Abstract
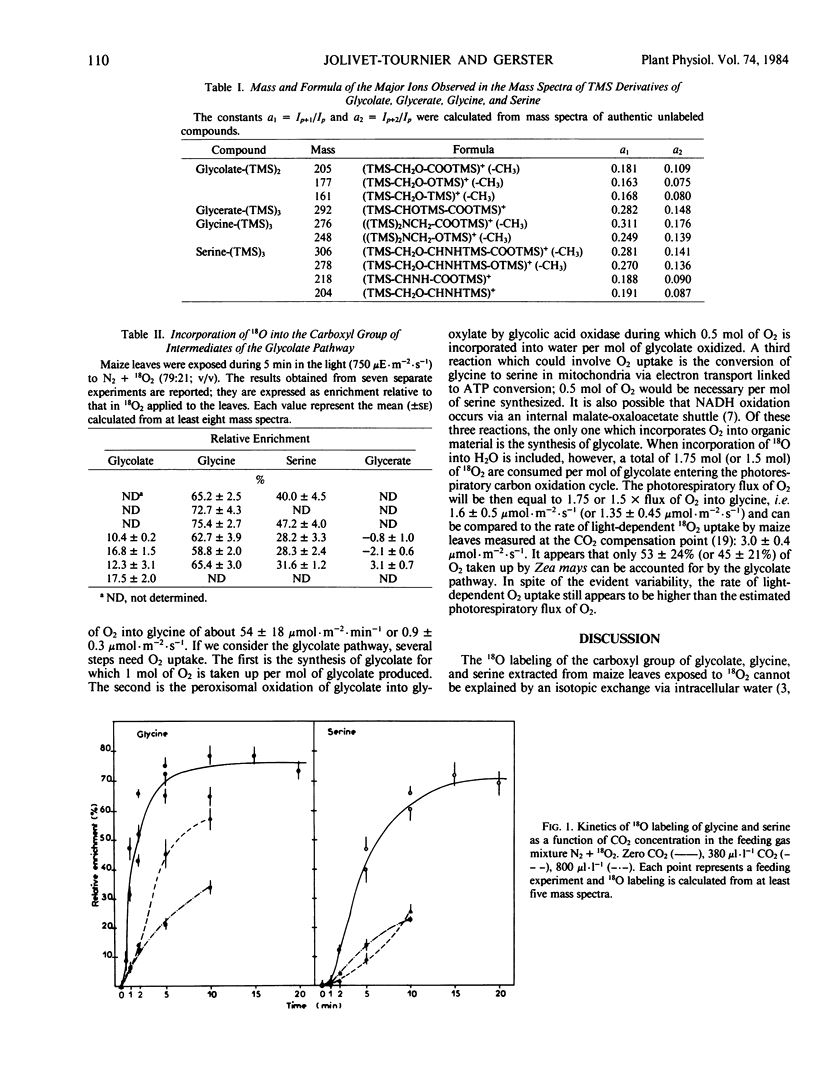
Glycolate, glycine, and serine extracted from excised Zea mays L. leaves which had been allowed to photosynthesize in the presence of 18O2 were analyzed by gas chromatography-mass spectrometry. In each case, only one of the oxygen atoms of the carboxyl group had become labeled. The maximum enrichment observed in glycine and serine was attained after 5 minutes and 15 minutes of exposure to 18O2 at the CO2 compensation point; the labeling was very high, reaching 70 to 73% of that in the applied O2. Thus, it appears that all or nearly all of the glycine and serine are synthesized in maize leaves via fixation of O2. In the presence of CO2 (380 or 800 microliters per liter), 18O-labeling was markedly slower.
Glycolate enrichment was variable and much lower than that in glycine and serine. It is possible that there are additional pathways of glycolate synthesis which do not result in the incorporation of 18O from molecular oxygen. An estimation of the metabolic flow of O2 through the photorespiratory cycle was made. It appeared that less than 75% of the O2 taken up by maize leaves is involved in this pathway. Therefore, other processes of O2 metabolism must occur in the light.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Incorporation of molecular oxygen into glycine and serine during photorespiration in spinach leaves. Biochemistry. 1971 Dec 7;10(25):4777–4782. doi: 10.1021/bi00801a027. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J., Wittingham C. P. The mechanism of inhibition of photosynthesis by high partial pressures of oxygen in Chlorella. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):511–520. doi: 10.1098/rspb.1966.0046. [DOI] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of Oxygen on Photosynthesis, Photorespiration and Respiration in Detached Leaves. II. Corn and other Monocotyledons. Plant Physiol. 1966 Mar;41(3):428–431. doi: 10.1104/pp.41.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. Photosynthesis and photorespiration in whole plants of wheat. Plant Physiol. 1979 Nov;64(5):735–738. doi: 10.1104/pp.64.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G., Canvin D. T. Determination of the Rate of CO(2) Evolution by Green Leaves in Light. Plant Physiol. 1969 May;44(5):662–670. doi: 10.1104/pp.44.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Andrews T. J., Tolbert N. E. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973 Jan 2;12(1):18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Osmond C. B., Akazawa T., Asami S. On the mechanism of glycolate synthesis by Chromatium and Chlorella. Arch Biochem Biophys. 1978 Jan 15;185(1):49–56. doi: 10.1016/0003-9861(78)90142-x. [DOI] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Ollinger O. Light-driven Uptake of Oxygen, Carbon Dioxide, and Bicarbonate by the Green Alga Scenedesmus. Plant Physiol. 1980 Apr;65(4):723–729. doi: 10.1104/pp.65.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R. J., Jackson W. A. Photorespiratory phenomena in maize: oxygen uptake, isotope discrimination, and carbon dioxide efflux. Plant Physiol. 1972 Feb;49(2):218–223. doi: 10.1104/pp.49.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]


