production at a lower cost compared to traditional battery materials. This scalability and cost-effectiveness make them attractive for commercialization and widespread adoption. Additionally, cellulose-based electrospun batteries have the potential to be biodegradable and environmentally friendly at the end of their life cycle. Researchers are exploring the development of cellulose-based electrodes that can be easily recycled or safely disposed of, which is particularly important as electronic waste continues to be a global concern. Eventually, the formation of cellulose-based electrospun fibers is challenging, requiring more investigations to approach novel strategies or substitution mechanisms.
Figure 8.
Advantages, current challenges, and future perspectives related to the application of electrospun cellulose-based components in LIB structure.
Acknowledgments
The authors would like to thank the Guangdong Province Science and Technology Foreign Talent Grant (Young Scientist, QN2022030021L). In addition, we thank the Shantou Listed Companies Development Promotion Association for the seed grant.
The authors declare no competing financial interest.
References
- Banitaba S. N.; Khademolqorani S.; Jadhav V. V.; Chamanehpour E.; Mishra Y. K.; Mostafavi E.; Kaushik A. ″Recent progress of bio-based smart wearable sensors for healthcare applications″. Materials Today Electronics 2023, 5, 100055. 10.1016/j.mtelec.2023.100055. [DOI] [Google Scholar]
- Yoshino A. ″The birth of the lithium-ion battery″. Angew. Chem., Int. Ed. 2012, 51 (24), 5798–5800. 10.1002/anie.201105006. [DOI] [PubMed] [Google Scholar]
- Manthiram A. ″An outlook on lithium ion battery technology″. ACS central science 2017, 3 (10), 1063–1069. 10.1021/acscentsci.7b00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banitaba S. N.; et al. ″Fabrication and performance evaluation of CuO, NiO, and Co3O4-embedded electrospun electrolytes: Suitable for lithium polymer solvent-free batteries″. J. Alloys Compd. 2022, 924, 166482. 10.1016/j.jallcom.2022.166482. [DOI] [Google Scholar]
- Banitaba S. N.; Ebadi S. V.; Salimi P.; Bagheri A.; Gupta A.; Arifeen W. U.; Chaudhary V.; Mishra Y. K.; Kaushik A.; Mostafavi E.; et al. ″Biopolymer-based Electrospun Fibers in Electrochemical Devices: Versatile Platform for Energy, Environment, and Health Monitoring″. Materials Horizons 2022, 9, 2914. 10.1039/D2MH00879C. [DOI] [PubMed] [Google Scholar]
- Lu L.; Han X.; Li J.; Hua J.; Ouyang M. ″A review on the key issues for lithium-ion battery management in electric vehicles″. J. Power Sources 2013, 226, 272–288. 10.1016/j.jpowsour.2012.10.060. [DOI] [Google Scholar]
- Chen Y.; et al. ″Electrospun cellulose polymer nanofiber membrane with flame resistance properties for lithium-ion batteries″. Carbohydr. Polym. 2020, 234, 115907. 10.1016/j.carbpol.2020.115907. [DOI] [PubMed] [Google Scholar]
- Wang W.; Sun Y.; Liu B.; Wang S.; Cao M. ″Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high performance lithium ion batteries″. Carbon 2015, 91, 56–65. 10.1016/j.carbon.2015.04.041. [DOI] [Google Scholar]
- Chun S.-J.; Choi E.-S.; Lee E.-H.; Kim J. H.; Lee S.-Y.; Lee S.-Y. ″Eco-friendly cellulose nanofiber paper-derived separator membranes featuring tunable nanoporous network channels for lithium-ion batteries″. J. Mater. Chem. 2012, 22 (32), 16618–16626. 10.1039/c2jm32415f. [DOI] [Google Scholar]
- Kishanji M. ″Preparation and characterization of cellulose/in situ generated silver nanoparticle composite films prepared using Pongamia pinnata leaf extract as a reducing and stabilizing agent″. Inorganic and Nano-Metal Chemistry 2021, 51 (9), 1207–1213. 10.1080/24701556.2020.1822869. [DOI] [Google Scholar]
- Wang X.; Yao C.; Wang F.; Li Z. ″Cellulose-based nanomaterials for energy applications″. Small 2017, 13 (42), 1702240. 10.1002/smll.201702240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. J.; Uddin M. J.; Alaboina P.. Review of Nanotechnology for Cathode Materials in Batteries. In Emerging Nanotechnologies in Rechargeable Energy Storage Systems, Rodriguez-Martinez L. M., Omar N., Eds.; Elsevier: Boston, MA, 2017; Chapter 3, pp 83–129. [Google Scholar]
- Liu Y.; et al. ″Nanostructured strategies towards boosting organic lithium-ion batteries″. Journal of Energy Chemistry 2021, 54, 179–193. 10.1016/j.jechem.2020.05.021. [DOI] [Google Scholar]
- Hao G.; Lai Q.; Zhang H. ″Nanostructured Mn-based oxides as high-performance cathodes for next generation Li-ion batteries″. Journal of Energy Chemistry 2021, 59, 547–571. 10.1016/j.jechem.2020.11.035. [DOI] [Google Scholar]
