Abstract

Cancer is a devastating disease with over 100 types, including lung and breast cancer. Cisplatin and metal-based drugs are limited due to their drug resistance and side effects. Iridium-based compounds have emerged as promising candidates due to their unique chemical properties and resemblance to platinum compounds. The objective of this study is to investigate the synthesis and categorization of iridium complexes, with a particular emphasis on their potential use as anticancer agents. The major focus of this research is to examine the synthesis of these complexes and their relevance to the field of cancer treatment. The negligible side effects and flexibility of cyclometalated iridium(III) complexes have garnered significant interest. Organometallic half-sandwich Ir(III) complexes have notable benefits in cancer research and treatment. The review places significant emphasis on categorizing iridium complexes according to their ligand environment, afterward considering the ligand density and coordination number. This study primarily focuses on several methods for synthesizing cyclometalated and half-sandwich Ir complexes, divided into subgroups based on ligand denticity. The coordination number of iridium complexes determines the number of ligands coordinated to the central iridium atom, which impacts their stability and reactivity. Understanding these complexes is crucial for designing compounds with desired properties and investigating their potential as anticancer agents. Cyclometalated iridium(III) complexes, which contain a meta-cycle with the E-M-C order σ bond, were synthesized in 1999. These complexes have high quantum yields, significant stock shifts, luminescence qualities, cell permeability, and strong photostability. They have been promising in biosensing, bioimaging, and phosphorescence of heavy metal complexes.
1. Introduction
Cancer remains a devastating disease, necessitating continuous research and advancements in treatment strategies. While platinum-based drugs have been widely used in cancer treatment, their associated side effects on vital organs have spurred the exploration of alternative compounds with improved safety profiles.1 Iridium-based complexes have recently gained popularity as viable possibilities since they may be more effective and have fewer negative side effects. Due to their distinct chemical characteristics and similarity to platinum compounds, these complexes have drawn much interest and are thus attractive for anticancer research.2
This comprehensive review aims to provide a detailed overview of the synthesis and classification of iridium complexes as potential anticancer agents. Specifically, this review focuses on the advancements made in the field of lung cancer treatment. In 2022, Zhang and his team published a seminal review paper discussing the efficacy of iridium complexes in combatting lung cancer. The paper explores the mechanisms through which iridium complexes exert their anticancer effects and delves into their synthesis methods and classifications.1,2
Iridium compounds’ anticancer efficacy may be fine-tuned by synthesizing their structures and characteristics. This review focuses on two forms of iridium complexes, cyclometalated iridium(III) complexes and organometallic half-sandwich iridium(III) complexes, and the various chemical techniques used to create them. Further subcategories were based on ligand denticity.
Cyclometalated iridium(III) complexes have gained significant attention in cancer research due to their minimal side effects and adaptability as potential anticancer drugs.3 The review discusses various synthesis approaches for cyclometalated complexes, elucidating the methods for achieving desired structures and properties. These synthesis techniques have enabled researchers to design cyclometalated complexes with specific functionalities such as inducing cell death, inhibiting protein–protein interactions, and disrupting the cellular balance. Unlike platinum-based drugs, cyclometalated iridium complexes interact with therapeutic targets in a noncovalent manner, reducing the likelihood of adverse side effects associated with platinum drugs. This unique approach to targeting cancer cells opens up new possibilities for safer and more effective cancer treatments.4,5
The second type of iridium complex explored in this review is organometallic half-sandwich Ir(III) complexes. These complexes offer distinct advantages in cancer research and therapy. The review examines various synthesis methods employed to customize the structures and properties of organometallic half-sandwich Ir(III) complexes. These methods include incorporating ferrocenyl groups, developing fluorescent complexes, utilizing Schiff base (O∧N) compounds, and introducing N-heterocyclic carbenes. These approaches allow for the design of organometallic half-sandwich Ir(III) complexes with multiple ligand sites, high lipid solubility, the ability to target specific organelles, and remarkable photostability. Such tailored properties enhance the potential of these complexes as effective anticancer agents.5
Additionally, the review emphasizes the classification of iridium complexes based on their ligand environment and coordination number. Iridium complexes can possess various ligands, such as nitrogen groups (N∧N type of ligand, N∧N∧N type of ligand, C∧N type of ligand, only N type of ligand, etc.), phosphorus (P∧P type of ligand), sulfur-based ligands (S∧S type of ligand), oxygen-based ligands (N∧O type of ligand), or carbon-based ligands (C∧C type of ligand, C∧N type of ligands, etc.), which significantly influence their chemical properties. Furthermore, the coordination number of iridium complexes determines the number of ligands coordinated to the central iridium atom, further impacting their stability and reactivity. Understanding the classification of iridium complexes based on these parameters is crucial for designing compounds with desired properties and investigating their potential as anticancer agents.
2. Discussion
Cyclometalated Iridium(III) Complexes
The cyclometalated compounds are those compounds that contain a meta-cycle with the E-M-C order σ bond where M is metal, E is the donor atom, and C is the carbon atom. In 1999, Pilloni and group synthesized the first cyclometalated iridium complex.6 These complexes have high quantum yields, significant stock shifts, excellent luminescence qualities, cell permeability, and robust photostability. In recent years, these complexes have become the most promising in biosensing, bioimaging, and phosphorescent heavy metal complexes. In this discussion, we elucidate cyclometalated compounds, focusing on their categorization according to the ligand density.
2.1. N∧N Type of Ligand
In 2014, Ye et al. introduced a cyclometalated Ir(III) complex as a targeted theranostic anticancer treatment.7 They combined this with HDAC inhibition and PDT, demonstrating the iridium(III) complexes with the potential of phosphorescence as a strategy for developing novel multifunctional metallo-anticancer therapeutics that specifically target tumors. The study identified four phosphorescent Ir(III) complexes, including one modeled after SAHA, which is a phenanthroline derivative. Ligand L, used in the study, was synthesized based on previous research. The study highlights the importance of molecularly targeted drug design methodologies in developing effective cancer treatments. These particular complexes were subjected to various analytical methods such as ESI-MS, 1H NMR, and elemental analysis to determine their characteristics and properties. Compounds 1–4 exhibited powerful cytotoxicity against the cancer cell lines tested, while demonstrating significantly lower levels of phototoxicity against healthy human cells. Heroine ligand = N1-hydroxy-N8-(1,10-phenanthrolin-5-yl) octane diamide and N–C = 2-phenylpyridine (ppy, 1a), 2-(2,4-difluorophenyl) pyridine (dfppy, 1d), 2-(2-thienyl) pyridine (thpy, 1c), and 3-(2-pyridinyl)-coumarin (coumarin, 1b)7 (Figure 1).
Figure 1.
Structure of complexes 1a–1d.
In 2015, Mao and colleagues synthesized four complexes (2–5) of carboline ligands with cyclometalated iridium(III).8 The authors claim that these compounds are agents and PSs for lysosome-targeted cell imaging and are pH-activatable in an acidic environment. Those complexes are β-carboline complexes with specific imaging properties and pH-dependent designs for producing singlet oxygen (1O2). [Ir(N∧C)2(N∧N)](PF6)] is the general formula of these four complexes for synthesis. They react to two equiv of β-carboline ligands with the corresponding cyclometalated Ir(III) dimers in CH2Cl2–CH3OH, purifying the product by anion exchange with NH4PF6 and then recrystallizing it. These complexes exhibit increased phosphorescent emission and oxygen production in acidic conditions (pH ≤ 6.5), such as those seen in tumors and lysosomes8 (Figure 2).
Figure 2.
Chemical structures complexes 2–5.
Later in the year 2015, Qiu et al. published a paper in which they synthesized four Ir(III)-based complexes as monitors of variations in the mitochondrial membrane potential in living cells.9 Ligands L1–L4 synthesized by the combined ammonium acetate, 4-morpholine-4-ylmethylbenzaldehyde, aniline, glacial acetic acid, and 1,10-phenanthroline-5,6-dione was refluxed for 12 h; after that, when the reaction mixture got cool, 20 mL of water was added to it, and then 25% ammonia was used for neutralization. DCM was added to the solution, and organic compounds were removed from the organic layer. By using rotary evaporation, they got the yellow crude product, and by using column chromatography, the crude product was purified. Here 6a = 4-(4-(1-phenyl-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)-benzyl)morpholine, 6b = 4-(4-(1-(p-tolyl)-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzyl)morpholine, 6c = 4-(4-(1-(4-(tert-butyl)phenyl)-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzyl)morpholine, and 6d = 4-(4-(1-(4-phenoxyphenyl)-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzyl)morpholine.
Synthesis of the complexes 6 was performed in a round-bottomed flask. A combination of ligands (L1–L4) and [Ir(2Fppy)2Cl]2 was mixed with a methanol and DCM solution. The mixture was heated with argon for 12 h at 65 °C. After this process, the solvent was removed by reduced pressure, and the resulting product was refined by column chromatography with DCM/ethanol. This process allowed for the isolation of iridium complexes, yielding a 50–55% success rate9 (Figure 3).
Figure 3.
Chemical structures of all Ir complexes.
A study by Cao et al. looked at using phosphorescent iridium(III) complexes to target cancer cell metabolism by immobilizing them in mitochondria. The study created four different complexes with specific ligands and found they could be effective mitochondrial-targeting anticancer drugs. This research was conducted in 2016. To synthesize these complexes, they used the corresponding cyclometalated iridium(III) dimers in CH2Cl2/CH3OH and 2 equiv of ligand followed by NH4PF6. For the purification of complexes, they use column chromatography, and recrystallization is also done. The cyclometalated Ir complex is [Ir(N∧C)2(N∧N)](PF6)] in N∧N = (2,2′-bipyridine)-4,4′-diyldimethanol for ligand 1 or 4,4′-bis(chloromethyl)-2,2′-bipyridine for ligand 2, and N∧C = 2-phenylpyridine or 2-(2,4-difluorophenyl)pyridine was also synthesized and characterized10 (Figure 4).
Figure 4.
Chemical structures of complexes 7–10.
Moving on to the year 2017, Tang et al. created a novel ligand and its iridium(III) complex. By directly reacting cyclohexane-1,2-dione, TTBD, and glacial acetic acid, the ligand THPDP was created. Combining dichloromethane and methanol, cis-[Ir(ppy)2Cl]2 and THPDP were combined to create the complex 13. Column chromatography on neutral alumina was used to clean the raw product. This complex shows cytotoxicity in vitro against cancer cells BEL-7402, A549, Eca-109, SGC-7901, and B16, and the average NIH 3T3 cell lines were evaluated using the MTT method11 (Scheme 1).
Scheme 1. Complex Preparation Reaction.
