Abstract
Background
Falls are frequent and devastating events for people with Parkinson’s disease (PD). Here, we investigated whether laboratory-based reactive step training combined with home-based volitional step training was effective in improving balance recovery and stepping ability in people with PD.
Methods
Forty-four people with idiopathic PD were randomized into intervention or control groups. Intervention participants performed unsupervised volitional step training using home-based exergames (80+ minutes/week) for 12 weeks and attended reactive step training sessions in which they were exposed to slip and trip perturbations at 4 and 8 weeks. Control participants continued their usual activities. Primary outcomes were balance recovery following an induced-trip/slip and choice stepping reaction time (CSRT) at the 12-week reassessment. Secondary outcomes comprised sensorimotor, balance, cognitive, psychological, complex stepping (inhibitory CSRT and Stroop Stepping Test [SST]), gait measures, and falls experienced in everyday life.
Results
At reassessment, the intervention group had significantly fewer total laboratory-induced falls and faster CSRT compared to the control group (P < .05). The intervention group also had significantly faster inhibitory CSRT and SST movement times and made fewer mistakes in the SST (P < .05). There were no significant differences in the rate of every day falls or other secondary outcome measures between the groups.
Conclusion
Combined volitional and reactive step training improved balance recovery from an induced-perturbation, voluntary stepping time, and stepping accuracy in cognitively challenging tests in people with PD. Further research is required to determine whether such combined step training can prevent daily-life falls in this population.
Keywords: Parkinson’s disease, falls, stepping, neurorehabilitation, training
Introduction
Over 50% of people with Parkinson’s disease (PD) fall in a given year,1-5 with a large proportion (50%-86%) falling recurrently. 6 The consequences of falls are devastating and include restriction of activities of daily living, fear of falling, high levels of caregiver stress, and injuries. 7 People with PD fall most often when walking due to internal factors such as freezing of gait and external factors such as trips and slips, especially outdoors.8-9 To avoid such externally-induced falls, effective stepping responses cued by the sensory detection of a postural threat (e.g. a trip and slip) and gait adjustments cued by the visual detection of hazards (e.g. uneven surfaces and wet floors) are required.10-12 Cumulative evidence suggests poor voluntary stepping and gait adaptability13,14 and impaired reactions to unexpected postural perturbations predispose people with PD to fall.15-17
Recent studies have reported promising effects of reactive step training (e.g. repeated perturbations) and volitional step training (e.g. stepping onto step targets in multiple directions) for reducing falls in people with PD.18-23 While both training modalities target impaired stepping performance, these interventions likely lead to reduced fall risk via distinctly different mechanisms. 10 Systematic review evidence in older adults10,24 has shown that reactive step training evokes task-specific sensorimotor adaptations for successful balance recovery with a relatively low dose (e.g. a few sessions),25-27 whereas volitional step training induces gradual musculoskeletal and functional improvements over longer timeframes (e.g. 12 weeks).28,29
Some studies in people with PD have incorporated reactive stepping induced by sudden treadmill starts and stops into multicomponent training.22,23 One study involving 18 participants used a reactive balance training dose of 1 hour/day, 3 times/week for 8 weeks, and reported a non-significant reduction in falls in the intervention group relative to the control group. 22 The other study, involving 45 participants, had a higher reactive step training dose, that is, 2 phases of 4-week physiotherapist-supervised training 3 times/week (60 minutes each session) along with home-based exercises 5 times/ week (60 minutes each session). 23 Fall rates were significantly reduced in the training group who completed the program compared to the control group during and beyond the trial period. While the above 2 studies demonstrated that combined voluntary and reactive step training could prevent falls, the high doses of both supervised and unsupervised training required may be impractical for many people with PD to undertake 30 and too resource intensive to implement at large scale.
Considering the above, we envisioned a less resource-intensive intervention comprising both volitional and reactive step training may also be efficacious in reducing fall risk in people with PD if (a) the volitional step training comprised enjoyable interactive stepping exergames that could be performed unsupervised at home, and (b) the reactive step training intervention had high ecological validity with respect to the nature of the slip and trip perturbations. In this regard, we considered training participants to successfully negotiate unexpected trip and slip hazards on an overground walkway would generalize better to such events that occur in everyday life than more artificial treadmill belt translations. Consequently, due to higher task specificity, only low training doses may be required to improve reactive balance, as previously found in studies involving older people.25-27,31-33
Thus, the primary aim of this study was to examine the effects of an intervention comprising 12 weeks of home-based high-dose unsupervised step training exergames (80+ minutes/week) interspersed with low-dose supervised laboratory-based reactive step training sessions on 2 key indices of fall risk: choice stepping reaction time (CSRT) and balance recovery to slip and trip perturbations in people with PD. Secondary aims were to (i) examine the effects of the intervention on sensorimotor, balance, cognitive, and clinical risk factors for falls, complex stepping tasks, and gait adaptability and (ii) obtain pilot data on the effect of this intervention approach for reducing daily-life falls.