- Zhang M.; et al. ″Free-standing and flexible CNT/(Fe@ Si@ SiO2) composite anodes with kernel-pulp-skin nanostructure for high-performance lithium-ion batteries″. J. Alloys Compd. 2021, 878, 160396. 10.1016/j.jallcom.2021.160396. [DOI] [Google Scholar]
- Banitaba S. N.; Semnani D.; Heydari-Soureshjani E.; Rezaei B.; Ensafi A. A.; Taghipour-Jahromi A. ″Novel electrospun polymer electrolytes incorporated with Keggin-type hetero polyoxometalate fillers as solvent-free electrolytes for lithium ion batteries″. Polym. Int. 2020, 69 (8), 675–687. 10.1002/pi.6001. [DOI] [Google Scholar]
- Banitaba S. N.; Semnani D.; Karimi M.; Heydari-Soureshjani E.; Rezaei B.; Ensafi A. A. ″A comparative analysis on the morphology and electrochemical performances of solution-casted and electrospun PEO-based electrolytes: The effect of fiber diameter and surface density″. Electrochim. Acta 2021, 368, 137339. 10.1016/j.electacta.2020.137339. [DOI] [Google Scholar]
- Hu X.; Zheng Y.; Howey D. A.; Perez H.; Foley A.; Pecht M. ″Battery warm-up methodologies at subzero temperatures for automotive applications: Recent advances and perspectives″. Prog. Energy Combust. Sci. 2020, 77, 100806. 10.1016/j.pecs.2019.100806. [DOI] [Google Scholar]
- Araldi da Silva B.; de Sousa Cunha R.; Valerio A.; De Noni Junior A.; Hotza D.; Gomez Gonzalez S. Y. ″Electrospinning of cellulose using ionic liquids: An overview on processing and applications″. Eur. Polym. J. 2021, 147, 110283. 10.1016/j.eurpolymj.2021.110283. [DOI] [Google Scholar]
- Motaung T. E.; Linganiso L. Z. ″Critical review on agrowaste cellulose applications for biopolymers″. International Journal of Plastics Technology 2018, 22 (2), 185–216. 10.1007/s12588-018-9219-6. [DOI] [Google Scholar]
- Bhat A.; Khan I.; Usmani M. A.; Umapathi R.; Al-Kindy S. M. ″Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose″. Int. J. Biol. Macromol. 2019, 129, 750–777. 10.1016/j.ijbiomac.2018.12.190. [DOI] [PubMed] [Google Scholar]
- Zhu S.; et al. ″Dissolution of cellulose with ionic liquids and its application: a mini-review″. Green Chem. 2006, 8 (4), 325–327. 10.1039/b601395c. [DOI] [Google Scholar]
- Banvillet G.; Depres G.; Belgacem N.; Bras J. ″Alkaline treatment combined with enzymatic hydrolysis for efficient cellulose nanofibrils production″. Carbohydr. Polym. 2021, 255, 117383. 10.1016/j.carbpol.2020.117383. [DOI] [PubMed] [Google Scholar]
- Putrino F. M.; Tedesco M.; Bodini R. B.; de Oliveira A. L. ″Study of supercritical carbon dioxide pretreatment processes on green coconut fiber to enhance enzymatic hydrolysis of cellulose″. Bioresour. Technol. 2020, 309, 123387. 10.1016/j.biortech.2020.123387. [DOI] [PubMed] [Google Scholar]
- Rol F.; Belgacem M. N.; Gandini A.; Bras J. ″Recent advances in surface-modified cellulose nanofibrils″. Prog. Polym. Sci. 2019, 88, 241–264. 10.1016/j.progpolymsci.2018.09.002. [DOI] [Google Scholar]
- Huang S.; Liu X.; Chang C.; Wang Y. ″Recent developments and prospective food-related applications of cellulose nanocrystals: A review″. Cellulose 2020, 27 (6), 2991–3011. 10.1007/s10570-020-02984-3. [DOI] [Google Scholar]
- Shi S.; Zhang M.; Ling C.; Hou W.; Yan Z. ″Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method″. Waste Management 2018, 82, 139–146. 10.1016/j.wasman.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Yang L.; et al. ″Marine biomass-derived composite aerogels for efficient and durable solar-driven interfacial evaporation and desalination″. Chemical Engineering Journal 2021, 417, 128051. 10.1016/j.cej.2020.128051. [DOI] [Google Scholar]
- Tian D.; et al. ″Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood″. Fuel 2019, 252, 589–597. 10.1016/j.fuel.2019.04.155. [DOI] [Google Scholar]
- Kamel R.; El-Wakil N. A.; Dufresne A.; Elkasabgy N. A. ″Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient″. Int. J. Biol. Macromol. 2020, 163, 1579–1590. 10.1016/j.ijbiomac.2020.07.242. [DOI] [PubMed] [Google Scholar]
- Xu J.; Krietemeyer E. F.; Boddu V. M.; Liu S. X.; Liu W.-C. ″Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover″. Carbohydr. Polym. 2018, 192, 202–207. 10.1016/j.carbpol.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Nang An V.; et al. ″Extraction of high crystalline nanocellulose from biorenewable sources of Vietnamese agricultural wastes″. Journal of Polymers and the Environment 2020, 28 (5), 1465–1474. 10.1007/s10924-020-01695-x. [DOI] [Google Scholar]
- Ventura-Cruz S.; Tecante A. ″Nanocellulose and microcrystalline cellulose from agricultural waste: Review on isolation and application as reinforcement in polymeric matrices″. Food Hydrocolloids 2021, 118, 106771. 10.1016/j.foodhyd.2021.106771. [DOI] [Google Scholar]
