Abstract
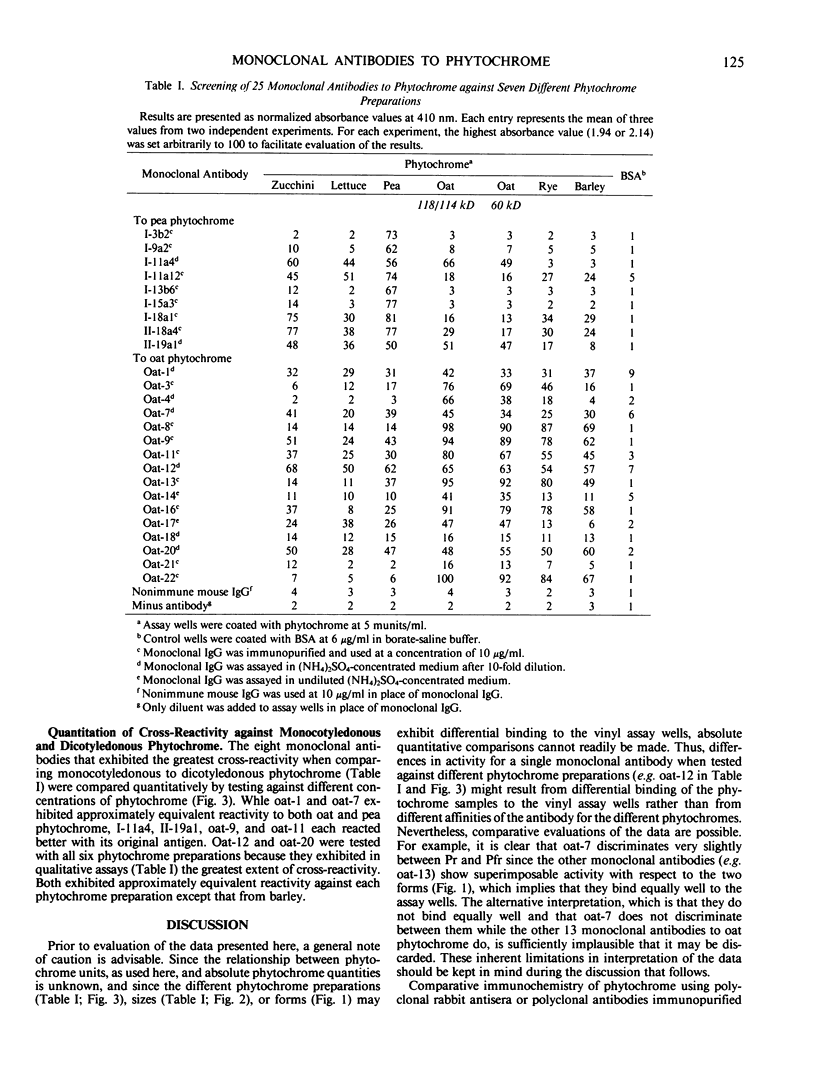
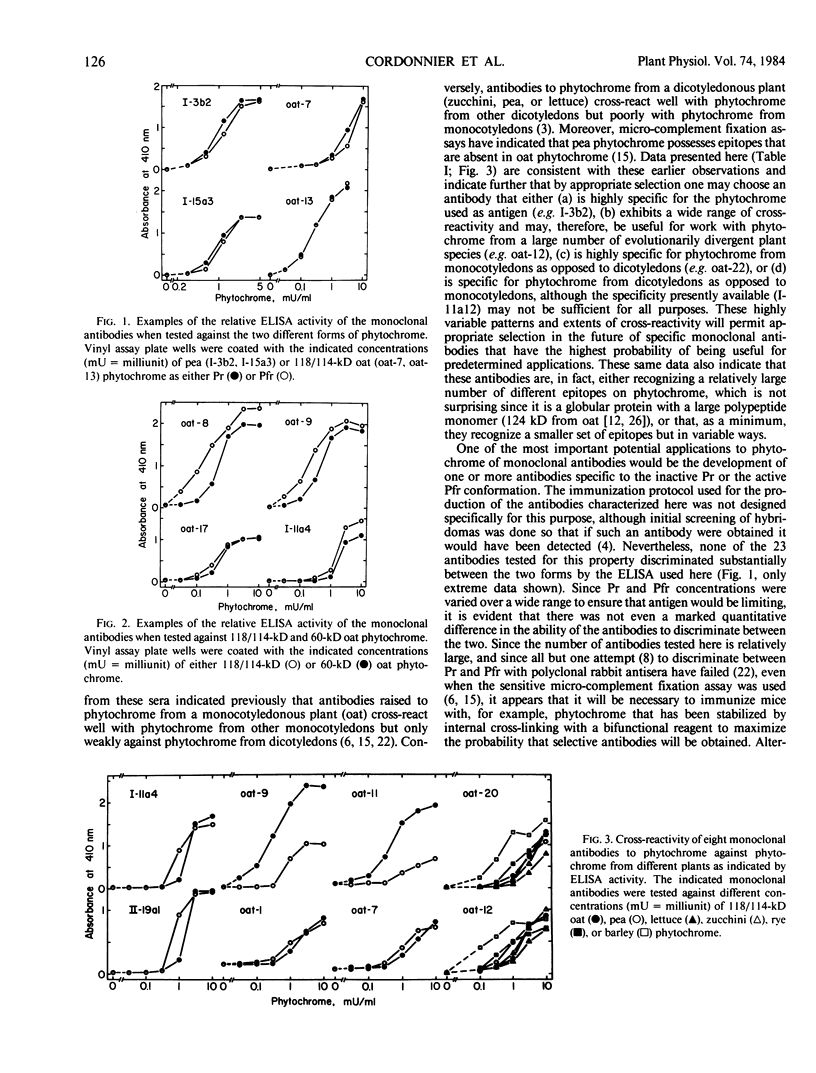
Nine monoclonal antibodies to pea (Pisum sativum L.) and 16 to oat (Avena sativa L.) phytochrome are characterized by enzyme-linked immunosorbent assay against phytochrome from six different sources: pea, zucchini (Cucurbita pepo L.), lettuce (Lactuca sativa L.), oat, rye (Secale cereale L.), and barley (Hordeum vulgare L.). All antibodies were raised against phytochrome with a monomer size near 120,000 daltons. Nevertheless, none of them discriminated qualitatively between 118/114-kilodalton oat phytochrome and a photoreversible, 60-kilodalton proteolytic degradation product derived from it. In addition, none of the 23 antibodies tested discriminated substantially between phytochrome—red-absorbing form and phytochrome—far red-absorbing form. Two antibodies to pea and six to oat phytochrome also bound strongly to phytochrome from the other species, even though these two plants are evolutionarily widely divergent. Of these eight antibodies, two bound significantly to all of the six phytochrome preparations tested, indicating that these two may recognize highly conserved regions of the chromoprotein. Since the molecular function of phytochrome is unknown, these two antibodies may serve as unique probes for regions of this pigment that are important to its mode of action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Norris K. H., Siegelman H. W., Hendricks S. B. DETECTION, ASSAY, AND PRELIMINARY PURIFICATION OF THE PIGMENT CONTROLLING PHOTORESPONSIVE DEVELOPMENT OF PLANTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1703–1708. doi: 10.1073/pnas.45.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier M. M., Pratt L. H. Comparative Phytochrome Immunochemistry as Assayed by Antisera against Both Monocotyledonous and Dicotyledonous Phytochrome. Plant Physiol. 1982 Sep;70(3):912–916. doi: 10.1104/pp.70.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier M. M., Pratt L. H. Immunopurification and initial characterization of dicotyledonous phytochrome. Plant Physiol. 