Methods
Design
We conducted an assessor-blinded, randomized, controlled trial involving 44 people with PD who were randomly allocated into intervention or control groups. A sample size calculation for a Poison regression analysis was conducted with the following assumptions: a 70% fall rate in the control group, a 10% dropout rate, a significance level of <.05 and a power of 0.8. This revealed that 44 eligible participants were required (22 in each group) to detect a 50% reduction in the number of laboratory-induced falls in the intervention group. Participants were enrolled between September 2018 and September 2019. We prospectively registered the trial protocol on the Australian New Zealand Clinical Trials Registry (ACTRN12618001515280). The University New South Wales Human Research Ethics Committee approved the study protocol, and written informed consent was obtained from all participants before their participation.
Participants
The study participants were recruited through the Neuroscience Research Australia (NeuRA) volunteer registry, Parkinson’s NSW (an organization that supports people with PD and their families in the state of New South Wales, Australia), the outpatient department at Calvary Hospital, and through social media and newsletter advertisements. Inclusion criteria were: diagnosis of idiopathic PD according to the UK PD Brain Bank criteria, 34 aged 40 years or older, ability to walk independently with or without a walking aid, stable antiparkinsonian medication for at least 4 weeks, PD Hoehn and Yahr stages 1 to 3 (mild to moderate), living independently and able to communicate in English. The exclusion criteria comprised a Montreal Cognitive Assessment 35 score <18 (indicative of dementia) 36 and any condition that would interfere with the safety and conduct of the home-based and/or laboratory-based training and testing protocol, including initial screening of frequency and severity of freezing of gait based on participant’s responses to the New Freezing of Gait questionnaire, 37 advanced PD (Hoehn and Yahr stages 4 and 5), significant pain and any fractures within the 6-month period prior to the study.
Demographic, PD-Related, Clinical, Physical, and Mobility Descriptors
Information regarding participant age, sex, fall status, use of assistive devices and medication use were obtained from structured interviews. Height and weight were measured, and body mass index was computed (Supplemental Material 1). Participants were assessed using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) 38 parts II (activities of daily living), III (motor score), and IV (motor complications). Participants’ stage of PD using the Hoehn and Yahr scale was extracted from the MDS-UPDRS. 39 Levodopa equivalency daily dosage intake, 40 PD duration since the date of diagnosis and information about participants’ experience on freezing of gait using the New Freezing of Gait questionnaire 37 were also recorded. Global cognition was assessed with the MOCA; 35 anxiety and depression were assessed with the Hospital Anxiety and Depression scale; 41 and weekly physical exercise was assessed with the Incidental and Planned Exercise Questionnaire. 42 The Physiological Profile Assessment was used as our sensorimotor assessment and to document participants’ risk of falling. 43 This assessment comprises 5 items assessing visual contrast sensitivity, lower limb proprioception, simple reaction time, quadriceps muscle strength, and postural sway. The 3 last measures were also used as secondary outcomes (Supplemental Material 2).
Randomization and Blinding
Participants were randomly allocated to either the intervention or control group following baseline assessments using randomization software with random block sizes (2, 4, or 6) and a 1:1 allocation ratio. All assessments, data verification, and statistical analyses for the primary outcome measures were conducted by research staff blinded to group allocation.
Intervention Group
The intervention group completed 2 supervised reactive step training sessions and undertook home-based volitional step training for 12 weeks. Participants were instructed to take their antiparkinsonian medications as usual during training. The training sessions were scheduled when the PD medications were working optimally during the “on” phase.
Volitional Step Training
Volitional step training involved playing home-based step training exergames (Supplemental Material 3). 44 A research assistant visited each participant’s home and installed the smart±step exergame system, which consisted of a wireless electronic step mat and a small computer connected to a television or computer screen. The step mat had 6 stepping panels (left, right, 2 back, and 2 fronts) around 2 central stance panels used to interact with 5 games. Games were designed to train directed and timed step responses with additional cognitive stimuli requiring working memory, visuospatial skills, inhibition, and attention. Participants could choose their preferred games and appropriate difficulty levels (beginner, easy, medium, hard, and expert) but were required to play a core game to promote rapid and timed stepping (Stepmania) each day before the remaining games were unlocked. They were instructed to play the games in a few separate sessions to reach at least 80 minutes of gameplay per week. The gameplay data were transferred to and monitored in a web application. If participants did less than 80 minutes of exercise per week for 2 weeks, they were contacted by telephone to encourage adherence and address any barriers to participation. Participants were given a booklet containing instructions and safety precautions, and additional visits and telephone support were provided for participants who experienced difficulties.