In 2017, Ouyang and collaborators synthesized six Ir(III) complexes with varying quantities of fluorine atoms. When tested against five cancer cell lines, these complexes showed much higher antiproliferation activity than the clinical chemotherapeutic drug cisplatin. Additionally, the antiproliferation actions corresponded with the total number of fluorine atoms replaced. In this study, the ligands are imidazole [4,5-f][1,10]phenanthroline derivative ligands (17a–17f). For the synthesis of complexes, they added an imidazole ligand along with [(ppy)2Ir(μ-Cl)]2 in dichloromethane, and methanol was refluxed for 12 h under argon. After cooling, the solvent was gently removed through reduced pressure, creating a refined and sophisticated orange–red solid through vacuum drying. For purifying, they use neutral alumina. For that, they use dichloromethane and ethanol12 (Scheme 2).
Scheme 2. Synthesis of the Ligands and Complexes 17a–17f.
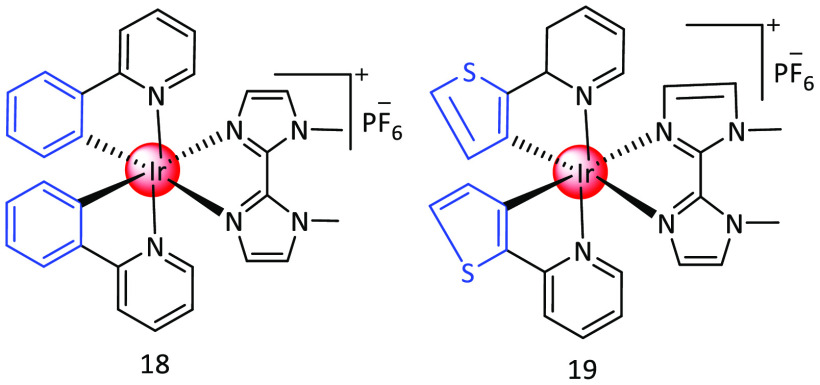
In 2015, Chen et al. published a paper titled “Light-Up Mitophagy in Live Cells with Dual-Functional Theranostic Phosphorescent Iridium(III) Complexes”. The paper showcases the innovative use of two phosphorescent cyclometalated Ir(III) complexes with the unique capability to gather in mitochondria and prompt mitophagy, as these complexes are fluorescent. Refluxing the matching cyclometalated Ir(III) dimer and the ligand in CH2Cl2/CH3OH (2:1, v/v) resulted in the high yield synthesis of 18 and 19, which were subsequently purified via anion exchange, column chromatography, and recrystallization. 18 and 19 triggered the process of mitophagy by lowering mitochondrial membrane potential, reducing ATP levels in cells, disrupting mitochondrial energy production, and leading to oxidative stress leading to oxidative stress13 (Figure 5).
Figure 5.
Structures of complex18 and 19.
Yi et al. have made a remarkable discovery regarding an Ir(III) compound with potent anticancer properties.14 This finding holds immense promise for the development of an efficacious cancer therapy. In this study, they present a novel Ir(III) complex that they designed and synthesized and assess its ability to inhibit the growth of cancer cells both in vitro and in vivo.
BDPIP was created in methanol by a direct reaction between the precursor complex cis-[Ir(ppy)2Cl]2 and BDPIP. Ammonium acetate, glacial acetic acid, 1,10-phenanthroline-5,6-dione, and 3-benzo[1,3]dioxo-5-yl-2-methylpropionaldehyde combined to create BDPIP, and DMSO dissolved the Ir complex. IR, ESI-MS, 13C, and 1H NMR confirmed the results. BDPIP killed specific cancerous cell lines. The complex killed SiHa, A549, SGC-7901, HepG2, HeLa, and BEL-7402 cells.14 (Scheme 3).
Scheme 3. Synthetic Route of Ligands and Complexes.
Transitioning toward 2018, Liang et al. synthesized four novel cyclometalated phosphorescent iridium complexes. For synthesizing these complexes, they used two types of ligands. The general formula of these ligands is [Ir(C∧N)2(N∧N)] (PF6), where N∧N = 9-methyl-1-(2-pyridyl)-β-carboline for ligand 1 or N∧N = 9-methyl-1-(2-quinolinyl)-β-carboline for ligand 2. For the preparation of the complex, with ligands L1 and L2 (2 equiv), dimeric Ir(III) precursors [Ir2(CN)4Cl2] in CH3OH–CH2Cl2 were used followed by NH4PF6 with anion exchange. Due to their positive charge and lipophilicity, these complexes are preferentially deposited in the mitochondria, as shown by cell imaging and ICP-MS data15 (Figure 6).
Figure 6.
Chemical structures of IrM1–IrM4.
In 2018, Liu and colleagues developed and synthesized iridium(III) complexes with the potential to serve as effective anticancer agents. These complexes were [Ir(ppy)2(adppz)]PF6 (27), [Ir(bzq)2(adppz)]PF6 (28), and [Ir(piq)2(adppz)]PF6 (29).16
For the synthesis of complex 27, a combination of adppz and cis-[Ir(ppy)2Cl]2 in methanol and dichloromethane (VCH2Cl2:VCH3OH = 2:1) produced a bright yellow solution at 6 h reflux. Then after dropwise addition of saturated aqueous NH4PF6 solution and stirring for 2 h at rt, a yellow precipitate was obtained after cooling. By using column chromatography, the complex was purified with a 79% yield. For the synthesis of complex 28, instead of [Ir(ppy)2Cl]2, they used [Ir(bzq)2Cl]2, and at last, for complex 29 synthesis, they used [Ir(piq)2Cl]2. The yields of these complexes were 71% for 28 and 72% for 29. The complexes have a stronger ability to fight cancer in A549 cells compared to cisplatin, and they are also effective at stopping cell invasion and proliferation. They work by targeting and increasing the permeability of lysosomes16 (Figure 7).
Figure 7.
Structures of complexes 27–29.
Three Ir compounds were synthesized by Zhang et al. These complexes are more cytotoxic than cisplatin against tumor cells like A549, BEL-7402, HeLa, HepG2, Eca-109, and SGC-7901. For synthesizing these complexes, they used one common ligand called NDPPZ. Along with that, for reagent they used cis-[Ir(ppy)2Cl]2 to synthesize Ir1 (33) in dichloromethane and methanol as a solvent in 1:2 ratio and at reflux for 6 h to obtain a red-brown transparent solution. Then ammonium hexafluorophosphate was dropwise added and stirred for 2 h. A yellow product formed with 82% yield. For the Ir2 (34) complex, they used [Ir(bzq)2Cl]2·2H2O instead of [Ir(ppy)2Cl]2 and obtained the product in 76% yield. For Ir3 (35) they used [Ir(piq)2Cl]2·2H2O with a yield of 78%17 (Scheme 4).
Scheme 4. Synthetic Procedure of Complexes Ir1–Ir3 (33–35).
Nowadays, PDT has demonstrated successful results in treating a range of cancers. Among the most used organometallic compounds for bioimaging and anticancer drugs are photoluminescent cyclometalated iridium(III) complexes. In 2019, Sun and colleagues synthesized three Ir complexes. Although they exhibited significant resistance to the human cancer cell lines studied, these complexes remain a fascinating area of research. These three novel cyclometalated Ir(III) complexes with guanidinium ligands showed excellent toxic impacts on several cancer cells when they were irradiated at 425 nm. Here [1,10]-1-(4-(1H-imidazo[4,5-f]phenanthroline-2-yl) phenyl) guanidine hydrochloride was taken as a ligand. Along with 2 equiv of ligands, they used 1 equiv of [Ir(bzq)2Cl]2, [Ir(tpy)2Cl]2, and [Ir(ppy)2Cl]2, and they got complexes 36, 37, and 38, respectively: 36 = [Ir(bzq)2(L)](PF6)2 with the yield 63.2%, 37 = [Ir(tpy)2(L)](PF6)2 with the yield 72.6%, and 38 = [Ir(ppy)2(L)](PF6)2 with the yield of 71.0%16 (Figure 8).
Figure 8.
Chemical structures of complexes 36–38.
A recent publication by Mao and colleagues has revealed that mitochondrial DNA could be a promising area of focus in the fight against cancer this year.19 This discovery presents a sophisticated and compelling approach to treating cancer. Following the methodology outlined in a 2014 research paper by Sharma and his team,18 we developed six exceptional cyclometalated iridium(III) complexes, known as Ir1–Ir6 (39–44), with a variety of elongated, flat diamine ligands. These complexes have been thoroughly evaluated and have shown great potential as highly effective anticancer agents. For the synthesis of the complex, they took methyl 5-bromo-2-picolinate and (4-dimesitylboryl)-phenylboronic acid, and they got triarylboryl (Mes2PhB) substituted 2-picolinic acid. After that reaction, they proceeded with the iridium dimeric compound. The synthesis of ligands was conducted by Sharma and his research group, as described in their publication. This study details the synthesis of three ligands, which were then used to form six complexes. These 6 Ir complexes can bind to DNA strongly in vitro because each one of them has a diamine ligand with an expanded planar region in its structure18,19 (Figure 9).
Figure 9.
Chemical structures of 39–44.
There is growing interest in the use of synthetic anion transporters to disrupt intracellular pH balance for tumor treatment. However, the biological mechanisms of these transporters are still not fully understood. To address this, Chen et al. have artfully crafted two phosphorescent cyclometalated iridium(III) complexes, with 2-ppy serving as the cyclometalated ligand. 45 and 46 were produced by directly reacting Hpyim or 2,20-bi-imidazole with the dimeric iridium(III) precursor [Ir(ppy)2Cl]2 in CH3OH/CH2Cl2. To purify the complexes, they useed the chromatography method. 45 and 46 were more effective against cancer cells than cisplatin when tested in vitro. They can also accumulate in lysosomes, making them useful for imaging these organelles20 (Figure 10).
Figure 10.
Schematic drawing of the structures of 45 and 46.
Central hypoxia in solid tumors is a common problem that slows progress in cancer treatment and makes people more resistant to drugs. Even though it is hard to make activated theranostic drugs that target hypoxic cancer cells specifically, Li et al. have risen to the task and have already released five Ir complexes in 2019. Here, ligand 47a is 2-(anthracene-9,10-dione-2-yl)-(1-p-tolyl)-imidazo[4,5f][1,10]phenanthroline; ligand 47b is 2-(anthracene-9,10-dione-2-yl)-(4-tert-butylphenyl)-imidazo[4,5-f][1,10]phenanthroline; ligand 47c is 2-(anthracene-9,10-dione-2-yl)-(4-fluorophenyl)-imidazo[4,5-f][1,10]phenanthroline; ligand 47d is 2-(anthracene-9,10-dione-2-yl)-(4-methoxyphenyl)-imidazo[4,5-f][1,10]phenanthroline; and ligand 47e is 2-(anthracene-9,10-dione-2-yl)-(4-phenoxyphenyl)-imidazo[4,5-f][1,10]phenanthroline. These five complexes were synthesized according to the method mentioned in Scheme 13. The complexes work well to turn on yellow phosphorescence when there is not enough oxygen. This was used to find hypoxia in 3D multiple tumor spheroids. In addition, the complexes kill HepG2, A549, A549R, and HeLa cell types21 (Scheme 5).