- Singh M.; Pahal V.; Ahuja D. ″Isolation and characterization of microfibrillated cellulose and nanofibrillated cellulose with “biomechanical hotspots”″. Carbohydr. Polym. 2020, 234, 115827. 10.1016/j.carbpol.2020.115827. [DOI] [PubMed] [Google Scholar]
- Kouadri I.; Satha H. ″Extraction and characterization of cellulose and cellulose nanofibers from Citrullus colocynthis seeds″. Industrial Crops and Products 2018, 124, 787–796. 10.1016/j.indcrop.2018.08.051. [DOI] [Google Scholar]
- Rasheed M.; Jawaid M.; Parveez B.; Zuriyati A.; Khan A. ″Morphological, chemical and thermal analysis of cellulose nanocrystals extracted from bamboo fibre″. Int. J. Biol. Macromol. 2020, 160, 183–191. 10.1016/j.ijbiomac.2020.05.170. [DOI] [PubMed] [Google Scholar]
- Hussain Z.; Sajjad W.; Khan T.; Wahid F. ″Production of bacterial cellulose from industrial wastes: a review″. Cellulose 2019, 26 (5), 2895–2911. 10.1007/s10570-019-02307-1. [DOI] [Google Scholar]
- Prakash Menon M.; Selvakumar R.; Suresh kumar P.; Ramakrishna S. ″Extraction and modification of cellulose nanofibers derived from biomass for environmental application″. RSC Adv. 2017, 7 (68), 42750–42773. 10.1039/C7RA06713E. [DOI] [Google Scholar]
- Abdul Khalil H.; et al. ″A review on plant cellulose nanofibre-based aerogels for biomedical applications″. Polymers 2020, 12 (8), 1759. 10.3390/polym12081759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Y.; Chen C.; Pastel G.; Li Y.; Song J.; Mi R.; Kong W.; Liu B.; Jiang Y.; Yang K.; Hu L.; et al. ″Conductive cellulose nanofiber enabled thick electrode for compact and flexible energy storage devices″. Adv. Energy Mater. 2018, 8 (33), 1802398. 10.1002/aenm.201802398. [DOI] [Google Scholar]
- Khademolqorani S.; Zeinal Hamadani A.; Tavanai H. ″Response Surface Modelling of Electrosprayed Polyacrylonitrile Nanoparticle Size″. Journal of Nanoparticles 2014, 2014, 146218. 10.1155/2014/146218. [DOI] [Google Scholar]
- Nagarajan K.; Balaji A.; Ramanujam N. ″Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment″. Carbohydr. Polym. 2019, 212, 312–322. 10.1016/j.carbpol.2019.02.063. [DOI] [PubMed] [Google Scholar]
- Supian M. A. F.; Amin K. N. M.; Jamari S. S.; Mohamad S. ″Production of cellulose nanofiber (CNF) from empty fruit bunch (EFB) via mechanical method″. Journal of Environmental Chemical Engineering 2020, 8 (1), 103024. 10.1016/j.jece.2019.103024. [DOI] [Google Scholar]
- Djafari Petroudy S. R.; Ranjbar J.; Rasooly Garmaroody E. ″Eco-friendly superabsorbent polymers based on carboxymethyl cellulose strengthened by TEMPO-mediated oxidation wheat straw cellulose nanofiber″. Carbohydr. Polym. 2018, 197, 565–575. 10.1016/j.carbpol.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Rana A. K.; Frollini E.; Thakur V. K. ″Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization″. Int. J. Biol. Macromol. 2021, 182, 1554–1581. 10.1016/j.ijbiomac.2021.05.119. [DOI] [PubMed] [Google Scholar]
- Ye J.; et al. ″Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium″. Bioresource technology 2019, 274, 518–524. 10.1016/j.biortech.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Reiniati I.; Hrymak A. N.; Margaritis A. ″Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals″. Critical reviews in biotechnology 2017, 37 (4), 510–524. 10.1080/07388551.2016.1189871. [DOI] [PubMed] [Google Scholar]
- Lavanya D.; Kulkarni P.; Dixit M.; Raavi P. K.; Krishna L. N. V. ″Sources of cellulose and their applications—A review″. International Journal of Drug Formulation and Research 2011, 2 (6), 19–38. [Google Scholar]
- Amini G.; Banitaba S. N.; Gharehaghaji A. A. ″Imparting strength into nanofiberous yarn by adhesive bonding″. Int. J. Adhes. Adhes. 2017, 75, 96–100. 10.1016/j.ijadhadh.2017.02.020. [DOI] [Google Scholar]
- Lizundia E.; Costa C. M.; Alves R.; Lanceros-Méndez S. ″Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties″. Carbohydrate Polymer Technologies and Applications 2020, 1, 100001. 10.1016/j.carpta.2020.100001. [DOI] [Google Scholar]
- Das A. M.; Ali A. A.; Hazarika M. P. ″Synthesis and characterization of cellulose acetate from rice husk: Eco-friendly condition″. Carbohydr. Polym. 2014, 112, 342–349. 10.1016/j.carbpol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Miyashiro D.; Hamano R.; Umemura K. ″A review of applications using mixed materials of cellulose, nanocellulose and carbon nanotubes″. Nanomaterials 2020, 10 (2), 186. 10.3390/nano10020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennells J.; Godwin I. D.; Amiralian N.; Martin D. J. ″Trends in the production of cellulose nanofibers from non-wood sources″. Cellulose 2020, 27 (2), 575–593. 10.1007/s10570-019-02828-9. [DOI] [Google Scholar]