Reactive Step Training
The reactive step training sessions were undertaken at NeuRA in weeks 4 and 8. Both sessions were 60 minutes long, supervised, and focused on balance recovery from 12 trips and 12 slips with progressive unpredictability. Each session consisted of 5 blocks of 6 trials (trips, slips, and washouts). The training was conducted on a 10-m Trip and Slip Walkway consisting of 50 cm × 50 cm wooden decking tiles (Figure 1). Participants wore comfortable exercise shoes and clothes, a ceiling-mounted full-body harness, and protectors on the toes, shins, and knees. In previous studies, we found that many participants become cautious and reduce their gait speed when exposed to repeated trip and slip hazards.45,46 To minimize between-participant variability in gait speed reduction due to anticipation of hazards, all participants performed the walking trials at approximately 81% of their usual gait speed. This was achieved by instructing participants to step on black and white target tiles positioned at 90% of their usual step length and a metronome set to 90% of their usual cadence. These usual gait parameters were based on a baseline walk over a 5-m electronic walkway (GAITRite mat, v4.0, 2010 CIR Systems). Trips were induced using a 14 cm height tripping board that flipped up from the walkway at mid-swing, cued by a hidden foot detection sensor. Slips were induced using a movable tile on 2 undetectable low-friction rails with linear bearings that could slide forward up to 40 cm on foot contact. Both the slipping tile and the tripping board were undetectable and could be positioned at various locations along the walkway to minimize predictive behaviors while training reactive stepping responses.31-33 At the end of each training block, participants provided ratings of their anxiety and perceived difficulty using 5-point Likert scales. 47 If these ratings indicated excessive anxiety or difficulty, gait speed (50%-80%) and slip distance (10-40 cm) in the following training block were reduced in consultation with each participant.
Figure 1.
Continuous images of a participant experiencing a trip (A) and a slip (B). A trip was induced using a 14-cm height tripping board that flipped up on the walkway. A slip was generated by a movable tile on 2 hidden low-friction rails with linear bearings that slide up to 70 cm after the participants’ foot landing.
Control Group
The control group was asked to continue with their usual health care and daily activities during the trial period.
Outcome Measures
A Physiotherapist experienced in PD assessment and rehabilitation and blinded to group allocation conducted all outcome assessments (except for prospective falls). Baseline assessments were conducted in a 3.5-hour session at a research laboratory at NeuRA. If requested, some clinical assessments were conducted at participants’ homes to reduce assessment length/participant burden during the visit. Reassessments were conducted in a 2.5-hour session at the same research laboratory at NeuRA. Participants completed the assessments while “on” their usual PD medication. The primary outcomes are detailed below. The secondary outcomes are provided in Supplementary Material 2.
Primary Outcomes
Volitional stepping performance was assessed with the CSRT using a customized system comprising a computerized mat (150 cm × 90 cm) and a computer screen. 48 The mat contained 8 panels: 2 central stance panels, a left panel, a right panel, 2 front panels, and 2 back panels (Figure 2). Participants were asked to stand on the 2 central panels. They were then instructed to step onto a panel as quickly as possible when the corresponding arrow on the screen changed color from white to green (Figure 2). Participants completed 6 practice trials followed by 24 randomly presented trials (4 trials each for the 6 stepping panels). The CSRT total time (ms), calculated as the sum of response time (i.e. stimulus presentation to foot lift-off) and movement time (i.e. foot lift-off to step-down on the correct panel), was used as a primary outcome.
Figure 2.
Stepping tests setup. Left panel: monitor displays of (1) choice stepping reaction time (CSRT) test: participants were required to step as possible onto the stepping mat panels corresponding to the location of the green arrow appearing on the screen (right/forward arrow on the mat); (2) inhibitory CSRT test: participants were required to step as quickly as possible onto mat panels corresponding to the location of the green arrow and to refrain from stepping when a purple arrow appears on the screen (refrain from stepping on the right panel); (3) Stroop Stepping Test (SST): participants were required to step as quickly as possible on the panel corresponding to the direction defined by the word in the arrow (right panel) and not to the direction the arrow is pointing (left panel). Right panel: graphic presentation of a participant performing the SST. This panel demonstrates a common mistake performed by participants who stepped in a different direction.
Reactive stepping performance was assessed when participants walked and responded to perturbations on the Trip and Slip Walkway (Figure 1).31-33 Participants were instructed to walk in time with a metronome and step onto target step tiles adjusted to 90% of regular step length and 90% of regular cadence (thus 81% of their average gait speed). No adjustment in gait speed was made during assessments. Participants completed at least 8 lengths of the walkway before exposure to the first perturbation, following which participants were exposed to 1 slip and 1 trip at baseline and 2 slips and 2 trips at reassessments in pre-determined pseudo-random order and locations.31-33 At least 1 washout walk was included between perturbation trials. If participants exhibited predictive behaviors (i.e. lifted their feet) or reduced their specified step length (i.e. heels not placed on the target step tiles) and cadence (i.e. steps not aligning with the metronome beats) in a walking trial, further instructions were provided, and additional washout walking trials were administered. Falls in the laboratory were defined by the harness-supported load of more than 30% of the participants’ body mass. 49 The number of slip falls (0-2), trip falls (0-2), and total (slip plus trip) falls (0-4) were used in the analysis.
Statistical Analyses
A blinded investigator conducted the statistical analyses according to a published statistical analysis plan (www.osf.io/s89zx). Student t-tests (continuous data), Mann–Whitney U tests (non-parametric data), and Chi-square tests (categorical data) were used for group comparisons at baseline. General linear models were used to examine between-group differences at reassessments while controlling for baseline scores for continuous outcomes (stepping, gait, sensorimotor, balance, cognition, and concern about falling). For gait adaptability, participant height was also entered as a covariate. Poisson regression was used with laboratory falls as a dependent variable, group as an independent variable, and the number of perturbations as an offset for the reassessment. Negative binomial regression was used to test for differences in the rate of falls in daily life between groups with the follow-up period in months entered as the exposure measure (offset). Extreme outlier scores (e.g. 5SD) were replaced with ±3SD from the average data. 50 Data missing at random (6.37% of data) were imputed using multiple imputations with 50 datasets.51,52 We set significance levels at P < .05. The data were analyzed using IBM SPSS Statistics 24 (SPSS, Inc., Chicago, IL).