Scheme 13. Synthetic Route of Ligands and Complexes.
Scheme 5. Chemical Structure of Hypoxia-Responsive Complexes.

In 2019, Mao and co-workers synthesized two iridium metal–NHC complexes. These complexes are mitochondria-targeting and photodynamic agents. They used two main ligands, LMe = 1,10-methylene-bis(3-methyl-1H-imidazol-3-ium) dichloride and LBu = 1,10-methylene-bis(3-butyl-1H-imidazole-3-ium) dichloride. To synthesize these complexes, they took both ligands one by one along with Ag2O and [Ir(dbpz)2]2Cl2 in CH2Cl2. Finally, they got complexes 49 = [Ir(dbpz)2(LMe)]Cl in 88.1% yield and 50 = [Ir(dbpz)2(LBu)]Cl in 90.3% yield22 (Figure 11).
Figure 11.
Structures of complexes 49 and 50.
Synthesis of a functional iridium(III) compound was performed by Ma et al. Ir-OXIME, a compound of iridium(III), was designed as a sensitive, rapid, and selective sensor for phosgene. Here they used oxime-modified bpy as a ligand, and for detection of phosgene Ir-OXIME was used. When phosgene was present, the phosphorescence was activated by the oxime’s quick conversion to the equivalent nitrile, creating a highly phosphorescent substance23 (Scheme 6).
Scheme 6. Schematic Representation of Phosphorescent Detection of Phosgene.
In the year 2020, Li et al. synthesized and characterized three phosphorescent cyclometalated iridium(III) complexes that are specific to mitochondria. For these complexes, 4,4′-bis(benzimidazolyl)-2,2′-bipyridine was used as a ligand. To synthesize the complexes, they used a mixture of Ir2(NC)4Cl2 and L (1:2 equiv) in CH3OH/CH2Cl2, and the mixture was heated until it started to bubble. After 4 h, the solution was cooled to room temperature, and a 6-fold excess of NH4PF6 was added. The mixture was stirred for another hour. The mixture was put through a filter, and the liquid that came out was evaporated until it was dry. The solid was dissolved in CH2Cl2 and cleaned by eluting it from a silica gel column with a 1:10 mixture of acetone and CH2Cl2. The compounds [Ir(thpy)2(L)]PF6 (55), [Ir(pq)2(L)]PF6 (54), and [Ir(ppy)2(L)]PF6 (53) were obtained in 86.1%, 81.3%, and 78.6% yield24 (Figure 12).
Figure 12.
Chemical structures of iridium(III) complexes 53–55.
Shifting to the next year, in 2021 Gu et al. created and synthesized a novel Ir(III) liposome as a possible therapeutic agent. This liposome has a functionalized iridium(III) complex. The produced liposomes were completely characterized by zeta potential, DLS, and the TEM analytical technique. For the synthetic route to this complex, they took a mixture of DQTT and [Ir(piq)2Cl]2 with 45 mL of dichloromethane, and methanol was stirred at 45 °C for 6 h under argon. After cooling, NH4PF6 was added drop by drop and stirred at rt for 2 h. They obtained a pure product with a 75% yield using neutral alumina25 (Figure 13).
Figure 13.
Structure of complex 56.
An article titled “Phosphorogenic dipyrrinato–iridium(III) complexes as photosensitizers for photodynamic therapy” was published in the same year, 2021, by Ortiz and his colleagues. In this study, the researchers synthesized a variety of iridium(III) complexes coordinated with two cyclometalated bisfluorophenylpyridine ligands. Ir(dipy)-57a, Ir(dipy)-57b, and Ir(dipy)-57c are all tertiary dipyrromethenes that have been functionalized with mesityl groups, chloroacetyl esters, and ammonium cations, respectively. The complete synthesis is described in Scheme 7 along with the complex structure26 (Scheme 7 and Figure 14).
Scheme 7. Synthesis of Ir(dipy)-57a, Ir(dipy)-57b, and Ir(dipy)-57c.
Figure 14.
Novel PSs based on the dipyrrinate–Ir(III) complex (Scheme 7).
A team led by Chen developed aptamer-cyclometalated iridium(III) complex conjugate (ApIrC) that serves as a highly targeted and effective anticancer agent.17 Their article titled “Design and synthesis of aptamer-cyclometalated iridium(III) complex conjugate targeting cancer cells” was published in 2022. Here they synthesized three types of ligands. For the synthesis of ligand-1 (65), (as shown in Scheme 8), they took 1,10-phenanthroline-5,6-dione with 4-formylbenzoic acid in the presence of ammonium acetate. For the synthesis of 66, they used EDCl with L1, and for the synthesis of ligand-3 (67), they used 6-aminohexanoic acid along with ligand-2 (66). For the complexation of Ir1–68, they performed a synthesis with 2 equiv of 67 and chloro-bridged iridium(III) dimers. Finally, ApIrC was obtained by adding aptamer AS1411 to the Ir2–69 complex17,27 (Scheme 8).
Scheme 8. Synthetic Route of ApIrC.
Recently, in the year 2022 Hao et al. synthesized three cyclometalated Ir complexes, and these complexes had remarkable cytotoxicity in the range of cancer cells. All three complexes were synthesized by direct reaction of their ligand FTTP in a mixture of dichloromethane and methanol. For 73, 74, and 75 they used [Ir(ppy)2Cl], [Ir(bzq)2Cl], or [Ir(piq)2Cl]2, respectively28 (Scheme 9).
Scheme 9. Synthetic Route of the Complexes.

In 2022, Yuan and co-workers synthesized the novel ligand (2-(1E,3E,5E,7E)-2,6-dimethyl-8-(2,6,6-trimethylcyclohexen-1-en-1-yl)octa-1,3,5,7-tetraene-1-yl)-1H-imidazo[4,5-f][1,10]phenanthroline) (DTOIP) and three extremely cytotoxic iridium(III) complexes.29 The complexes can permeate the cell and accumulate in the mitochondria at high concentrations. The synthesis of these complexes are shown in Scheme 19. Here, 78 = [Ir(ppy)2(DTOIP)](PF6), 79 = [Ir(piq)2(DTOIP)](PF6), and 80 = [Ir(bzq)2(DTOIP)](PF6)28,29 (Scheme 10).
Scheme 19. Synthetic Routes of Ligands and Complexes.
Scheme 10. Synthetic Route for the Ligands and Complexes.

In 2022, Wang et al. skillfully synthesized the ligand HMSPIP and its iridium(III) complexes, which have demonstrated remarkable efficacy in inducing cell death or apoptosis through four distinct pathways. Through in vitro toxicity assays, the team has discovered that 83 exhibits superior cellular toxicity against the HeLa cell line compared to 84. By using 1,10-phenanthroline-5,6-dione, they synthesized the HMSPIP ligand and its Ir complexes. Here 83 is [Ir(ppy)2(HMSPIP)]PF6 with a 68.6% yield, and 84 is [Ir(bzq)2(HMSPIP)]PF6 with 56.1% yield. The detailed synthesis is shown in Scheme 11.30
Scheme 11. Synthetic Route for Ligands and Complexes.

Zhao et al. developed new iridium(III)–NBDHEX conjugates in 2023.32 These complexes were developed to enhance the biological and pharmacological effects of cyclometalated iridium(III)-based anticancer medicines. In Scheme 12 we describe two syntheses: the synthesis of complexes 85 and 87 and the synthesis of 86 and 88. 85 and 87 were synthesized by combining cyclometalated ligands with iridium(III)-GST inhibitor conjugates based on Figure 15, and the final compound chemistry was confirmed by NMR. As controls, two unfunctionalized iridium complexes, 86 and 88, were made according to Scheme 12 and characterized by 1H and 13C NMR spectra and HRMS (ESI) analysis.31,32
Scheme 12. Synthesis of Complexes 85–88.
Figure 15.
Chemical structures of the Ir(III) complexes (see Scheme 12).31,32
Two novel Ir polypyridyl compounds were synthesized in 2023 by Liu and his colleagues. Here the Ir1 (95) complex is [Ir(bzq)2(DIPH)](PF6), and the Ir2 (97) complex is [Ir(piq)2(DIPH)](PF6). For the synthesis of DIPH they used a mixture of 1,10-phenanthroline-5,6-dione, 2,5-dibromo-1,4-benzenedicarboxaldehyde, and NH4Ac, and the mixture was heated after being dissolved in glacial acetic acid. For the synthesis of 95 they used cis-[Ir(bzq)2Cl]2 with DIPH, and for synthesis of 97 they took cis-[Ir(piq)2Cl]2 with DIPH. The yield of both of complexes was 77% (95) and 79% (97)31 (Scheme 13).
2.1.1. N∧N∧N Type of Ligand
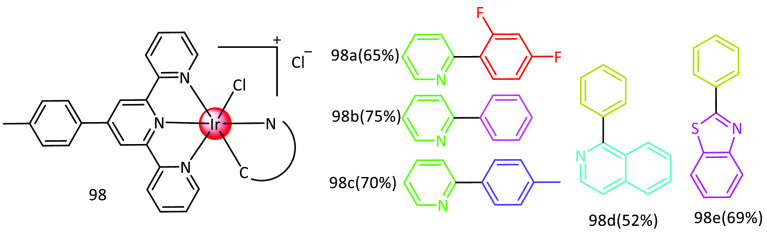
In 2016, Huang et al. published a paper in which they describe the production of five iridium(III) complexes based on real-time monitoring of mitochondrial dynamic remodeling using two-photon phosphorescent probes. 2-Phenylpyridine, 2-(2,4-difluorophenyl) pyridine, 1-phenylpyrazole, 2-phenylbenzothiazole, 1-phenylisoquinoline, and IrCl3·4H2O are included in the C∧N ligand during the the preparation of [Ir(ttpy)(CN)Cl](PF6). A clear solution was created by stirring a mixture of [Ir(ttpy)Cl3] and the corresponding cyclometalated ligand in glycol under an Arat temperature of 180 °C overnight. The solution was then cooled and poured into another substance. A 50 mL solution of NH4PF6 was used to create precipitates, which were then separated using filtration and washed three times with water and diethyl ether. The yield of complexes 98a–98e was 52% to 75%. Here 98a = [Ir(ttpy)(dfppy)Cl](PF6), 98b = [Ir(ttpy)(ppyCH3)Cl](PF6), 98c = Ir(ttpy)(ppz)Cl](PF6), 98d = [Ir(ttpy)(pbt)Cl](PF6), and 98e = [Ir(ttpy)(pbt)Cl](PF6). The complexes displayed profound absorption of high energy within the range of 250–350 nm, accompanied by subtle absorption between 350 and 480 nm33 (Figure 16).