- Rezaei A.; Nasirpour A.; Fathi M. ″Application of cellulosic nanofibers in food science using electrospinning and its potential risk″. Comprehensive Reviews in Food Science and Food Safety 2015, 14 (3), 269–284. 10.1111/1541-4337.12128. [DOI] [PubMed] [Google Scholar]
- Xue J.; Wu T.; Dai Y.; Xia Y. ″Electrospinning and electrospun nanofibers: Methods, materials, and applications″. Chem. Rev. 2019, 119 (8), 5298–5415. 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M. W. ″Electrospinning cellulose and cellulose derivatives″. Polym. Rev. 2008, 48 (2), 378–391. 10.1080/15583720802022281. [DOI] [Google Scholar]
- Khoo W.; Koh C. T. ″A review of electrospinning process and microstructure morphology control″. ARPN J. Eng. Appl. Sci. 2016, 11 (12), 7774–7781. [Google Scholar]
- Phan D.-N.; Lee H.; Huang B.; Mukai Y.; Kim I.-S. ″Fabrication of electrospun chitosan/cellulose nanofibers having adsorption property with enhanced mechanical property″. Cellulose 2019, 26 (3), 1781–1793. 10.1007/s10570-018-2169-5. [DOI] [Google Scholar]
- Ahmadian S.; Ghorbani M.; Mahmoodzadeh F. ″Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing″. Int. J. Biol. Macromol. 2020, 162, 1555–1565. 10.1016/j.ijbiomac.2020.08.059. [DOI] [PubMed] [Google Scholar]
- Kim M.; Lee H.; Kim M.; Park Y. ″Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin. Nanomaterials 2021, 11, 222. 10.3390/nano11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Q.; Peng B.; Xie Z.; Du S.; Zhang L.; Zhu J. ″Moist-induced electricity generation by electrospun cellulose acetate membranes with optimized porous structures″. ACS Appl. Mater. Interfaces 2020, 12 (51), 57373–57381. 10.1021/acsami.0c17931. [DOI] [PubMed] [Google Scholar]
- Thakur V. K.Nanocellulose polymer nanocomposites: fundamentals and applications; John Wiley & Sons, 2014. [Google Scholar]
- Kim C.-W.; Kim D.-S.; Kang S.-Y.; Marquez M.; Joo Y. L. ″Structural studies of electrospun cellulose nanofibers″. Polymer 2006, 47 (14), 5097–5107. 10.1016/j.polymer.2006.05.033. [DOI] [Google Scholar]
- Zhang K.; et al. ″Preparation and characterization of tree-like cellulose nanofiber membranes via the electrospinning method″. Carbohydr. Polym. 2018, 183, 62–69. 10.1016/j.carbpol.2017.11.032. [DOI] [PubMed] [Google Scholar]
- El-Kafrawy A. ″Investigation of the cellulose/lithium chloride/dimethylacetamide and cellulose/lithium chloride/N-methyl-2-pyrrolidinone solutions by 13C NMR spectroscopy″. J. Appl. Polym. Sci. 1982, 27, 2435–2443. 10.1002/app.1982.070270714. [DOI] [Google Scholar]
- Swatloski R. P.; Spear S. K.; Holbrey J. D.; Rogers R. D. ″Dissolution of cellose with ionic liquids″. Journal of the American chemical society 2002, 124 (18), 4974–4975. 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- Zhang H.; et al. ″Facile cellulose dissolution and characterization in the newly synthesized 1, 3-diallyl-2-ethylimidazolium acetate ionic liquid″. Polymers 2017, 9 (10), 526. 10.3390/polym9100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli L.; Miao J.; Dordick J. S.; Linhardt R. J. ″Electrospinning from room temperature ionic liquids for biopolymer fiber formation″. Green Chem. 2010, 12 (11), 1883–1892. 10.1039/c0gc00283f. [DOI] [Google Scholar]
- Polaskova M.; et al. ″Preparation of microfibers from wood/ionic liquid solutions″. Carbohydr. Polym. 2013, 92 (1), 214–217. 10.1016/j.carbpol.2012.08.089. [DOI] [PubMed] [Google Scholar]
- Viswanathan G.; Murugesan S.; Pushparaj V.; Nalamasu O.; Ajayan P. M.; Linhardt R. J. ″Preparation of biopolymer fibers by electrospinning from room temperature ionic liquids″. Biomacromolecules 2006, 7 (2), 415–418. 10.1021/bm050837s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru N.; Anghel N.; Pascariu P.; Ailiesei G. ″Synthesis and testing of cellulose acetate nicotinate as adsorbent for rhodamine B dye″. J. Appl. Polym. Sci. 2019, 136 (29), 47772. 10.1002/app.47772. [DOI] [Google Scholar]
- Chen W.; Ma H.; Xing B. ″Electrospinning of multifunctional cellulose acetate membrane and its adsorption properties for ionic dyes″. Int. J. Biol. Macromol. 2020, 158, 1342–1351. 10.1016/j.ijbiomac.2020.04.249. [DOI] [PubMed] [Google Scholar]
- Liu K.; et al. ″Fabrication of amino-modified electrospun nanofibrous cellulose membrane and adsorption for typical organoarsenic contaminants: behavior and mechanism″. Chemical Engineering Journal 2020, 382, 122775. 10.1016/j.cej.2019.122775. [DOI] [Google Scholar]