Results
Participants
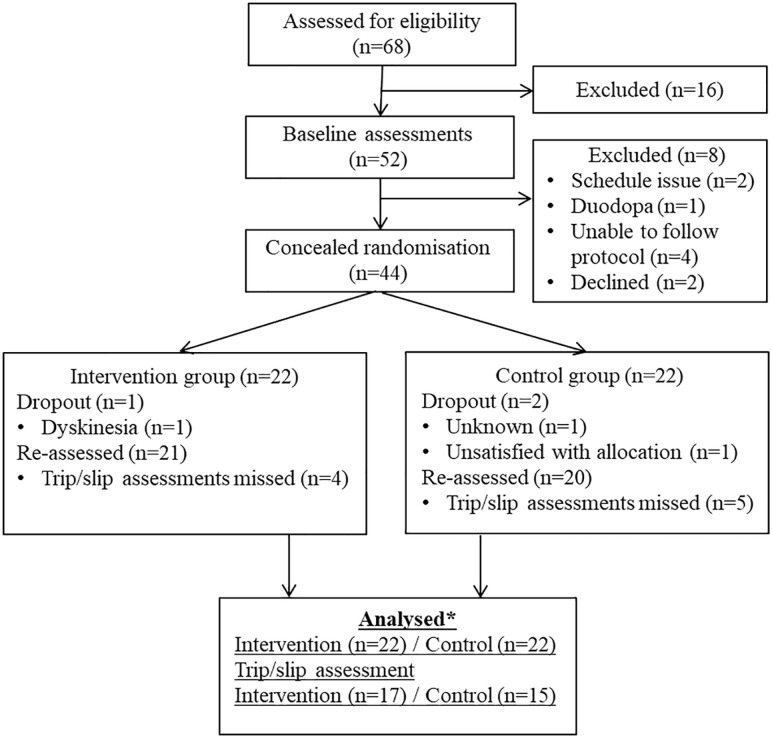
Fifty-two participants who met the initial eligibility criteria determined via screening questions administered during a telephone call were invited for baseline assessment. Eight participants were subsequently excluded due to scheduling issues (n = 2), use of a Duodopa pump (due to the risk of damaging the intestinal tube resulting from falls into the safety harness; n = 1), being unable to follow assessment protocols (n = 4) or declining to participate further (n = 2). Thus, 44 participants were enrolled and randomized to the intervention (n = 22) or control (n = 22) groups (Figure 3). No significant between-group differences were found in the demographic measures, clinical characteristics, and physical and mobility measures at baseline (Supplemental Material 1). Nine participants were not assessed for reactive balance in the trip and slip perturbations protocol due to health concerns (e.g. foot pain, shoulder injury, and anxiety).
Figure 3.
Consort flow chart showing participants involved in the trial.
*One intervention and 2 control participants who missed reassessments were included in the analysis with multiple imputations. Trip and slip data and daily-life fall data were not imputed.
Intervention and Adherence
Of the 22 intervention participants, 17 (77%) completed at least 75% of the prescribed dose of volitional stepping training. Median (interquartile range) weekly adherence for months 1, 2, and 3 were 81.8 (60.3), 81.8 (83.6), and 77.3 (53.5) minutes/week, respectively. Seventeen participants (77%) completed the 2 reactive-step training sessions. Four participants (18%) did not participate in 1 reactive step training session due to illness, ankle sprain, or hip and shoulder pain unrelated to the study, and 1 participant (5%) withdrew before any training due to illness.
Adverse Events
No participants fell during the home-based volitional step training. One participant felt knee discomfort during volitional step training that resolved after a 1-week training break. One participant felt increased hip pain (due to a car accident the previous week), and 1 reported high anxiety, requiring early termination of reactive step training.
Primary Outcomes
The intervention group had significantly faster CSRT total times than the control group at reassessment (P = .004; Table 1). In addition, the intervention group experienced significantly fewer total laboratory-induced falls, which is the sum of slip and trip falls compared to the control group (incidence rate ratio [IRR]: 0.51, 95% CI 0.30-0.89, P = .017). There was also a trend for fewer slip falls in the intervention group (IRR: 0.48, 95% CI 0.24-1.00 P = .05; Table 2). Trip falls did not differ significantly between the groups (IRR: 0.60, 95% CI 0.26-1.37, P = .226).
Table 1.