Figure 16.
Chemical structures of 98a–98e.
Three iridium compounds were synthesized (in 2015) and tested for their antiproliferative effects by Liang and colleagues. The deformed octahedral geometry of six coordinates was used by iridium(III) in the center of these complexes. Three iridium(III) complexes, [Ir(4-MeO-Phtpy)Cl3], [Ir(2-MeO-Phtpy)Cl3], and [Ir(3-MeO-Phtpy)Cl3], with 4′-(4-methoxyphenyl)-2,2′,6′,2′-terpyridine (4-MeO-Phtpy), 4′-(2-methoxyphenyl)-2,2′:6′,2″-terpyridine (2-MeO-Phtpy), and 4′-(3-methoxyphenyl)-2,2′:6′,2″-terpyridine (3-MeO-Phtpy) as ligands, respectively, were synthesized and characterized. IrCl3 was incubated with each of the three terpyridine ligands in CH3OH and DMSO at 80 °C for 48 h to produce the appropriate Ir(III) metal complexes 102a–102c. The compounds tested specifically killed tumor cells, and experiments with Hep-G2 tumor cells showed that the compounds acted as a telomerase inhibitor by targeting the c-myc promoter elements34 (Scheme 14).
Scheme 14. Synthetic Routes for Three Ligands and the Ir Complexes 102a–102c.
Zheng et al. successfully crafted four exquisite mixed-ligand phosphorescent iridium(III) complexes in 2017. In this study, they used two types of ligands: 2,6-bis(2-benzimidazolyl)pyridine, bpy = 2,2′-bipyridine, as one ligand and 2,6-bis(1-methyl-benzimidazol-2-yl)pyridine, ppy = 2-phenylpyridine, as the second ligand. The complete synthesis method is shown in Scheme 15. Here Complex 107a = [Ir(L1)(bpy)Cl](PF6)2 (yield 65%), 107b = [Ir(L1)(ppy)Cl] (PF6) (yield 78%), 107c = [Ir(L2)(bpy)Cl](PF6)2 (yield 75%), and 107d = [Ir(L2)(ppy)Cl](PF6) (yield 78%)35 (Scheme 15).
Scheme 15. Structures and Synthetic Routes of Ir Complexes.
In 2019, Bo Yuan et al. synthesized iridium(III) complexes (111–113), and those complexes are coordinated to a range of bidentate ligands with an increasingly expansive conjugated area.16 For the synthesis of these complexes, [Ir(tpy)(bhqd)Cl]PF6 was used as ligand, where tpy = 2,2′:6′,2″-terpyridine and bhqd = benzo[h]quinoline-5,6-dione.
For the synthesis of Ir complex 110 they used a mixture of ethylene diamine and ligand in 10 mL of methanol, followed by reflux at 65 °C for 5 h, and the yield was 42%. For the synthesis of complex 111, they followed the same procedure, but instead of ethylene diamine they used o-phenylenediamine. They yield was 48%. For the synthesis of 112, they used 3-diaminonaphthalene instead of ethylene diamine, and the yield was 35%. These complexes are coordinated bidentate ligands, and they are specially accumulated for the endoplasmic reticulum36 (Scheme 16).
Scheme 16. Chemical Structures and Synthesis Route of 111–113.

2.1.2. C∧C Type of Ligand
In 2012, José Ruiz et al. synthesized a new steroidal complex [Ir(η5-C5Me5)(LEV-ppy)Cl]. They were able to get the novel complex [Ir(5-C5Me5)-(LEV-ppy)Cl] by utilizing [M(5-C5Me5)Cl2]2 in conjunction with 2 equiv of LEV-ppy and stirring the mixture at room temperature. This complex is comparatively more active than cis-platin in the human breast cancer cell line T47D and has a deficient resistance factor against an A2780 cell line37 (Scheme 17).
Scheme 17. Reaction to the Complex Preparation of 114.
In 2014, a team of esteemed researchers led by Zong-Wan Mao pioneered the creation of three exquisite cyclometalated iridium(III) complexes, complemented by the addition of bis-NHC ligands.38 These extraordinary compounds were ingeniously designed to serve as both theranostic and photodynamic agents with a specific affinity for the mitochondria. The meticulous process of crafting these ligands involved subjecting 60 mmol of 1-alkyl-1H-imidazole and 20 mmol of CH2Cl2 to a carefully controlled temperature of 85 °C for a duration of 24 h. This elegant synthesis showcases the team’s unwavering commitment to pushing the boundaries of scientific innovation and holds immense promise for future advancements in this field. After the solution was cooled to room temperature, the solvent was removed under air pressure by evaporation. It was then redissolved in CH3OH. Two further hours were spent stirring in THF. This suspension was filtered. After the sample was vacuum-dried, the resultant substance was purified with the aid of THF, a potent solvent, to achieve a refined and pristine final product. The yield of L1, L2, and L3 was 42.6%, 44.8%, and 44.8%. To synthesize the complex strategy for making complexes in general, CH2Cl2, Ag2O, ligand, and [Ir(ppy)2]2Cl2 were boiled in a sealed reaction container overnight at 95 °C. The yields of complex 1, complex 2, and complex 3 were 84.7%, 96.2%, and 96.9%. These complexes are more effective than cisplatin in inhibiting the growth of several cancer cells, including cisplatin-resistant A549 cells. Here, complex 115a = [Ir(ppy)2(L1)]Cl and L1 is 1,10-methylenebis(3-methyl-1H-imidazol-3-ium) dichloride; complex 115b = [Ir(ppy)2(L2)]Cl and L2 is 1,10-methylenebis(3-ethyl-1H-imidazol-3-ium) dichloride; and complex 115c = [Ir(ppy)2(L2)]Cl and L3 is 1,10-methylenebis(3-butyl-1H-imidazol-3-ium) dichloride38 (Figure 17).
Figure 17.
Ir complex structures.
In 2017, Mao and his team accomplished a remarkable feat by synthesizing numerous N-heterocyclic complexes that incorporate carbene. These Ir complexes contained nitrogen heterocyclic ligand L, [Ir(pq)2]2Cl2 (0.1 mmol), and Ag2O (0.21 mmol) in CH2Cl2 (10 mL) at 95 °C and were heated overnight in the sealed reaction container. The mixture was filtrated after cooling and washing with CH2Cl2. Here 116 is [Ir(pq)2(LMe)]Cl with 84.7% yield; 117 is [Ir(thpy)2(LMe)]Cl with 90.3% yield; 118 is [Ir(pq)2(LBu)]Cl with 82.6% yield; and 119 is [Ir(thpy)2(LBu)]Cl with 76.4% yield. When tested against a panel of cancer cell lines, including cisplatin-resistant A549 cells, these complexes demonstrated antiproliferative effects greater than cisplatin. The complexes’ cytotoxicity is proportional to their accessory ligands’ lipophilicity and cellular absorption efficiencies39 (Figure 18).
Figure 18.
Chemical structures of all complexes 116–119.
2.1.3. C∧N Type of Ligand
Filippo Monti and group made charged biscyclometalated iridium(III) complexes in 2013. These complexes have been reported in previous studies. The synthesis procedure is mentioned in Scheme 18. They used silver(I) oxide, an aqueous KPF6 solution, and 1,2-dichloroethane, which were heated and refluxed overnight at 95 °C. Both series of complexes have one common ligand (C∧C) and four different bidentate ancillary ligands (N∧C). The photophysical characteristics of these complexes were analyzed in different states and environments. Although the two series have similar decay rates, the N∧C complexes have significantly higher nonradiative decay rates in solution compared to the C∧C analogs, indicating the existence of other higher-energy nonradiative paths40 (Scheme 18).
Scheme 18. General Synthetic Scheme for Charged Complexes.
In 2018, Yang et al. accomplished the comprehensive characterization and synthesis of a set of luminous cyclometalated Ir(III) anticancer compounds, belonging to the category of [Ir(ppy)2(CN)]PF6. In luminous cyclometalated Ir(III), [Ir(ppy)2(C–N)]PF6 (where C–N = imine-NHC ligands with different kinds of substituents and ppy is 2-phenyl pyridine) was produced. The detailed method is shown below in Scheme 19, and the results showed that the order of in vitro anticancer activity for the tested complexes was Ir5 > Ir4 > Ir3 > Ir2 > Ir1 > cisplatin.41
2.1.4. N/O∧N/O Type of Ligand
In the same year, Lucas et al. successfully created a series of Ir(III) complexes utilizing sophisticated bidentate ligands, namely, (N, O), (N, N), and (O, O), to develop potential anticancer medications. Their groundbreaking research was soon followed by another significant study by Sadler et al. In this subsequent investigation, they unveiled the remarkable properties of half-sandwich organometallic Ir(III) complexes, characterized by the overall structure [(5Cp)Ir(XY)Cl]0/+, wherein Cp represents an enhanced version of either Cp or Cp*. Their groundbreaking research was soon followed by another significant study conducted by Sadler et al. In this subsequent investigation, they unveiled the remarkable properties of half-sandwich organometallic Ir(III) complexes, characterized by the overall structure [(5Cp)Ir(XY)Cl]0/+, wherein Cp represents either an enhanced version of Cp or Cp*. The addition of phenyl to the cyclopentadienyl ligand improves its potency against A2780 human ovarian cancer cells. They have made four different iridium arene complexes, each with a different coordinating ligand (N, N), (O, O), or (N, O). The steps involved in the synthesis are outlined in Scheme 20. Both were targets of piano-stool complexes. The most potent complexes (N, O) showed action against HT-29 and MCF 7 cell lines equivalent to cisplatin.42
Scheme 20. Synthesis of all 4 Cp* Complexes.
2.1.5. P∧P Type of Ligand
In 2019, Hao at el. reported mitochondrial viscosity monitored using anticancer phosphorescent iridium(III) complexes and two-photon lifetime imaging. For the synthesis of the complexes, the bisdiphenyl phosphine ligand (2 equiv) and the corresponding cyclometalated iridium(III) dimer were heated in a mixture of CH2Cl2 and CH3OH for 6 h for complexes 135–137 or in dimethylformamide for 72 h for the formation of complexes 138–140. [Ir(ppy)4Cl2] and [Ir(ppy-CHO)4Cl2] were used as L1 and L2. Here 135 = [Ir(ppy)2L1](PF6) in 78% yield; 136 = [Ir(ppy)2L2](PF6) in 64% yield; 137 = [Ir(ppy)2L3](PF6) in 72% yield, 138 = [Ir(ppy-CHO)2L1](PF6) in 75% yield; 139 = [Ir(ppy-CHO)2L2](PF6) in 61% yield; and 140 = [Ir(ppy-CHO)2L3](PF6) with 67% yield43 (Figure 19).