- Ahmed F.; et al. ″Ultrasonic-assisted deacetylation of cellulose acetate nanofibers: A rapid method to produce cellulose nanofibers″. Ultrasonics sonochemistry 2017, 36, 319–325. 10.1016/j.ultsonch.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Silva P.; et al. ″Food-grade hydroxypropyl methylcellulose-based formulations for electrohydrodynamic processing: Part I–Role of solution parameters on fibre and particle production″. Food Hydrocolloids 2021, 118, 106761. 10.1016/j.foodhyd.2021.106761. [DOI] [Google Scholar]
- Wu X.; Wang L.; Yu H.; Huang Y. ″Effect of solvent on morphology of electrospinning ethyl cellulose fibers″. J. Appl. Polym. Sci. 2005, 97 (3), 1292–1297. 10.1002/app.21818. [DOI] [Google Scholar]
- Zhuang Z.; et al. ″Metal–Organic Framework-Derived ZnO, N Dually Doped Nanocages as an Efficient Host for Stable Li Metal Anodes″. ACS Appl. Mater. Interfaces 2023, 15 (32), 38530–38539. 10.1021/acsami.3c08766. [DOI] [PubMed] [Google Scholar]
- Goriparti S.; Miele E.; De Angelis F.; Di Fabrizio E.; Proietti Zaccaria R.; Capiglia C. ″Review on recent progress of nanostructured anode materials for Li-ion batteries″. J. Power Sources 2014, 257, 421–443. 10.1016/j.jpowsour.2013.11.103. [DOI] [Google Scholar]
- Nitta N.; Wu F.; Lee J. T.; Yushin G. ″Li-ion battery materials: present and future″. Mater. Today 2015, 18 (5), 252–264. 10.1016/j.mattod.2014.10.040. [DOI] [Google Scholar]
- Banitaba S. N.; Ehrmann A. ″Application of Electrospun Nanofibers for Fabrication of Versatile and Highly Efficient Electrochemical Devices: A Review″. Polymers 2021, 13 (11), 1741. 10.3390/polym13111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson H. O.Handbook of carbon, graphite, diamond and fullerenes; Noyes Publications: Park Ridge, NJ, 1993; p 43. [Google Scholar]
- Banitaba S. N.Application of electrospun fibers for the fabrication of high performance all-solid-state fibrous batteries. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Ehrmann A., Nguyen T. A., Tri P. N., Eds.; Elsevier, 2021; Chapter 14, pp 229–244. [Google Scholar]
- Agubra V.; Fergus J. ″Lithium ion battery anode aging mechanisms″. Materials 2013, 6 (4), 1310–1325. 10.3390/ma6041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Liu Y.; Shao R.; Wu J.; Jiang R.; Jin Z. ″Recent progress and future perspective on practical silicon anode-based lithium ion batteries″. Energy Storage Materials 2022, 46, 482. 10.1016/j.ensm.2022.01.042. [DOI] [Google Scholar]
- Liu C.; Sun J.; Zheng P.; Jiang L.; Liu H.; Chai J.; Liu Q.; Liu Z.; Zheng Y.; Rui X. ″Recent advances of non-lithium metal anode materials for solid-state lithium-ion batteries″. Journal of Materials Chemistry A 2022, 10, 16761. 10.1039/D2TA03905B. [DOI] [Google Scholar]
- Wei Z.; Wang J.; Liu Y.; Yuan J.; Liu T.; Du G.; Zhu S.; Nie S.; et al. ″Sustainable triboelectric materials for smart active sensing systems″. Adv. Funct. Mater. 2022, 32 (52), 2208277. 10.1002/adfm.202208277. [DOI] [Google Scholar]
- Zeng Q.; et al. ″Sulfur-Bridged Bonds Boost the Conversion Reaction of the Flexible Self-Supporting MnS@ MXene@ CNF Anode for High-Rate and Long-Life Lithium-Ion Batteries″. ACS Appl. Mater. Interfaces 2022, 14 (5), 6958–6966. 10.1021/acsami.1c24417. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Yu K.; Wang D.; Zhang Y.; Li L.; Liang C. ″Core-shell structured Fe2P@ TiO2/CNF anode nanocomposite fibers for efficient lithium/sodium-ion storage″. Colloids Surf., A 2022, 653, 129953. 10.1016/j.colsurfa.2022.129953. [DOI] [Google Scholar]
- Tao L.; et al. ″Flexible anode materials for lithium-ion batteries derived from waste biomass-based carbon nanofibers: I. Effect of carbonization temperature″. RSC Adv. 2018, 8 (13), 7102–7109. 10.1039/C7RA13639K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Yue L.; Wang C.; Li D.; Tang L.; Ma R.; Li B.; Yang T.; Liu X.; Xu Q.; Wang J.; Gao M.; et al. ″Free-Standing Carbon Nanofiber Composite Networks Derived from Bacterial Cellulose and Polypyrrole for Ultrastable Potassium-Ion Batteries″. ACS Appl. Mater. Interfaces 2023, 15 (11), 14865–14873. 10.1021/acsami.3c01401. [DOI] [PubMed] [Google Scholar]
- Li W.; Yang Z.; Cheng J.; Zhong X.; Gu L.; Yu Y. ″Germanium nanoparticles encapsulated in flexible carbon nanofibers as self-supported electrodes for high performance lithium-ion batteries″. Nanoscale 2014, 6 (9), 4532–4537. 10.1039/C4NR00140K. [DOI] [PubMed] [Google Scholar]
- Li S.; Huang J. ″A nanofibrous silver-nanoparticle/titania/carbon composite as an anode material for lithium ion batteries″. Journal of Materials Chemistry A 2015, 3 (8), 4354–4360. 10.1039/C4TA06562J. [DOI] [Google Scholar]
- Liu Y.; et al. ″Facile fabrication of Fe3O4 nanoparticle/carbon nanofiber aerogel from Fe-ion cross-linked cellulose nanofibrils as anode for lithium-ion battery with superhigh capacity″. J. Alloys Compd. 2020, 829, 154541. 10.1016/j.jallcom.2020.154541. [DOI] [Google Scholar]