Stepping Outcome Measures at Baseline and Reassessment. Data are Mean (SD). P-value refers to Group Comparison at Reassessment While Controlling for Baseline Data.
| Outcome | Baseline | Reassessment | P | ||
|---|---|---|---|---|---|
| Intervention (n = 22) | Control (n = 22) | Intervention (n = 22) | Control (n = 22) | ||
| CSRT | |||||
| Response time, ms | 909 (202) | 992 (171) | 834 (161) | 902 (102) | .269 |
| Movement time, ms | 330 (68) | 357 (93) | 275 (65) | 326 (61) | .020 |
| Total time, ms | 1238 (233) | 1352 (226) | 1034 (214) | 1233 (144) | .004*** |
| Number of mistakes | 0.27 (0.70) | 0.27 (0.55) | 0.31 (0.52) | 0.24 (0.61) | .797 |
| iCSRT | |||||
| Response time, ms | 988 (215) | 1083 (189) | 923 (161) | 1019 (152) | .177 |
| Movement time, ms | 322 (95) | 392 (105) | 275 (64) | 338 (82) | .029 |
| Total time, ms | 1310 (203) | 1497 (308) | 1186 (192) | 1287 (143) | .175 |
| Number of mistakes | 0.77 (1.60) | 1.14 (1.88) | 0.27 (0.46) | 0.29 (0.86) | .710 |
| SST | |||||
| Response time, ms | 1370 (438) | 1489 (444) | 1325 (440) | 1391 (286) | .991 |
| Movement time, ms | 364 (104) | 398 (123) | 343 (87) | 461 (219) | .041 |
| Total time, ms | 1735 (477) | 1885 (502) | 1668 (461) | 1891 (533) | .296 |
| Number of mistakes | 1.14 (1.61) | 1.32 (1.29) | 0.40 (1.00) | 1.77 (3.03) | <.001 |
Abbreviation: CSRT, choice stepping reaction time test; iCSRT, inhibitory choice stepping reaction time test; SST, Stroop Stepping Test.
Registered primary outcome.
P-values in bold reflect they were statistically significant.
Table 2.
Laboratory-Induced Falls and Daily-Life Prospectively Recorded Falls in Daily Life.
| Outcome | IRR (CI 95%) | P |
|---|---|---|
| Laboratory falls | ||
| Baseline | ||
| Trip falls | 0.698 (0.197-2.475) | .578 |
| Slip falls | 1.857 (0.741-4.655) | .187 |
| Total falls | 1.308 (0.635-2.692) | .467 |
| Reassessment a | ||
| Trip falls | 0.600 (0.263-1.371) | .226 |
| Slip falls | 0.484 (0.235-0.999) | .050 |
| Total falls | 0.514 (0.298-0.888) | .017 |
| Daily-life falls | ||
| Rate of falls (number of falls/ up to 6 months) | 0.863 (0.231-3.223) | .826 |
IRR, incidence rate ratio.
Registered primary outcome.
Secondary Outcomes
At reassessment, intervention group participants had significantly faster movement times during the CSRT (P = .020), inhibitory CSRT (iCSRT; P = .029), and Stroop stepping test (SST; P = .041) and made fewer errors in the SST (P < .001) compared to the control group participants (Table 1). No between-group differences were observed at reassessment for the remaining stepping, gait, sensorimotor, balance, and psychological outcome measures (P > .05; Supplemental material 2).
Participants in the intervention group reported 45 falls during a mean (SD) follow-up period of 6.2 (1.2) months (0.33 falls per person per month), IRR: 0.86, 95% CI 0.23-3.32, P = .59. Thirteen participants reported no falls, 4 participants reported a single fall, and 5 participants reported multiple falls (3-19 falls)). Participants in the control group reported 62 falls during 6.3 (0.7) months (0.45 falls per person per month). Fourteen participants reported no falls, 2 reported a single fall, and 6 reported multiple falls (2 to 37 falls). Details of predisposing factors were reported for 99 of 107 falls. There were: (i) 19 falls from trips in the intervention group and 17 in the control group; (ii) 1 fall from a slip in the intervention group and 3 in the control group; (iii) and 21 falls from other reasons (e.g. loss of balance, freezing of gait, and dizziness) in the intervention group and 38 in the control group.
Discussion
The study findings suggest that a program comprising home-based volitional step training and reactive step training can improve balance recovery and voluntary stepping reaction times and errors in people with PD. At the end of the 12-week program, the intervention group experienced fewer laboratory-induced total falls in the laboratory, demonstrated faster step movement times in the cognitively challenging stepping tests and made fewer stepping errors in the SST. These findings indicate that people with PD can learn important motor skills related to fall prevention.
The participants randomized to the intervention arm received both voluntary and reactive step training, so it is not possible to determine whether specific intervention components led to improvements in the outcome measures observed. However, with this caveat, the following 2 discussion points are proffered in line with the construct of task specificity in that improved performance likely relates to the task practiced. 53
First, our study builds on a previous trial 42 that implemented a 12-week home-based step training intervention (1 exergame with a minimum dose of three 15-minute sessions per week) that found CSRT improved only in participants with lower disease severity (Hoehn and Yahr 1-3). It is possible that increased emphasis on executive function factors such as selective attention and inhibition, greater recommended dose, and better adherence to the home-based voluntary step training program led to improved step movement times and reduced errors in the CSRT, iCSRT, and SST found in the current study. These are encouraging findings given that poor performances in these tests are predictors of falls in older people 54 and people with PD. 13
In a similar vein, the reactive step training component likely improved balance recovery, as evidenced by fewer laboratory-induced falls in the intervention group relative to the control group. This is consistent with previous studies reporting short-term positive outcomes from reactive balance training in people with PD. For example, Barajas and Peterson 20 found that 2 days of training involving repeated anterior platform surface translations improved the margin of stability during balance recovery from an unexpected perturbation test. In addition, Peterson et al. 21 found that a program of 25 forward and 25 backward translations of the support surface improved postural responses in people with PD, and the adaptations were maintained for 24 hours. Our study builds on this work by demonstrating that a low dose of reactive step training involving repeated trips and slips may enhance balance recovery responses over 3 months.