1982 Feb;69(2):360–365. doi: 10.1104/pp.69.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Immunological Determination of the Relationship between Large and Small Sizes of Phytochrome. Plant Physiol. 1973 Jan;51(1):210–213. doi: 10.1104/pp.51.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Immunological and physical characterization of the products of phytochrome proteolysis. Plant Physiol. 1975 Feb;55(2):212–217. doi: 10.1104/pp.55.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Phytochrome Characterization by Rabbit Antiserum against High Molecular Weight Phytochrome. Plant Physiol. 1975 Feb;55(2):207–211. doi: 10.1104/pp.55.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D. W., Butler W. L. Immunochemical and spectroscopic evidence for protein conformational changes in phytochrome transformations. Plant Physiol. 1970 May;45(5):567–570. doi: 10.1104/pp.45.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Partial characterization of undegraded oat phytochrome. Biochemistry. 1980 Jan 22;19(2):390–394. doi: 10.1021/bi00543a022. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome immunoaffinity purification. Plant Physiol. 1979 Aug;64(2):332–336. doi: 10.1104/pp.64.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome radioimmunoassay. Plant Physiol. 1979 Aug;64(2):327–331. doi: 10.1104/pp.64.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Immunochemistry of phytochrome. Plant Physiol. 1973 May;51(5):939–945. doi: 10.1104/pp.51.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Partial characterization of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):927–938. doi: 10.1104/pp.51.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H. Native phytochrome: Inhibition of proteolysis yields a homogeneous monomer of 124 kilodaltons from Avena. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5272–5276. doi: 10.1073/pnas.79.17.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]