Figure 19.

Chemical structures of 135–140.
2.1.6. S∧S Type
As we look to the year 2022, Wu et al. designed four (143–146) neutral cyclometalated Ir(III) complexes which contain dithioformic acid. Neutral iridium(III) dithioformic acid was created specifically for human nonsmall cell lung cancer. Synthesis of ([(ppy)2Ir(SS)]) was carried out in CH3OH at ambient temperature (A.T.) by reacting [(ppy)2IrCl]2 (dimer) with Di thiocarboxylic acid (2 equiv) derivatives. The ligand structure and a detailed synthesis are shown below in Scheme 21 and Figure 20.44
Scheme 21. Synthesis Process of Cyclometalated Ir(III) Dithioformic Acid Complexes.
Figure 20.
Structures of designed cyclometalated Ir(III) dithioformic acid complexes ([(ppy)2Ir(S∧S)]) and ligands used in Scheme 16.
2.2. Organometallic Half-Sandwich Ir(III) Complex
Half-sandwich iridium complexes have emerged as a fascinating and vibrant area of research in the field of coordination chemistry. These compounds, characterized by a central iridium atom or ion coordinated by a single cyclic ligand, exhibit unique structural, electronic, and reactivity properties that have captivated the interest of chemists worldwide. These compounds may also be classified based on their ligand density.45
Half-sandwich iridium complexes were first discovered and studied in the middle of the 20th century, when scientists realized that transition metal complexes might have a wide range of interesting features. Iridium is used as the main metal atom in these complexes because of its distinctive electronic structure, which enables a variety of oxidation states and ligand-binding options. These complexes get specific qualities through the usage of cyclic ligands such as cyclopentadienyl (Cp) or cyclooctadiene (cod), leading to a wide range of structures and features. Structurally, half-sandwich iridium complexes are characterized by the coordination of the cyclic ligand to the central iridium atom, forming a “pincer” or “half-sandwich” motif. The coordination environment around the metal center can vary depending on the ligand used and the geometry of the complex. The ligand can bind in different modes, such as η∧5 (pentahapto) or η∧2 (dihapto), resulting in different bonding arrangements and electronic properties. These structural aspects play a crucial role in dictating the reactivity and properties of the complex. Over the years, scientists have developed a wide variety of approaches for synthesizing half-sandwich iridium complexes. The controlled reactivity of iridium precursors with the appropriate ligands is a popular method. The stereochemistry, regiochemistry, and selectivity of the resultant complex can be modified by adjusting the ligand and reaction conditions. As a means of fine-tuning these systems’ characteristics, here we classify the iridium complex II as a half-sandwich complex and further divide it into subclasses based on the dative nature of its ligands.6,46
The remarkable reactivity of half-sandwich iridium complexes has found applications in various scientific disciplines. These complexes have been employed in organic synthesis as catalysts for various transformations, including C–H activation and C–C bond formation. The development of organometallic half-sandwich compounds as anticancer drugs and the study of bio-organometallic chemistry are both areas of rising interest.47
2.2.1. C∧N Type of Ligand
Liu et al. began their research in 2011, when they synthesized and designed a [(η5-Cpx)Ir (XY)Cl]0/+ type of a half-sandwich iridium complex that exhibited anticancer properties. This is the first reported organic “half-sandwich” iridium(III) complex. In this complex, Cpx and Cp* are either phenyl Cpxph or biphenyl Cpxbiph derivatives, and XY is an N,N-chelating ligand, 1,10-phenanthroline, 2,20-ethylenediamine, bipyridine, or N,O-chelating picolinate. They synthesized 14 complexes by using 2,2′-bipyridine, 1,10-phenanthroline, 2-picolinate, or ethylenediamine with the appropriate dimer [(η5-Cpx)IrCl2]2 in methanol. The detailed structure is given in Figure 21.48
Figure 21.

Iridium cyclopentadienyl complexes studied.
In 2017, Zhang and colleagues successfully synthesized a Schiff base Ir(III) half-sandwich complex and investigated an in vitro investigation against the K562 cell line. Here ketone 161 was treated with aniline 162, which produced ketimine 163. Then, ketimine was reacted with [Cp*IrCl2]2 in the presence of NaOAc in dichloromethane for 24 h49 (Scheme 22).
Scheme 22. Synthetic Route to Ir(III) Complexes.

In 2018, Li et al. used confocal microscopy to demonstrate that it is possible to easily image half-sandwich iridium anticancer complexes inside cells. Confocal microscopy can provide an understanding of cellular distribution, uptake, and interaction with biological targets that is challenging to acquire using other approaches. The compounds dimer 1 and dimer 2 were created by heating IrCl3 and a cyclopentadienyl ligand using microwave technology. Dimer 1 has two iridium atoms connected by a dichloro bridge and is made with an η5Cp* ligand. Dimer 2 also has two iridium atoms connected by a dichloro bridge and is made with an η5-Cpxbiph ligand. Six Ir complexes (1–6) were created by combining a N∧N-chelating ligand called (triphenylmethyl)(pyridin-2-ylmethylene) amine with binuclear iridium precursor dimers in methanol at room temperature. Here dimer 1 = [(η5-Cp*) IrCl2]2, and dimer 2 = [(η5-Cpxbiph)IrCl2]250 (Scheme 23).
Scheme 23. Synthesis of the N∧N-Chelating Ligand and Complex.
In 2018, Yang et al. discovered novel half-sandwich Ir(III) complexes that are characterized by their versatile imine-NHC ligands.51 These complexes exhibit luminescence properties and display potent anticancer and antibacterial activity. Their synthesis and characterization are a testament to the group’s scientific acumen, and these findings represent a significant contribution to the field of chemistry. This particular framework is distinguished from other complexes, as it offers the ability to adjust complex cytotoxicity through the alteration of substituents in four distinct locations. A remarkable collection of new half-sandwich iridium(III) complexes, identified by the general formula [(5-Cpx)Ir (CN)Cl], have been produced and thoroughly examined. The processes for creating imine-NHC ligands L1–L11 and half-sandwich iridium(III) complexes 174–185 are depicted in the following diagram. Through a successful coupling reaction of imidoyl chlorides and imidazole with varying substituents, imidazolium salts were synthesized in good yields. Additionally, the classical transmetalation method was utilized to synthesize high-yield (75–97%) half-sandwich iridium(III) complexes 174–185. These sophisticated and efficient processes highlight the potential for groundbreaking advancements in the field51,52 (Scheme 24).
Scheme 24. Synthetic Routes for Ligands and Complexes.

2.2.2. C∧C Type of Ligand
2017 was also the year when Wang et al. synthesized the half-sandwich Ir complex of N-heterocyclic carbene anticancer complexes. The article describes the synthesis and characterization of anticancer drugs called pseudo-octahedral pentamethylcyclopentadienyl Ir(III) complexes. These complexes contain different N-heterocyclic carbene ligands and are similar to those of a previously reported complex. Here they use a [(η5-Cpx)Ir(C∧C) Cl]PF6 type of complex in Cpx = pentamethylcyclopentadienyl (Cp*), biphenyl (Cpxbiph = C5Me4C6H4C6H5), or phenyl (Cpxph = C5Me4C6H5), and the C∧C-chelating ligands are different NHC ligands. To create a half-sandwich complex, a mixture of N-ethyl or N-aryl imidazole, dichloromethane, and PEG 400 was heated in an autoclave for 24 h at 110 °C. The resulting white precipitate was washed three times with THF using a sonicator to produce a white, hygroscopic powder. To create binuclear dicarbene silver(I) complexes (2d and 4d), a round-bottomed flask was used. 189a or 189c and deionized H2O were added to the flask. Then, an excess of Ag2O was added, and the solution was stirred for 5 h at room temperature. After the solution was filtered, NH4PF6 was added, which caused the complexes to precipitate as a white powder. The powder was filtered and dried at 50 °C overnight. The compounds dimer 147, dimer 148, and dimer 149, which contain iridium and different carbon rings, were made using a microwave synthesizer. This method resulted in a 15% increase in yield compared to the traditional heating reflux method53 (Scheme 25).
Scheme 25. Structure of the Dimers and Ligands and Synthesis of Complexes.
In 2018, Liu and colleagues synthesized Ir half-sandwich complexes. These complexes included two different types of aminopyridine chelating ligands. The complexes that were conceived and manufactured displayed greater anticancer efficacy against A549 cancer cells in comparison to clinically utilized cisplatin. Six Ir(III) half-sandwich complexes [(5-Cpx)Ir(N∧N)Cl]PF6 were created and made with two different aminopyridine chelating ligands. A mixture of a compound called chloride-bridged dimer [(η5-Cpx)IrCl2]2 and an aminopyridine ligand was heated under reflux in MeOH for 10 h in a N2 atmosphere, filtered, and then slowly reduced in volume. NH4PF6 was added, and the solution was left to stand at room temperature, resulting in the formation of microcrystals. These were collected, washed, and recrystallized from CH2Cl2/diethyl ether54 (Scheme 26 and Figure 22).
Scheme 26. Synthesis of Ir(III) Complexes.
Figure 22.

Cyclopentadienyl iridium aminopyrinedine complexes 191–196 (Scheme 26).
Han et al. created and analyzed a series of Ir(III) N-heterocyclic carbene complexes with the potential to combat cancer in 2018. The team examined these compounds closely and found that they could be used in new ways to fight cancer. The half-sandwich Ir(III) NHC complexes of the [(Cp*)IrC Cl] type were expertly made in DCM at room temperature using silver oxide (Ag2O) as a catalyst. New iridium(III) NHC complexes were created with high yields using NHC chelating ligands derived from the reaction of imidazole and iodine hydrocarbons. The quality of the intermediates and final complexes was confirmed through rigorous testing using techniques such as 1H NMR, MS, and elemental analysis. A group of antitumor complexes containing NHC chelating ligands with phenyl rings at different positions have been made and studied. These complexes are not affected by moisture and can dissolve in organic solvents like dichloromethane, chloroform, and dimethyl sulfoxide but only partially dissolve in methanol and cannot dissolve in hexane or petroleum ether55 (Scheme 27).
Scheme 27. Selected NHC Chelating Ligands (198a–198d) and the Process of Ir(III) NHC Complexes.
2.2.3. N∧O/S Type of Ligand
Liu and team synthesized six fluorescent half-sandwich Ir(III) complexes in the year 2020.56 The general formula of these complexes is [(η5-Cp*)Ir(ON)Cl]), and these are the coumarin-salicylaldehyde Schiff base compounds. For the synthesis of the Schiff base ligand (210a-L1), they used 7-amino-4-methyl coumarin, anhydrous methanol, and salicylaldehyde along with a drop of formic acid that was added after reflux for 12 h. In the end, they washed diethyl ether and cold methanol. The ligand was synthesized using salicylaldehyde and 7-amino-4-trifluoromethylcoumarin followed by the same method which is used for ligand 1 (210a).