A strength of our study was the inclusion of various engaging step training games that provided player feedback, reinforcement, and rewards, which likely contributed to good adherence. The trips and slip perturbations used in our reactive step training had high ecological validity. They were highly analogous to trip and slip-falls experienced in daily life by people with PD. 55 Our current trial also offered valuable insights into the efficacy, feasibility, and safety of a combined volitional and reactive step training intervention for people with PD. However, while our study suggests the potential application of such a training paradigm, more development is required to maximize participant acceptability and adherence, and it would be preferable if reactive step training could be implemented in a clinic with minimal equipment.
We also acknowledge some study limitations. First, we did not conduct the laboratory-fall assessment on 9 participants due to concerns regarding their health. Thus, our results are biased toward healthier participants who could undertake this assessment. Second, as indicated above, the study design precluded us from teasing out the effects of individual components of the combined program. Future studies could examine the positive impacts of combined voluntary and reactive balance training versus voluntary and reactive balance training administered as single interventions. Third, our primary study outcomes were restricted to falls following the trip and slip perturbations. Future studies could include complementary kinematic and electromyography measures of balance recovery ability, such as the margin of stability, trunk sway, step height 47 and muscle activation latency. 56 Fourth, we had less contact with control participants compared with intervention participants during the trial. These observations indicate strong task-specificity and limited transferability from the step training to other performance outcomes. There was also no indication from our pilot data that the intervention reduced falls in daily life over 6 months (the 95% confidence interval for the incident rate ratio contrasting the group’s fall rates was too large to make any meaningful conclusions in this regard). This may indicate that training programs should specifically address some of our secondary outcome measures known as risk factors for falls (balance, strength, cognition, and gait adaptability) in addition to voluntary and reactive step training in a multifactorial fall prevention intervention. It is also possible that the reactive step training dose (two 60-minute sessions) was insufficient for some participants, and additional training sessions may be beneficial. Future studies could consider more tailored reactive step training and the optimal doses of the 2 types of stepping training.
Conclusions
A combined volitional and reactive step training can improve balance recovery from an induced-perturbation, step movement time, and reduce errors in cognitively challenging stepping tests in people with PD. The training appeared to impact fall avoidance from slips more than trips in the laboratory. Larger trials powered for prospective falls in daily life are required to determine whether combined volitional and reactive balance training can prevent falls in daily life in this population.
Supplemental Material
Supplemental material, sj-docx-1-nnr-10.1177_15459683231206743 for Combined Reactive and Volitional Step Training Improves Balance Recovery and Stepping Reaction Time in People With Parkinson’s Disease: A Randomised Controlled Trial by Paulo H. S. Pelicioni, Stephen R. Lord, Jasmine C. Menant, Carly Chaplin, Collen Canning, Matthew A. Brodie, Daina L. Sturnieks and Yoshiro Okubo in Neurorehabilitation and Neural Repair
Acknowledgments
We express thanks to Ms Bethany Halmy, Mr Matthew Hand, Mr Beyond Yoon Min, Ms Natassia Smith, Mr Cameron Hicks, Mr Benjamin Barros, Mr Alex Neito, and Mr Julius Kinne for their support in conducting this study. Finally, we thank all volunteers who participated in this study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a Parkinson’s NSW seed grant. Dr Paulo H. S. Pelicioni was a recipient of a Coordenação de Aperfiçoamento de Pessoal de Nivel Superior PhD scholarship (Grant number: BEX 2194/15-5). Prof Stephen R. Lord is supported by NHMRC Investigator Grant.