- Huang Y.; et al. ″Amorphous Fe2O3 nanoshells coated on carbonized bacterial cellulose nanofibers as a flexible anode for high-performance lithium ion batteries″. J. Power Sources 2016, 307, 649–656. 10.1016/j.jpowsour.2016.01.026. [DOI] [Google Scholar]
- Wang M.; Li S.; Zhang Y.; Huang J. ″Hierarchical SnO2/carbon nanofibrous composite derived from cellulose substance as anode material for lithium-ion batteries″. Chemistry–A European Journal 2015, 21 (45), 16195–16202. 10.1002/chem.201502833. [DOI] [PubMed] [Google Scholar]
- Caballero Á.; Morales J.; Sánchez L. ″Tin nanoparticles formed in the presence of cellulose fibers exhibit excellent electrochemical performance as anode materials in lithium-ion batteries″. Electrochem. Solid-State Lett. 2005, 8 (9), A464. 10.1149/1.1993388. [DOI] [Google Scholar]
- Kim J. M.; Guccini V.; Seong K.-d.; Oh J.; Salazar-Alvarez G.; Piao Y. ″Extensively interconnected silicon nanoparticles via carbon network derived from ultrathin cellulose nanofibers as high performance lithium ion battery anodes″. Carbon 2017, 118, 8–17. 10.1016/j.carbon.2017.03.028. [DOI] [Google Scholar]
- Kim J. M.; et al. ″TEMPO-oxidized cellulose nanofibers as versatile additives for highly stable silicon anode in lithium-ion batteries″. Electrochim. Acta 2021, 369, 137708. 10.1016/j.electacta.2020.137708. [DOI] [Google Scholar]
- Wang K.; Huang J. ″Natural cellulose derived nanofibrous Ag-nanoparticle/SnO2/carbon ternary composite as an anodic material for lithium-ion batteries″. J. Phys. Chem. Solids 2019, 126, 155–163. 10.1016/j.jpcs.2018.11.025. [DOI] [Google Scholar]
- Wang K.; Huang J. ″A bioinspired three-dimensional nanofibrous Cu-nanoparticle/SnO2/carbon composite as an anodic material for lithium-ion battery″. Appl. Surf. Sci. 2019, 476, 293–302. 10.1016/j.apsusc.2019.01.094. [DOI] [Google Scholar]
- Ma Y.; et al. ″Three-dimensional bacterial cellulose and MOF-74 derivatives as anode for high performance potassium-ion batteries″. J. Alloys Compd. 2023, 946, 169365. 10.1016/j.jallcom.2023.169365. [DOI] [Google Scholar]
- Han W.; Xiao Y.; Yin J.; Gong Y.; Tuo X.; Cao J. ″Fe3O4@Carbon Nanofibers Synthesized from Cellulose Acetate and Application in Lithium-Ion Battery″. Langmuir 2020, 36 (38), 11237–11244. 10.1021/acs.langmuir.0c01399. [DOI] [PubMed] [Google Scholar]
- Qiu L.; et al. ″Study on effects of carboxymethyl cellulose lithium (CMC-Li) synthesis and electrospinning on high-rate lithium ion batteries″. Cellulose 2014, 21 (1), 615–626. 10.1007/s10570-013-0108-z. [DOI] [Google Scholar]
- Shi Q.; Liu D.; Wang Y.; Zhao Y.; Yang X.; Huang J. ″High-Performance Sodium-Ion Battery Anode via Rapid Microwave Carbonization of Natural Cellulose Nanofibers with Graphene Initiator″. Small 2019, 15 (41), 1901724. 10.1002/smll.201901724. [DOI] [PubMed] [Google Scholar]
- Zhang S.; et al. ″Fabricating Fe3O4/Fe/Biocarbon Fibers using Cellulose Nanocrystals for High-Rate Li-ion Battery Anode″. Electrochim. Acta 2015, 174, 1175–1184. 10.1016/j.electacta.2015.06.098. [DOI] [Google Scholar]
- Cao S.; et al. ″In Situ Carbonized Cellulose-Based Hybrid Film as Flexible Paper Anode for Lithium-Ion Batteries″. ACS Appl. Mater. Interfaces 2016, 8 (2), 1073–1079. 10.1021/acsami.5b10648. [DOI] [PubMed] [Google Scholar]
- Wu C.-Y.; Lin T.-Y.; Duh J.-G. ″Constructing cellulose nanofiber/Si composite 3D-Net structure from waste rice straw via freeze drying process for lithium-ion battery anode materials″. Mater. Chem. Phys. 2022, 285, 126107. 10.1016/j.matchemphys.2022.126107. [DOI] [Google Scholar]
- Illa M. P.; Khandelwal M.; Sharma C. S. ″Bacterial cellulose-derived carbon nanofibers as anode for lithium-ion batteries″. Emergent Materials 2018, 1 (3), 105–120. 10.1007/s42247-018-0012-2. [DOI] [Google Scholar]
- Guo S.; Zhang P.; Feng Y.; Wang Z.; Li X.; Yao J. ″Rational design of interlaced Co9S8/carbon composites from ZIF-67/cellulose nanofibers for enhanced lithium storage″. J. Alloys Compd. 2020, 818, 152911. 10.1016/j.jallcom.2019.152911. [DOI] [Google Scholar]
- Zhang W.; et al. ″Wasp nest-imitated assembly of elastic rGO/p-Ti3C2Tx MXene-cellulose nanofibers for high-performance sodium-ion batteries″. Carbon 2019, 153, 625–633. 10.1016/j.carbon.2019.07.040. [DOI] [Google Scholar]
- Wang Z.; et al. ″Comparative effects of electrospinning ways for fabricating green, sustainable, flexible, porous, nanofibrous cellulose/chitosan carbon mats as anode materials for lithium-ion batteries″. Journal of Materials Research and Technology 2021, 11, 50–61. 10.1016/j.jmrt.2021.01.009. [DOI] [Google Scholar]
