Abstract
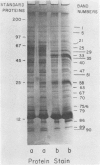
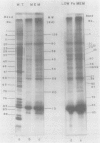
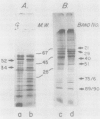
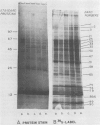
Cultures of the cyanobacterium Anacystis nidulans were grown under iron-deficient conditions and then restored by the addition of iron. Membrane proteins from iron-deficient and iron-restored cells were analyzed by lithium dodecyl sulfate-polyacrylamide gradient gel electrophoresis. The incorporation of [35S]sulfate into membrane proteins and lactoperoxidase-catalyzed 125I iodination were used to monitor the rates of polypeptide biosynthesis and surface exposure of membrane proteins, respectively. These polypeptide profiles revealed major differences in the membrane composition of iron-deficient and normal cells. Iron deficiency caused a decrease in the amount of certain important membrane proteins, reflecting a decreased rate of biosynthesis of these peptides. Several photosystem II peptides also showed an increase in surface exposure after iron stress. In addition, iron deficiency led to the synthesis of proteins at 34 and 52 kilodaltons which were not present in normal cells. When iron was restored to a deficient culture, a metabolic sequence was initiated within the first 12 h after the addition of iron which led to phenotypically normal cells. Pulse labeling with [35S]sulfate during this period demonstrated that iron addition initiates a coordinated pattern of synthesis that leads to the assembly of normal membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berezin F. B., Bogoslovskii V. A. O novom podkhode k terapii nekotorykh paroksizmal'nykh narushenii ritma serdtsa. Ter Arkh. 1976;48(10):44–48. [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Shochat S., Malkin S., Ohad I. Functional Organization of the Chlorophyll-Containing Complexes of Chlamydomonas reinhardi: A Study of Their Formation and Interconnection with Reaction Centers in the Greening Process of the y-1 Mutant. Plant Physiol. 1982 Sep;70(3):637–644. doi: 10.1104/pp.70.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Chlorophyll-protein organization of membranes from the cyanobacterium Anacystis nidulans. Arch Biochem Biophys. 1983 Jan;220(1):155–166. doi: 10.1016/0003-9861(83)90396-x. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F. H., Börner T., Hagemann R. Biosynthesis of thylakoids and the membrane-bound enzyme systems of photosynthesis. Results Probl Cell Differ. 1980;10:147–177. doi: 10.1007/978-3-540-38255-3_5. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Heller K., Coulton J. W., Braun V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J Bacteriol. 1980 Jul;143(1):256–264. doi: 10.1128/jb.143.1.256-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold O. Lamellar proteins of green and chlorotic chloroplasts as affected by iron deficiency and antibiotics. Biochim Biophys Acta. 1971 May 13;238(2):324–331. doi: 10.1016/0005-2787(71)90099-2. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. A developmental study of photosystem I peripheral chlorophyll proteins. Plant Physiol. 1980 May;65(5):823–827. doi: 10.1104/pp.65.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. P., Lean D. R., Nalewajko C. Blue-green algae: their excretion of iron-selective chelators enables them to dominate other algae. Science. 1976 May 28;192(4242):900–902. doi: 10.1126/science.818707. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Sherman L. A. Isolation and characterization of photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochim Biophys Acta. 1978 Aug 8;503(2):343–361. doi: 10.1016/0005-2728(78)90193-7. [DOI] [PubMed] [Google Scholar]
- Sadewasser D. A., Sherman L. A. Internal and external membrane proteins of the cyanobacterium, Synechococcus cedrorum. Biochim Biophys Acta. 1981 Jan 8;640(1):326–340. doi: 10.1016/0005-2736(81)90556-3. [DOI] [PubMed] [Google Scholar]