ORCID iDs: Paulo H. S. Pelicioni  https://orcid.org/0000-0003-3168-3388
https://orcid.org/0000-0003-3168-3388
Jasmine C. Menant  https://orcid.org/0000-0001-8686-0500
https://orcid.org/0000-0001-8686-0500
Yoshiro Okubo  https://orcid.org/0000-0003-1767-0230
https://orcid.org/0000-0003-1767-0230
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
References
- 1. Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248:950-958. [DOI] [PubMed] [Google Scholar]
- 2. Latt MD, Lord SR, Morris JGL, Fung VSC. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov Disord. 2009;24:1280-1289. [DOI] [PubMed] [Google Scholar]
- 3. Paul SS, Sherrington C, Canning CG, Fung VS, Close JC, Lord SR. The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: a large prospective cohort study. Neurorehabil Neural Repair. 2014;28:282-290. [DOI] [PubMed] [Google Scholar]
- 4. Pelicioni PHS, Menant JC, Latt MD, Lord SR. Falls in Parkinson’s disease subtypes: risk factors, locations and circumstances. Int J Environ Res Public Health. 2019;16:2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelicioni PHS, Menant JC, Henderson EJ, Latt MD, Brodie MAD, Lord SR. Mild and marked executive dysfunction and falls in people with Parkinson’s disease. Braz J Phys Ther. 2021;25:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: a systematic review. Parkinsons Dis. 2013;2013:906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fasano A, Canning CG, Hausdorff JM, Lord S. Falls in Parkinson’s disease: a complex and evolving picture. Mov Disord. 2017;32:1524-1536. [DOI] [PubMed] [Google Scholar]
- 8. Gazibara T, Kisic-Tepavcevic D, Svetel M, et al. Indoor and outdoor falls in persons with Parkinson’s disease after 1 year follow-up study: differences and consequences. Neurol Sci. 2016;37:597-602. [DOI] [PubMed] [Google Scholar]
- 9. Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of falls diaries to facilitate reporting. Disabil Rehabil. 2008;30:1205-1212. [DOI] [PubMed] [Google Scholar]
- 10. Okubo Y, Schoene D, Lord SR. Step training improves reaction time, gait and balance and reduces falls in older people: a systematic review and meta-analysis. Br J Sports Med. 2017;51:586-593. [DOI] [PubMed] [Google Scholar]
- 11. Schoene D, Delbaere K, Lord SR. Impaired response selection during stepping predicts falls in older people-a cohort study. J Am Med Dir Assoc. 2017;18:719-725. [DOI] [PubMed] [Google Scholar]
- 12. Patla AE. Strategies for dynamic stability during adaptive human locomotion. IEEE Eng Med Biol Mag. 2003;22:48-52. [DOI] [PubMed] [Google Scholar]
- 13. Caetano MJD, Lord SR, Allen NE, et al. Stepping reaction time and gait adaptability are significantly impaired in people with Parkinson’s disease: implications for fall risk. Parkinsonism Relat Disord. 2018;47:32-38. [DOI] [PubMed] [Google Scholar]
- 14. Pelicioni PHS, Lord SR, Okubo Y, Menant JC. Cortical activation during gait adaptability in people with Parkinson’s disease. Gait Posture. 2022;91:247-253. [DOI] [PubMed] [Google Scholar]
- 15. Valkovic P, Brozova J, Botzel K, Ruzicka E, Benetin J. Push-and-release test predicts Parkinson fallers and non fallers better than the pull test: comparison in OFF and ON medication states. Mov Disord. 2008;23:1453-1457. [DOI] [PubMed] [Google Scholar]
- 16. Peterson DS, Lohse KR, Mancini M. Relating anticipatory postural adjustments to step outcomes during loss of balance in people with Parkinson’s disease. Neurorehabil Neural Repair. 2018;32:887-898. [DOI] [PubMed] [Google Scholar]
- 17. Beretta VS, Vitorio R, Santos PCR, Orcioli-Silva D, Gobbi LTB. Postural control after unexpected external perturbation: effects of Parkinson’s disease subtype. Hum Mov Sci. 2019;64:12-18. [DOI] [PubMed] [Google Scholar]
- 18. Mirelman A, Rochester L, Maidan I, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet. 2016;388:1170-1182. [DOI] [PubMed] [Google Scholar]
- 19. Mansfield A, Wong JS, Bryce J, Knorr S, Patterson KK. Does perturbation-based balance training prevent falls? Systematic review and meta-analysis of preliminary randomized controlled trials. Phys Ther. 2015;95:700-709. [DOI] [PubMed] [Google Scholar]
- 20. Barajas JS, Peterson DS. First-trial protective step performance before and after short-term perturbation practice in people with Parkinson’s disease. J Neurol. 2018;265:1138-1144. [DOI] [PubMed] [Google Scholar]
- 21. Peterson DS, Djikstra BW, Horak FB. Postural motor learning in people with Parkinson’s disease. J Neurol. 2016;263:1518-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Protas EJ, Mitchell K, Williams A, Qhreshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20:183-190. [PubMed] [Google Scholar]
- 23. Shen X, Mak MK. Technology-assisted balance and gait training reduces falls in patients with Parkinson’s disease: a randomized controlled trial with 12-month follow-up. Neurorehabil Neural Repair. 2015;29:103-111. [DOI] [PubMed] [Google Scholar]