- Jadhav V. V.; Zhuang Z.; Banitaba S. N.; Khademolqorani S.; Gandla D.; Zhang F.; Tan D. Q. ″Tailoring Performance of the LiNi0. 8Mn0. 1Co0. 1O2 Cathode by Al2O3 and MoO3 artificial cathode electrolyte interphase (CEI) layers through plasma-enhanced atomic layer deposition (PEALD) Coating,″. Dalton Trans. 2023, 52, 14564. 10.1039/D3DT02865H. [DOI] [PubMed] [Google Scholar]
- Jadhav V. ″Hierarchical Nanoneedles Grown on Nickel Foam As Binder-Free Electrode for High Energy Density Asymmetric Supercapacitors″. ECS Meeting Abstracts 2020, MA2020-02, 646. 10.1149/MA2020-023646mtgabs. [DOI] [Google Scholar]
- Lu G.; Wang Z.; Zhang S.; Ding J.; Luo J.; Liu X. ″Halide aqueous redox flow batteries cathode materials: Recent progress and future perspectives″. Nanoscale 2023, 15 (4), 1. 10.1039/D2NR07291B. [DOI] [PubMed] [Google Scholar]
- Li J.; Klöpsch R.; Nowak S.; Kunze M.; Winter M.; Passerini S. ″Investigations on cellulose-based high voltage composite cathodes for lithium ion batteries″. J. Power Sources 2011, 196 (18), 7687–7691. 10.1016/j.jpowsour.2011.04.030. [DOI] [Google Scholar]
- Zolin L.; et al. ″Flexible cellulose-based electrodes: Towards eco-friendly all-paper batteries″. Chemical Engineering Transactions 2014, 41, 361–366. 10.3303/CET1441061. [DOI] [Google Scholar]
- Park S.; et al. ″Facile preparation of cellulose nanofiber derived carbon and reduced graphene oxide co-supported LiFePO4 nanocomposite as enhanced cathode material for lithium-ion battery″. Electrochim. Acta 2020, 354, 136707. 10.1016/j.electacta.2020.136707. [DOI] [Google Scholar]
- Wang C.-Y.; Zheng Z.-J.; Feng Y.-Q.; Ye H.; Cao F.-F.; Guo Z.-P. ″Topological design of ultrastrong MXene paper hosted Li enables ultrathin and fully flexible lithium metal batteries″. Nano Energy 2020, 74, 104817. 10.1016/j.nanoen.2020.104817. [DOI] [Google Scholar]
- Yoo G.; et al. ″Highly reliable quinone-based cathodes and cellulose nanofiber separators: toward eco-friendly organic lithium batteries″. Cellulose 2020, 27 (12), 6707–6717. 10.1007/s10570-020-03266-8. [DOI] [Google Scholar]
- Jiang Z.; et al. ″A “two-for-one” strategy to construct a LiFePO4@ C cathode with 3D porous framework for high-energy Li-on batteries″. J. Alloys Compd. 2023, 937, 168402. 10.1016/j.jallcom.2022.168402. [DOI] [Google Scholar]
- Barbosa J. C.; et al. ″Lithium-ion battery separator membranes based on poly(L-lactic acid) biopolymer″. Materials Today Energy 2020, 18, 100494. 10.1016/j.mtener.2020.100494. [DOI] [Google Scholar]
- Gwon H.; et al. ″A safe and sustainable bacterial cellulose nanofiber separator for lithium rechargeable batteries″. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (39), 19288–19293. 10.1073/pnas.1905527116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R.; Xu X.; Sun R.; Wang Z.; Lindh J.; Edstrom K.; Strømme M.; Nyholm L.; et al. ″Nanocellulose modified polyethylene separators for lithium metal batteries″. Small 2018, 14 (21), 1704371. 10.1002/smll.201704371. [DOI] [PubMed] [Google Scholar]
- Huang F.; et al. ″Coaxial Electrospun Cellulose-Core Fluoropolymer-Shell Fibrous Membrane from Recycled Cigarette Filter as Separator for High Performance Lithium-Ion Battery″. ACS Sustainable Chem. Eng. 2015, 3 (5), 932–940. 10.1021/acssuschemeng.5b00032. [DOI] [Google Scholar]
- Wang S.; Zhang D.; Shao Z.; Liu S. ″Cellulosic materials-enhanced sandwich structure-like separator via electrospinning towards safer lithium-ion battery″. Carbohydr. Polym. 2019, 214, 328–336. 10.1016/j.carbpol.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Boriboon D.; Vongsetskul T.; Limthongkul P.; Kobsiriphat W.; Tammawat P. ″Cellulose ultrafine fibers embedded with titania particles as a high performance and eco-friendly separator for lithium-ion batteries″. Carbohydr. Polym. 2018, 189, 145–151. 10.1016/j.carbpol.2018.01.077. [DOI] [PubMed] [Google Scholar]
- Xie X.; et al. ″Multi-functional groups decorated composite nanofiber separator with excellent chemical stability in ester-based electrolyte for enhancing the lithium-ion transport″. J. Power Sources 2023, 555, 232431. 10.1016/j.jpowsour.2022.232431. [DOI] [Google Scholar]
- Liu X.; et al. ″Study on cellulose nanofibers/aramid fibers lithium-ion battery separators by the heterogeneous preparation method″. Int. J. Biol. Macromol. 2023, 225, 1476–1486. 10.1016/j.ijbiomac.2022.11.204. [DOI] [PubMed] [Google Scholar]
- Yang R.; et al. ″Microfibril Aramid-Nanocellulose Fiber-Based Hybrid Separator for High-Performance Lithium-Ion Batteries″. J. Electrochem. Soc. 2023, 170 (2), 020519. 10.1149/1945-7111/acb8e6. [DOI] [Google Scholar]