Moving to the synthesis of six complexes, they used Ir(III) dimer solution along with sodium acetate and ligands and then stirred it overnight at ambient temperature. The addition of a coumarin unit to six iridium complexes resulted in an increase in their antitumor activity, with the best performing compound being almost two times as effective as the clinical drug cisplatin56 (Scheme 28).
Scheme 28. Structure of the Iridium Complex and Synthetic Procedure of the Half-Sandwich Complex.
The year 2021 saw the publication of a study conducted by Sadler and his team, which revealed the potential of iridium(III) complexes with a half-sandwich structure as effective organometallic anticancer agents.58 As part of their research, the team created four fluorescent iridium(III) TSC anticancer complexes that were modified by using triphenylamine. Four new triphenylamine-modified half-sandwich iridium(III) TSC complexes were created that had an unusual dimeric structure, which was caused by the “enol” orientation of the TSC ligands. These complexes were different from typical half-sandwich complexes due to the way the TSC Schiff base ligands were configured. They have successfully synthesized N∧S pro-ligands (214a–214d) using a combination of 4-formyl-4,4′-dimethylaniline, thiosemicarbazide, methanol, and a small amount of formic acid as a catalyst. The mixture was carefully refluxed for 6 h in a flask and monitored using thin-layer chromatography. Following vacuum distillation, a yellow coarse product was obtained and purified through column chromatography, resulting in the creation of 214a. Compounds 214b–214d were created using the same method as for 214a, by combining 4-formyl-4,4′-dimethylaniline with 4-methyl thiosemicarbazide or 4-phenyl thiosemicarbazide or 4-benzyl thiosemicarbazide. Compound 214b and 214c were obtained in 92% yield, and for 214d the yield was 88%. Through the use of fluorescence properties, research has discovered that complexes can enter cancerous cells in a way that requires energy, gather in lysosomes, and cause harm to the integrity of the lysosome57 (Scheme 29).
Scheme 29. Synthetic Process of TSC Ligands and Target Complexes.

2.2.4. N∧N or N Type of Ligand
In the year 2021, Chellan et al. successfully synthesized a new compound called a dipyridyl ester derivative, which was incorporated into complexes with a wide range of Ir(III) and sandwich-shaped atoms. This process resulted in the creation of six cationic complexes, labeled 1–6, each with different cyclopentadienyl ligand substituents. The reaction of DHA with 4-methyl-4′-carboxy-2,2′-bipyridine resulted in the production of a novel ester derivative that was designated as 223. Within the scope of this research project, six novel classes of organometallic half-sandwich chlorido Ir(III) complexes were designed, synthesized, and investigated. These complexes contain different types of cyclopentadienyl groups and a bipyridyl group of ligand 223 that is chelated with nitrogen. In order to synthesize Ir(III) complexes, researchers utilized bipyridylartemisinyl complexes. To begin the process, 223 was dissolved in methanol, and an iridium dimer solution was added to the mixture in DCM. The reaction solution was then stirred for 16 h at room temperature. After a period of stirring, ammonium hexafluorophosphate was added to the mixture and allowed to react for an additional hour. Following this step, the solvent was evaporated, which resulted in the formation of an orange-yellow residue. This residue was next dissolved in acetone and filtered in order to eliminate any inorganic salts that were insoluble. The volume of the liquid that was produced as a consequence was brought down to around 3 mL in volume. After that, the product was isolated by a procedure that used diethyl ether, which was added to the solution in order to remove it from the solution. After that, the product was filtered through a vacuum, and the product that had been made was dried after being rinsed with diethyl ether58 (Scheme 30).
Scheme 30. Synthesis of Ligand (223) and complexes 226–228.

In 2023, Wang and colleagues created ten new pyridine complexes using fluorescent half-sandwich iridium(III) and triphenylamine modifications. These complexes, with the general formula [(η5-Cpx)Ir(L)Cl2], displayed potential in fighting cell proliferation by inhibiting the migration of A549 cells. Ir6 was shown to be the most effective of these 10, and it also displayed remarkable fluorescence. TPA-modified pyridine proligands (229–233) were coupled with either dimer 147 or dimer 148, while the reaction was carried out at room temperature, which are made up of [(η5-Cp*)IrCl2]2 or [(η5-Cpxph)IrCl2]2, respectively (as shown in Scheme 31). The Suzuki reaction between TPA boric acid and 4-bromopyridine produced pyridine pro-ligands 229–230. Using the Wittig reaction and condensation, molecules 231–233 were synthesized from 4-formylpyridine and either 4-methyl TPA phosphide reagent or 4-amino-TPA. NMR spectroscopy, HRMS, and elemental analysis were used to learn more about the composition and structure of these complexes and pyridine pro-ligands. MeOH, ligands, and 24 h of reflux are used to produce the complexes59 (Scheme 31).
Scheme 31. Design and Synthesis of Complexes.
2.2.5. P∧P Type of Ligand
In 2023, Liu et al. conducted research on the potential use of half-sandwich- and ferrocene-containing organometallic complexes in the field of anticancer treatment. The study found that these complexes were more effective at blocking cell migration and halting the growth of A549 cell lines compared to cisplatin. To prepare the complexes, dimers 1–3 and dimer 4 were ground with two dppf pro-ligands in CH3OH at 65 °C, resulting in complexes 249–252. The complexes were extensively characterized using various techniques such as NMR, MS, and elemental analysis60 (Scheme 32).
Scheme 32. Synthesis Design of Complexes.
3. Conclusions
In summary, recent progress in the synthesis of Ir(III) complexes has shown encouraging outcomes in the field of cancer treatment. A comprehensive compilation of 51 methodologies for the synthesis of iridium(III) has been presented in this review. Additionally, it highlights the adaptability of these frames in creating new classes of molecules from diverse moieties and synthetic approaches. In addition, studies have revealed that some iridium(III) complexes are not toxic to healthy cells but are very dangerous to cancer cells. This allows cancer cells to absorb the complexes selectively while avoiding their effects on healthy cells. These Ir(III) complexes can be categorized into cyclometallic iridium compounds and half-sandwich iridium(III) compounds. Cyclometallic structures of iridium compounds are particularly effective against tumors and exhibit optical properties superior to those with half-sandwich structures. From a chemical standpoint, the N∧N ligand for cyclometalated complexes and the C∧N ligand for half-sandwich complexes exhibit a high degree of modifiability and synthetic feasibility, enabling the creation of a diverse array of molecules with distinct structural characteristics. The aforementioned qualities make these compounds appealing as fundamental constituents for advancing medicines targeting specific molecules. This finding has the potential for developing iridium-based reagents for phototherapy. The review will help synthetic chemists understand the potential of this reaction and its synthetic characteristics, enabling them to employ these data in their study.
Acknowledgments
The authors would like to give a very sincere thanks to VIT Vellore for research fellowships, respectively. The authors are also thankful to VIT Vellore for financial support in the form of a seed grant.
Glossary
ABBREVIATION
- LEV-ppy
17-[2-phenylpyridyl-4-ethynyl]-19-nortestosterone
- PDT
photodynamic therapy
- HDAC
histone deacetylases
- THF
tetrahydrofuran
- NHC
N-heterocyclic carbene
- DCM
dichloromethane
- THPDP
11-(6,7,8,9-tetrahydrophenazin-2-yl) dipyrido[3,2-a:2′,3′-c] phenazine
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- BDPIP
(2-(1-benzo[d]dioxol-5-yl)propan-2-yl)-1H-imidazo[4,5-f][1,10]phenanthrene
- bzq
benzo[h]quinolone
- adppz
7-aminodipyrido[3,2-a:2′,3′-c] phenazine
- ppy
phenylpyridine
- byp
bipyridine
- TEM
transmission electron microscopy
- DLS
dynamic light scattering
- AT
ambident temp
- EDCl
N-(3-(dimethylamino)propyl)-N′-ethyl carbodiimide hydrochloride
- ApIrC
aptamer-cyclometalated iridium(III) complex conjugate
- FTTP
2-(3-fluoronaphthalen-2-yloxy)-1,4,8,9-tetraazatriphenylene
- piq
1-phenylisoquinoline
- HMSPIP
2-(4-(methylsulfonyl) phenyl)-1H-imidazo[4,5-f][1,10]phenanthroline
- DIPH
4-(2,5-dibromo-4-(1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)-4-hydroxybutan-2-one)
- piq
deprotonated 1-phenylisoquinoline
- TSC
thiosemicarbazone
- rt
room temperature
The authors declare no competing financial interest.
References
- Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer (accessed 2023-05-06).