- 24. Gerards MHG, Meijer K, Karaminidis K, et al. Adaptability to balance perturbations during walking as a potential marker of falls history in older adults. Front Sports Act Living. 2021;3:682861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pai YC, Bhatt T, Yang F, Wang E. Perturbation training can reduce community-dwelling older adults’ annual fall risk: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69:1586-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parijat P, Lockhart TE. Effects of moveable platform training in preventing slip-induced falls in older adults. Ann Biomed Eng. 2012;40:1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lurie JD, Zagaria AB, Ellis L, et al. Surface perturbation training to prevent falls in older adults: a highly pragmatic, randomized controlled trial. Phys Ther. 2020;100:1153-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pichierri G, Coppe A, Lorenzetti S, Murer K, de Bruin ED. The effect of a cognitive-motor intervention on voluntary step execution under single and dual task conditions in older adults: a randomized controlled pilot study. Clin Interv Aging 2012;7:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoene D, Lord SR, Delbaere D, Severino C, Davies TA, Smith ST. A randomized controlled pilot study of home-based step training in older people using videogame technology. PLoS One 2013;8:e57734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen NE, Sherrington C, Suriyarachchi GD, Paul SS, Song J, Canning CG. Exercise and motor training in people with Parkinson’s disease: a systematic review of participant characteristics, intervention delivery, retention rates, adherence, and adverse events in clinical trials. Parkinsons Dis. 2012;2012:854328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okubo Y, Sturnieks DL, Brodie MAD, Duran L, Lord SR. Effect of reactive balance training involving repeated slips and trips on balance recovery among older adults: a blinded randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2019;74:1489-1496. [DOI] [PubMed] [Google Scholar]
- 32. Okubo Y, Brodie MA, Sturnieks DL, et al. Exposure to trips and slips with increasing unpredictability while walking can improve balance recovery responses with minimum predictive gait alterations. PLoS One. 2018;13:e0202913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okubo Y, Brodie MAD, Sturnieks DL, Hicks C, Lord SR. A pilot study of reactive balance training using trips and slips with increasing unpredictability in young and older adults: biomechanical mechanisms, falls and clinical feasibility. Clin Biomech. 2019;67:171-179. [DOI] [PubMed] [Google Scholar]
- 34. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nasreddine ZS, Phillips NA, Bedirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. [DOI] [PubMed] [Google Scholar]
- 36. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129-2170. [DOI] [PubMed] [Google Scholar]
- 38. Hoenh MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427-442. [DOI] [PubMed] [Google Scholar]
- 39. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disorder. 2010;25:2649-2653. [DOI] [PubMed] [Google Scholar]
- 40. Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30:459-463. [DOI] [PubMed] [Google Scholar]
- 41. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [DOI] [PubMed] [Google Scholar]
- 42. Delbaere K, Hauer K, Lord SR. Evaluation of the incidental and planned activity questionnaire (IPEQ) for older people. Br J Sports Med. 2010;44:1029-1034. [DOI] [PubMed] [Google Scholar]
- 43. Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237-252. [PubMed] [Google Scholar]
- 44. Sturnieks DL, Menant JC, Valenzuela M, et al. Effect of cognitive-only and cognitive-motor training on preventing falls in community-dwelling older people: protocol for the smart±step randomised controlled trial. BMJ Open. 2019;9:e029409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhatt T, Wening JD, Pai YC. Adaptive control of fait stability in reducing slip-related backward loss of balance. Exp Brain Res. 2006;170:61-73. [DOI] [PubMed] [Google Scholar]
- 46. Wang TY, Bhatt T, Yang F, Pai YC. Adaptive control reduces trip-induced forward gait instability among young adults. J Biomech. 2012;45:1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okubo Y, Sturnieks DL, Brodie MA, Duran L, Lord SR. Effect of reactive balance training involving repeated slips and trips on balance recovery among older adults: a blinded randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2019;74:1489-1496. [DOI] [PubMed] [Google Scholar]
- 48. Pelicioni PHS, Lord SR, Okubo Y, Sturnieks DL, Menant JC. People with Parkinson’s disease exhibit reduced cognitive and motor cortical activity when undertaking complex stepping tasks requiring inhibitory control. Neurorehabil Neural Repair. 2020;34:1088-1098. [DOI] [PubMed] [Google Scholar]
- 49. Yang F, Pai YC. Automatic recognition of falls in gait-slip training: harness load cell based criteria. J Biomech. 2011;44:2243-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kwak SK, Kim JH. Statistical data preparation: management of missing values and outliers. Korean J Anesthesiol. 2017;70:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rochester L, Baker K, Hetherington V, et al. Evidence for motor learning in Parkinson’s disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010;1319:103-111. [DOI] [PubMed] [Google Scholar]
- 54. Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risk in older people. J Gerontol A Biol Sci Med Sci. 2001;56:M627-M632. [DOI] [PubMed] [Google Scholar]
- 55. Gazibara T, Pekmezovic T, Tepavcevic T, et al. Circumstances of falls and fall-related injuries among patients with Parkinson’s disease in an outpatient setting. Geriatr Nurs. 2014;35:364-369. [DOI] [PubMed] [Google Scholar]
- 56. Phu S, Sturnieks DL, Lord SR, Okubo Y. Impact of ageing, fell history and exercise on postural reflexes following unpredictable perturbations: a systematic review and meta-analyses. Mech Ageing Dev. 2022;2023:111634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-nnr-10.1177_15459683231206743 for Combined Reactive and Volitional Step Training Improves Balance Recovery and Stepping Reaction Time in People With Parkinson’s Disease: A Randomised Controlled Trial by Paulo H. S. Pelicioni, Stephen R. Lord, Jasmine C. Menant, Carly Chaplin, Collen Canning, Matthew A. Brodie, Daina L. Sturnieks and Yoshiro Okubo in Neurorehabilitation and Neural Repair