- Li W.; Wang S.; Fan Z.; Li S.; Newman N. ″Oxidized bacterial cellulose functionalized with SiO2 nanoparticles as a separator for lithium-metal and lithium–sulfur batteries″. Cellulose 2023, 30 (1), 481–493. 10.1007/s10570-022-04931-w. [DOI] [Google Scholar]
- Si J.; Zhao M.; Marcelle S. s. a.; Wang Q.; Cui Z.; Liu X. ″Design and Modification of Janus Polyvinylidene Fluoride/Deacetylated Cellulose Acetate Nanofiber Membrane and its Multifunctionality″. Advanced Materials Interfaces 2023, 10, 2201550. 10.1002/admi.202201550. [DOI] [Google Scholar]
- Dong G.; Li H.; Wang Y.; Jiang W.; Ma Z. ″Electrospun PAN/cellulose composite separator for high performance lithium-ion battery″. Ionics 2021, 27 (7), 2955–2965. 10.1007/s11581-021-04073-2. [DOI] [Google Scholar]
- Yang N.; Liang Y.; Jia S. ″Enhanced Thermal Stability and Electrochemical Performance of Polyacrylonitrile/Cellulose Acetate-Electrospun Fiber Membrane by Boehmite Nanoparticles: Application to High-Performance Lithium-Ion Batteries″. Macromol. Mater. Eng. 2021, 306 (10), 2100300. 10.1002/mame.202100300. [DOI] [Google Scholar]
- Laezza A.; et al. ″Cellulose Nanocrystals as Additives in Electrospun Biocompatible Separators for Aprotic Lithium-Ion Batteries″. ACS Applied Polymer Materials 2023, 5 (2), 1453–1463. 10.1021/acsapm.2c01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicy K.; et al. ″Sustainable lithium-ion battery separators derived from polyethylene oxide/lignocellulose coated electrospun P(VDF-TrFE) nanofibrous membranes″. Surfaces and Interfaces 2022, 29, 101716. 10.1016/j.surfin.2021.101716. [DOI] [Google Scholar]
- Deng J.; Cao D.; Yang X.; Zhang G. ″Cross-linked cellulose/carboxylated polyimide nanofiber separator for lithium-ion battery application″. Chemical Engineering Journal 2022, 433, 133934. 10.1016/j.cej.2021.133934. [DOI] [Google Scholar]
- Banitaba S. N.; et al. ″Nanocomposite with Fast Li+ Ion Conductivity: A Solvent-Free Polymer Electrolyte Reinforced with Decorated Fe3O4 Nanoparticles″. ACS Applied Energy Materials 2023, 6 (9), 4704–4714. 10.1021/acsaem.3c00042. [DOI] [Google Scholar]
- Narwade S. H.; Ghule B. G.; Shinde N. M.; Kamble C.; Jadhav V. V.; Mane R. S.. Electrochemical synthesis for metal oxide/hydroxide nanostructures,. In Solution Methods for Metal Oxide Nanostructures. Mane R., Jadhav V., Al-Enizi A., Eds.; Elsevier, 2023; Chapter 17, pp 393–418. [Google Scholar]
- Liu M.; et al. ″A cross-linked gel polymer electrolyte employing cellulose acetate matrix and layered boron nitride filler prepared via in situ thermal polymerization″. J. Power Sources 2021, 484, 229235. 10.1016/j.jpowsour.2020.229235. [DOI] [Google Scholar]
- Lalia B. S.; Samad Y. A.; Hashaikeh R. ″Nanocrystalline cellulose-reinforced composite mats for lithium-ion batteries: electrochemical and thermomechanical performance″. J. Solid State Electrochem. 2013, 17 (3), 575–581. 10.1007/s10008-012-1894-1. [DOI] [Google Scholar]
- Kale S. B.; et al. ″Cellulose-derived flame-retardant solid polymer electrolyte for lithium-ion batteries″. ACS Sustainable Chem. Eng. 2021, 9 (4), 1559–1567. 10.1021/acssuschemeng.0c06309. [DOI] [Google Scholar]
- Samad Y. A.; Asghar A.; Hashaikeh R. ″Electrospun cellulose/PEO fiber mats as a solid polymer electrolytes for Li ion batteries″. Renewable Energy 2013, 56, 90–95. 10.1016/j.renene.2012.09.015. [DOI] [Google Scholar]
- Abdulkadir B. A.; Dennis J. O.; Adam A. A.; Al-Dhahebi A. M.; Shukur M. F. ″Novel electrospun separator-electrolyte based on PVA-K2CO3-SiO2-cellulose nanofiber for application in flexible energy storage devices″. J. Appl. Polym. Sci. 2022, 139 (23), 52308. 10.1002/app.52308. [DOI] [Google Scholar]
- Bhute M. V.; Kondawar S. B. ″Electrospun poly (vinylidene fluoride)/cellulose acetate/AgTiO2 nanofibers polymer electrolyte membrane for lithium ion battery″. Solid State Ionics 2019, 333, 38–44. 10.1016/j.ssi.2019.01.019. [DOI] [Google Scholar]
- Zhu M.; Lan J.; Tan C.; Sui G.; Yang X. ″Degradable cellulose acetate/poly-l-lactic acid/halloysite nanotube composite nanofiber membranes with outstanding performance for gel polymer electrolytes″. Journal of Materials Chemistry A 2016, 4 (31), 12136–12143. 10.1039/C6TA05207J. [DOI] [Google Scholar]
- Yue L.; et al. ″Sulfonated bacterial cellulose/polyaniline composite membrane for use as gel polymer electrolyte″. Compos. Sci. Technol. 2017, 145, 122–131. 10.1016/j.compscitech.2017.04.002. [DOI] [Google Scholar]
- Li Y.; et al. ″Bacterial cellulose composite solid polymer electrolyte with high tensile strength and lithium dendrite inhibition for long life battery″. Energy & Environmental Materials 2021, 4 (3), 434–443. 10.1002/eem2.12122. [DOI] [Google Scholar]