- Yang T.; Zhu M.; Jiang M.; Yang F.; Zhang Z.. Current Status of Iridium-Based Complexes against Lung Cancer. Frontiers in Pharmacology; Frontiers Media S.A., September 23, 2022. 10.3389/fphar.2022.1025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oun R.; Moussa Y. E.; Wheate N. J.. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Transactions; Royal Society of Chemistry, 2018; pp 6645–6653. 10.1039/c8dt00838h. [DOI] [PubMed] [Google Scholar]
- Lee S.; Han W. S.. Cyclometalated Ir(Iii) Complexes towards Blue-Emissive Dopant for Organic Light-Emitting Diodes: Fundamentals of Photophysics and Designing Strategies. Inorganic Chemistry Frontiers; Royal Society of Chemistry, June 21, 2020; pp 2396–2422. 10.1039/d0qi00001a. [DOI] [Google Scholar]
- Kralj J.; Bolje A.; Polančec D. S.; Steiner I.; Gržan T.; Tupek A.; Stojanović N.; Hohloch S.; Urankar D.; Osmak M.; Sarkar B.; Brozovic A.; Košmrlj J. Half-Sandwich Ir(III) and Os(II) Complexes of Pyridyl-Mesoionic Carbenes as Potential Anticancer Agents. Organometallics 2019, 38 (21), 4082–4092. 10.1021/acs.organomet.9b00327. [DOI] [Google Scholar]
- Longato B.; Riello L.; Bandoli G.; Pilloni G. Iridium(III, 0, and – I) Complexes Stabilized by 1,1‘-Bis(Diphenylphosphino)Ferrocene (Dppf): Synthesis and Characterization. Crystal Structures of [Na(THF) 5][Ir(Dppf) 2]·THF and [Ir(Dppf) 2]. Inorg. Chem. 1999, 38 (12), 2818–2823. 10.1021/ic981166s. [DOI] [PubMed] [Google Scholar]
- Ye R. R.; Tan C. P.; He L.; Chen M. H.; Ji L. N.; Mao Z. W. Cyclometalated Ir(Iii) Complexes as Targeted Theranostic Anticancer Therapeutics: Combining Hdac Inhibition with Photodynamic Therapy. Chem. Commun. 2014, 50 (75), 10945–10948. 10.1039/C4CC05215C. [DOI] [PubMed] [Google Scholar]
- He L.; Li Y.; Tan C. P.; Ye R. R.; Chen M. H.; Cao J. J.; Ji L. N.; Mao Z. W. Cyclometalated Iridium(III) Complexes as Lysosome-Targeted Photodynamic Anticancer and Real-Time Tracking Agents. Chem. Sci. 2015, 6 (10), 5409–5418. 10.1039/C5SC01955A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K.; Huang H.; Liu B.; Liu Y.; Zhang P.; Chen Y.; Ji L.; Chao H. Mitochondria-Specific Imaging and Tracking in Living Cells with Two-Photon Phosphorescent Iridium(III) Complexes. J. Mater. Chem. B 2015, 3 (32), 6690–6697. 10.1039/C5TB01091H. [DOI] [PubMed] [Google Scholar]
- Cao J. J.; Tan C. P.; Chen M. H.; Wu N.; Yao D. Y.; Liu X. G.; Ji L. N.; Mao Z. W. Targeting Cancer Cell Metabolism with Mitochondria-Immobilized Phosphorescent Cyclometalated Iridium(Iii) Complexes. Chem. Sci. 2017, 8 (1), 631–640. 10.1039/C6SC02901A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.; Wan D.; Wang Y. J.; Yi Q. Y.; Guo B. H.; Liu Y. J. An Iridium (III) Complex as Potent Anticancer Agent Induces Apoptosis and Autophagy in B16 cells through Inhibition of the AKT/MTOR Pathway. Eur. J. Med. Chem. 2018, 145, 302–314. 10.1016/j.ejmech.2017.12.087. [DOI] [PubMed] [Google Scholar]
- Ouyang M.; Zeng L.; Huang H.; Jin C.; Liu J.; Chen Y.; Ji L.; Chao H. Fluorinated Cyclometalated Iridium(III) Complexes as Mitochondria-Targeted Theranostic Anticancer Agents. Dalton Transactions 2017, 46 (20), 6734–6744. 10.1039/C7DT01043E. [DOI] [PubMed] [Google Scholar]
- Chen M. H.; Wang F. X.; Cao J. J.; Tan C. P.; Ji L. N.; Mao Z. W. Light-Up Mitophagy in Live Cells with Dual-Functional Theranostic Phosphorescent Iridium(III) Complexes. ACS Appl. Mater. Interfaces 2017, 9 (15), 13304–13314. 10.1021/acsami.7b01735. [DOI] [PubMed] [Google Scholar]
- Yi Q. Y.; Wan D.; Tang B.; Wang Y. J.; Zhang W. Y.; Du F.; He M.; Liu Y. J. Synthesis, Characterization and Anticancer Activity in Vitro and in Vivo Evaluation of an Iridium (III) Polypyridyl Complex. Eur. J. Med. Chem. 2018, 145, 338–349. 10.1016/j.ejmech.2017.11.091. [DOI] [PubMed] [Google Scholar]
- He L.; Wang K. N.; Zheng Y.; Cao J. J.; Zhang M. F.; Tan C. P.; Ji L. N.; Mao Z. W. Cyclometalated Iridium(Iii) Complexes Induce Mitochondria-Derived Paraptotic Cell Death and Inhibit Tumor Growth: In Vivo. Dalton Transactions 2018, 47 (20), 6942–6953. 10.1039/C8DT00783G. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Liu J.; Guan R.; Jin C.; Ji L.; Chao H. Endoplasmic Reticulum Targeted Cyclometalated Iridium(Iii) Complexes as Efficient Photodynamic Therapy Photosensitizers. Dalton Transactions 2019, 48 (19), 6408–6415. 10.1039/C9DT01072F. [DOI] [PubMed] [Google Scholar]
- Chen W.; Cai X.; Sun Q.; Guo X.; Liang C.; Tang H.; Huang H.; Luo H.; Chen L.; Chen J. Design and Synthesis of Aptamer-Cyclometalated Iridium(III) Complex Conjugate Targeting Cancer Cells. Eur. J. Med. Chem. 2022, 236, 114335. 10.1016/j.ejmech.2022.114335. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Kim H.; Lee Y. H.; Kim T.; Lee Y. S.; Lee M. H. Heteroleptic Cyclometalated Iridium(III) Complexes Supported by Triarylborylpicolinate Ligand: Ratiometric Turn-on Phosphorescence Response upon Fluoride Binding. Inorg. Chem. 2014, 53 (16), 8672–8680. 10.1021/ic501286m. [DOI] [PubMed] [Google Scholar]
- Cao J. J.; Zheng Y.; Wu X. W.; Tan C. P.; Chen M. H.; Wu N.; Ji L. N.; Mao Z. W. Anticancer Cyclometalated Iridium(III) Complexes with Planar Ligands: Mitochondrial DNA Damage and Metabolism Disturbance. J. Med. Chem. 2019, 62 (7), 3311–3322. 10.1021/acs.jmedchem.8b01704. [DOI] [PubMed] [Google Scholar]
- Chen M. H.; Zheng Y.; Cai X. J.; Zhang H.; Wang F. X.; Tan C. P.; Chen W. H.; Ji L. N.; Mao Z. W. Inhibition of Autophagic Flux by Cyclometalated Iridium(Iii) Complexes through Anion Transportation. Chem. Sci. 2019, 10 (11), 3315–3323. 10.1039/C8SC04520H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Chen H.; Zeng L.; Rees T. W.; Xiong K.; Chen Y.; Ji L.; Chao H. Mitochondria-Targeting Cyclometalated Iridium(Iii) Complexes for Tumor Hypoxic Imaging and Therapy. Inorg. Chem. Front 2019, 6 (4), 1003–1010. 10.1039/C9QI00081J. [DOI] [Google Scholar]
- Li Y.; Wang K. N.; He L.; Ji L. N.; Mao Z. W. Synthesis, Photophysical and Anticancer Properties of Mitochondria-Targeted Phosphorescent Cyclometalated Iridium(III) N-Heterocyclic Carbene Complexes. J. Inorg. Biochem 2020, 205, 110976. 10.1016/j.jinorgbio.2019.110976. [DOI] [PubMed] [Google Scholar]
- Ma B.; Wang X.; Gao S.; Qi L.; Xu Y.; Yang J.; Zuo G. Iridium(III) Complex-Based Phosphorescent Probe for Rapid, Specific, and Sensitive Detection of Phosgene. Dyes Pigm. 2020, 177, 108279. 10.1016/j.dyepig.2020.108279. [DOI] [Google Scholar]
- Li Y.; Liu B.; Xu C. X.; He L.; Wan Y. C.; Ji L. N.; Mao Z. W. Mitochondria-Targeted Phosphorescent Cyclometalated Iridium(III) Complexes: Synthesis, Characterization, and Anticancer Properties. Journal of Biological Inorganic Chemistry 2020, 25 (4), 597–607. 10.1007/s00775-020-01783-2. [DOI] [PubMed] [Google Scholar]
- Gu Y.; Wen H.; Zhang Y.; Bai L.; Zhou Y.; Zhang H.; Tian L.; Hao J.; Liu Y. Studies of Anticancer Activity in Vivo and in Vitro Behaviors of Liposomes Encapsulated Iridium(III) Complex. Journal of Biological Inorganic Chemistry 2021, 26 (1), 109–122. 10.1007/s00775-020-01841-9. [DOI] [PubMed] [Google Scholar]
- Prieto-Castañeda A.; Lérida-Viso A.; Avellanal-Zaballa E.; Sola-Llano R.; Bañuelos J.; Agarrabeitia A. R.; Martínez-Máñez R.; Ortiz M. J. Phosphorogenic Dipyrrinato-Iridium(III) Complexes as Photosensitizers for Photodynamic Therapy. Dyes Pigm. 2022, 197, 109886. 10.1016/j.dyepig.2021.109886. [DOI] [Google Scholar]
- Wu Y.; Liu J.; Shao M.; Zhang P.; Song S.; Yang G.; Liu X.; Liu Z. Cyclometalated Iridium(III) Dithioformic Acid Complexes as Mitochondria-Targeted Imaging and Anticancer Agents. J. Inorg. Biochem 2022, 233, 111855. 10.1016/j.jinorgbio.2022.111855. [DOI] [PubMed] [Google Scholar]
- Hao J.; Liu H.; Wang J.; Wang X.; Huang C.; Liang L.; Chen J.; Wang Y.; Liu Y. Iridium (III) Complexes Induce Cervical Carcinoma Apoptosis via Disturbing Cellular Redox Homeostasis Disorder and Inhibiting PI3K/AKT/MTOR Pathway. J. Inorg. Biochem 2022, 235, 111946. 10.1016/j.jinorgbio.2022.111946. [DOI] [PubMed] [Google Scholar]
- Yuan Y.; Zhang Y.; Chen J.; Huang C.; Liu H.; Li W.; Liang L.; Wang Y.; Liu Y. Synthesis, Biological Evaluation of Novel Iridium(III) Complexes Targeting Mitochondria toward Melanoma B16 Cells. Eur. J. Med. Chem. 2023, 247, 115046. 10.1016/j.ejmech.2022.115046. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li Y.; Chen J.; Liu H.; Zhou Y.; Huang C.; Liang L.; Liu Y.; Wang X. Anticancer Effect Evaluation of Iridium(III) Complexes Targeting Mitochondria and Endoplasmic Reticulum. J. Inorg. Biochem 2023, 238, 112054. 10.1016/j.jinorgbio.2022.112054. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Gu Y.; Hu H.; Liu H.; Li W.; Huang C.; Chen J.; Liang L.; Liu Y. Design, Synthesis and Biological Evaluation of Liposome Entrapped Iridium(III) Complexes toward SGC-7901 Cells. J. Inorg. Biochem 2023, 241, 112134. 10.1016/j.jinorgbio.2023.112134. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Gao Y.; He W.; Wang W.; Hu W.; Sun Y. Synthesis, Characterization and Biological Evaluation of Two Cyclometalated Iridium(III) Complexes Containing a Glutathione S-Transferase Inhibitor. J. Inorg. Biochem 2023, 238, 112050. 10.1016/j.jinorgbio.2022.112050. [DOI] [PubMed] [Google Scholar]
- Huang H.; Yang L.; Zhang P.; Qiu K.; Huang J.; Chen Y.; Diao J. J.; Liu J.; Ji L.; Long J.; Chao H. Real-Time Tracking Mitochondrial Dynamic Remodeling with Two-Photon Phosphorescent Iridium (III) Complexes. Biomaterials 2016, 83, 321–331. 10.1016/j.biomaterials.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Qin Q. P.; Meng T.; Tan M. X.; Liu Y. C.; Luo X. J.; Zou B. Q.; Liang H. Synthesis and in Vitro Biological Evaluation of Three 4′-(4-Methoxyphenyl)-2,2′:6′,2″-Terpyridine Iridium(III) Complexes as New Telomerase Inhibitors. Eur. J. Med. Chem. 2018, 143, 1387–1395. 10.1016/j.ejmech.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; He L.; Zhang D. Y.; Tan C. P.; Ji L. N.; Mao Z. W. Mixed-Ligand Iridium(III) Complexes as Photodynamic Anticancer Agents. Dalton Transactions 2017, 46 (34), 11395–11407. 10.1039/C7DT02273E. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Qiu K.; Liu C.; Huang H.; Rees T. W.; Ji L.; Zhang Q.; Chao H. Tracking Mitochondrial Dynamics during Apoptosis with Phosphorescent Fluorinated Iridium(Iii) Complexes. Dalton Transactions 2018, 47 (37), 12907–12913. 10.1039/C8DT02918K. [DOI] [PubMed] [Google Scholar]
- Ruiz J.; Rodríguez V.; Cutillas N.; Samper K. G.; Capdevila M.; Palacios O.; Espinosa A. Novel C,N-Chelate Rhodium(Iii) and Iridium(Iii) Antitumor Complexes Incorporating a Lipophilic Steroidal Conjugate and Their Interaction with DNA. Dalton Transactions 2012, 41 (41), 12847–12856. 10.1039/c2dt31654d. [DOI] [PubMed] [Google Scholar]
- Li Y.; Tan C. P.; Zhang W.; He L.; Ji L. N.; Mao Z. W. Phosphorescent Iridium(III)-Bis-N-Heterocyclic Carbene Complexes as Mitochondria-Targeted Theranostic and Photodynamic Anticancer Agents. Biomaterials 2015, 39, 95–104. 10.1016/j.biomaterials.2014.10.070. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu B.; Lu X. R.; Li M. F.; Ji L. N.; Mao Z. W. Cyclometalated Iridium(III) N-Heterocyclic Carbene Complexes as Potential Mitochondrial Anticancer and Photodynamic Agents. Dalton Transactions 2017, 46 (34), 11363–11371. 10.1039/C7DT01903C. [DOI] [PubMed] [Google Scholar]
- Monti F.; Kessler F.; Delgado M.; Frey J.; Bazzanini F.; Accorsi G.; Armaroli N.; Bolink H. J.; Ortí E.; Scopelliti R.; Nazeeruddin M. K.; Baranoff E. Charged Bis-Cyclometalated Iridium(III) Complexes with Carbene-Based Ancillary Ligands. Inorg. Chem. 2013, 52 (18), 10292–10305. 10.1021/ic400600d. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Guo L.; Ge X.; Tian Z.; Gong Y.; Zheng H.; Du Q.; Zheng X.; Liu Z. Novel Lysosome-Targeted Cyclometalated Iridium(III) Anticancer Complexes Containing Imine-N-Heterocyclic Carbene Ligands: Synthesis, Spectroscopic Properties and Biological Activity. Dyes Pigm. 2019, 161, 119–129. 10.1016/j.dyepig.2018.09.044. [DOI] [Google Scholar]
- Lucas S. J.; Lord R. M.; Wilson R. L.; Phillips R. M.; Sridharan V.; McGowan P. C. Synthesis of Iridium and Ruthenium Complexes with (N,N), (N,O) and (O,O) Coordinating Bidentate Ligands as Potential Anti-Cancer Agents. Dalton Transactions 2012, 41 (45), 13800–13802. 10.1039/c2dt32104a. [DOI] [PubMed] [Google Scholar]
- Hao L.; Li Z. W.; Zhang D. Y.; He L.; Liu W.; Yang J.; Tan C. P.; Ji L. N.; Mao Z. W. Monitoring Mitochondrial Viscosity with Anticancer Phosphorescent Ir(Iii) Complexes: Via Two-Photon Lifetime Imaging. Chem. Sci. 2019, 10 (5), 1285–1293. 10.1039/C8SC04242J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Liu J.; Shao M.; Zhang P.; Song S.; Yang G.; Liu X.; Liu Z. Cyclometalated Iridium(III) Dithioformic Acid Complexes as Mitochondria-Targeted Imaging and Anticancer Agents. J. Inorg. Biochem 2022, 233, 111855. 10.1016/j.jinorgbio.2022.111855. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wu X.; Iggo J. A.; Xiao J. Half-Sandwich Iridium Complexes-Synthesis and Applications in Catalysis. Coord. Chem. Rev. 2008, 252, 782–809. 10.1016/j.ccr.2008.01.015. [DOI] [Google Scholar]
- Gichumbi J. M.; Friedrich H. B. Half-Sandwich Complexes of Platinum Group Metals (Ir, Rh, Ru and Os) and Some Recent Biological and Catalytic Applications. J. Organomet. Chem. 2018, 866, 123–143. 10.1016/j.jorganchem.2018.04.021. [DOI] [Google Scholar]
- Blakemore J. D.; Schley N. D.; Balcells D.; Hull J. F.; Olack G. W.; Incarvito C. D.; Eisenstein O.; Brudvig G. W.; Crabtree R. H. Half-Sandwich Iridium Complexes for Homogeneous Water-Oxidation Catalysis. J. Am. Chem. Soc. 2010, 132 (45), 16017–16029. 10.1021/ja104775j. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Habtemariam A.; Pizarro A. M.; Fletcher S. A.; Kisova A.; Vrana O.; Salassa L.; Bruijnincx P. C. A.; Clarkson G. J.; Brabec V.; Sadler P. J. Organometallic Half-Sandwich Iridium Anticancer Complexes. J. Med. Chem. 2011, 54 (8), 3011–3026. 10.1021/jm2000932. [DOI] [PubMed] [Google Scholar]
- Mou Z. D.; Deng N.; Zhang F.; Zhang J.; Cen J.; Zhang X. "Half-Sandwich” Schiff-Base Ir(III) Complexes as Anticancer Agents. Eur. J. Med. Chem. 2017, 138, 72–82. 10.1016/j.ejmech.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Li J.; Guo L.; Tian Z.; Zhang S.; Xu Z.; Han Y.; Li R.; Li Y.; Liu Z. Half-Sandwich Iridium and Ruthenium Complexes: Effective Tracking in Cells and Anticancer Studies. Inorg. Chem. 2018, 57 (21), 13552–13563. 10.1021/acs.inorgchem.8b02161. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Guo L.; Tian Z.; Gong Y.; Zheng H.; Zhang S.; Xu Z.; Ge X.; Liu Z. Novel and Versatile Imine-N-Heterocyclic Carbene Half-Sandwich Iridium(III) Complexes as Lysosome-Targeted Anticancer Agents. Inorg. Chem. 2018, 57 (17), 11087–11098. 10.1021/acs.inorgchem.8b01656. [DOI] [PubMed] [Google Scholar]
- Du Q.; Yang Y.; Guo L.; Tian M.; Ge X.; Tian Z.; Zhao L.; Xu Z.; Li J.; Liu Z. Fluorescent Half-Sandwich Phosphine-Sulfonate Iridium(III) and Ruthenium(II) Complexes as Potential Lysosome-Targeted Anticancer Agents. Dyes Pigm. 2019, 162, 821–830. 10.1016/j.dyepig.2018.11.009. [DOI] [Google Scholar]
- Wang C.; Liu J.; Tian Z.; Tian M.; Tian L.; Zhao W.; Liu Z. Half-Sandwich Iridium N-Heterocyclic Carbene Anticancer Complexes. Dalton Transactions 2017, 46 (21), 6870–6883. 10.1039/C7DT00575J. [DOI] [PubMed] [Google Scholar]
- Kong D.; Tian M.; Guo L.; Liu X.; Zhang S.; Song Y.; Meng X.; Wu S.; Zhang L.; Liu Z. Novel Iridium(III) Iminopyridine Complexes: Synthetic, Catalytic, and in Vitro Anticancer Activity Studies. Journal of Biological Inorganic Chemistry 2018, 23 (5), 819–832. 10.1007/s00775-018-1578-0. [DOI] [PubMed] [Google Scholar]
- Han Y.; Tian Z.; Zhang S.; Liu X.; Li J.; Li Y.; Liu Y.; Gao M.; Liu Z. Half-Sandwich IridiumIII N-Heterocyclic Carbene Antitumor Complexes and Biological Applications. J. Inorg. Biochem 2018, 189, 163–171. 10.1016/j.jinorgbio.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Liu C.; Liu X.; Ge X.; Wang Q.; Zhang L.; Shang W.; Zhang Y.; Yuan X. A.; Tian L.; Liu Z.; You J. Fluorescent Iridium(Iii) Coumarin-Salicylaldehyde Schiff Base Compounds as Lysosome-Targeted Antitumor Agents. Dalton Transactions 2020, 49 (18), 5988–5998. 10.1039/D0DT00627K. [DOI] [PubMed] [Google Scholar]
- Wang L.; Liu X.; Wu Y.; He X.; Guo X.; Gao W.; Tan L.; Yuan X. A.; Liu J.; Liu Z. In Vitro and In Vivo Antitumor Assay of Mitochondrially Targeted Fluorescent Half-Sandwich Iridium(III) Pyridine Complexes. Inorg. Chem. 2023, 62 (8), 3395–3408. 10.1021/acs.inorgchem.2c03333. [DOI] [PubMed] [Google Scholar]
- Chellan P.; Avery V. M.; Duffy S.; Land K. M.; Tam C. C.; Kim J. H.; Cheng L. W.; Romero-Canelón I.; Sadler P. J. Bioactive Half-Sandwich Rh and Ir Bipyridyl Complexes Containing Artemisinin. J. Inorg. Biochem 2021, 219, 111408. 10.1016/j.jinorgbio.2021.111408. [DOI] [PubMed] [Google Scholar]
- Wang L.; Liu X.; Wu Y.; He X.; Guo X.; Gao W.; Tan L.; Yuan X. A.; Liu J.; Liu Z. In Vitro and In Vivo Antitumor Assay of Mitochondrially Targeted Fluorescent Half-Sandwich Iridium(III) Pyridine Complexes. Inorg. Chem. 2023, 62 (8), 3395–3408. 10.1021/acs.inorgchem.2c03333. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wu Y.; Yang G.; Liu Z.; Liu X. Mitochondrial Targeting Half-Sandwich Iridium(III) and Ruthenium(II) Dppf Complexes and in Vitro Anticancer Assay. J. Inorg. Biochem 2023, 239, 112069. 10.1016/j.jinorgbio.2022.112069. [DOI] [PubMed] [Google Scholar]