Abstract
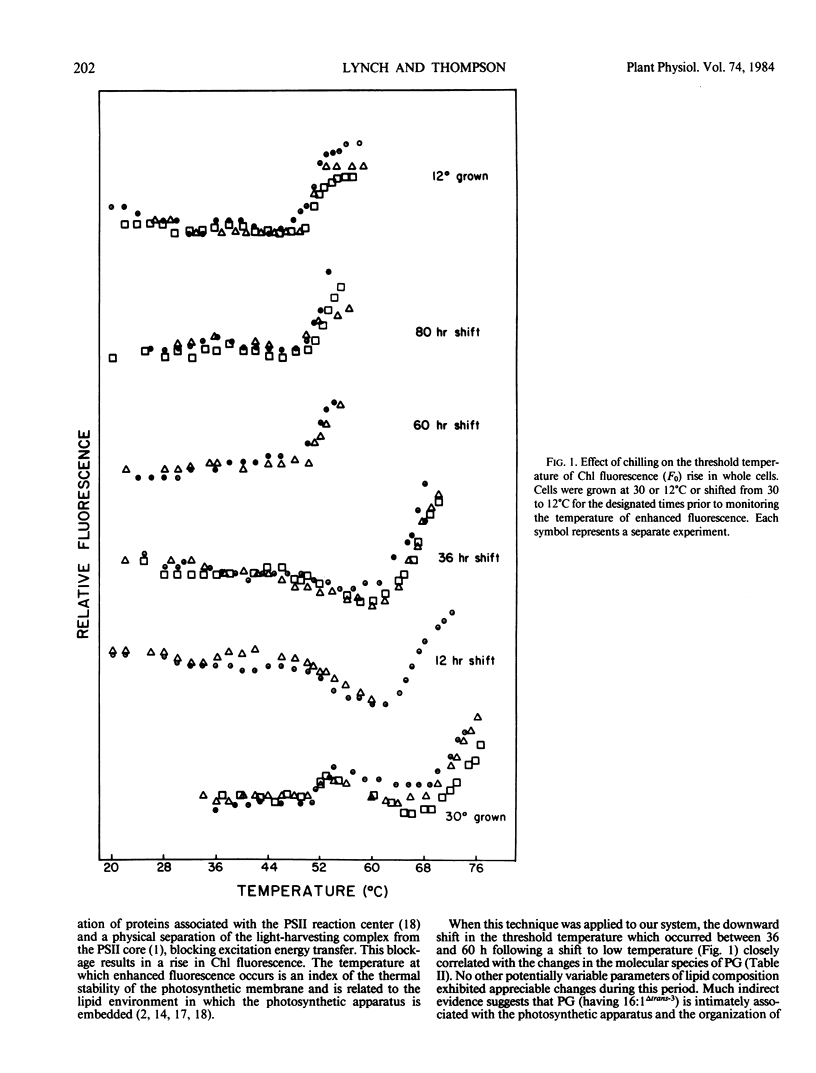
The alterations in chloroplast phospholipid acyl chain composition and phospholipid molecular species composition of Dunaliella salina (UTEX 1644) were monitored during acclimation to low temperature. Chlorophyll fluorescence yield, an indicator of chloroplast membrane stability, was used as a physical means of following the acclimation process.
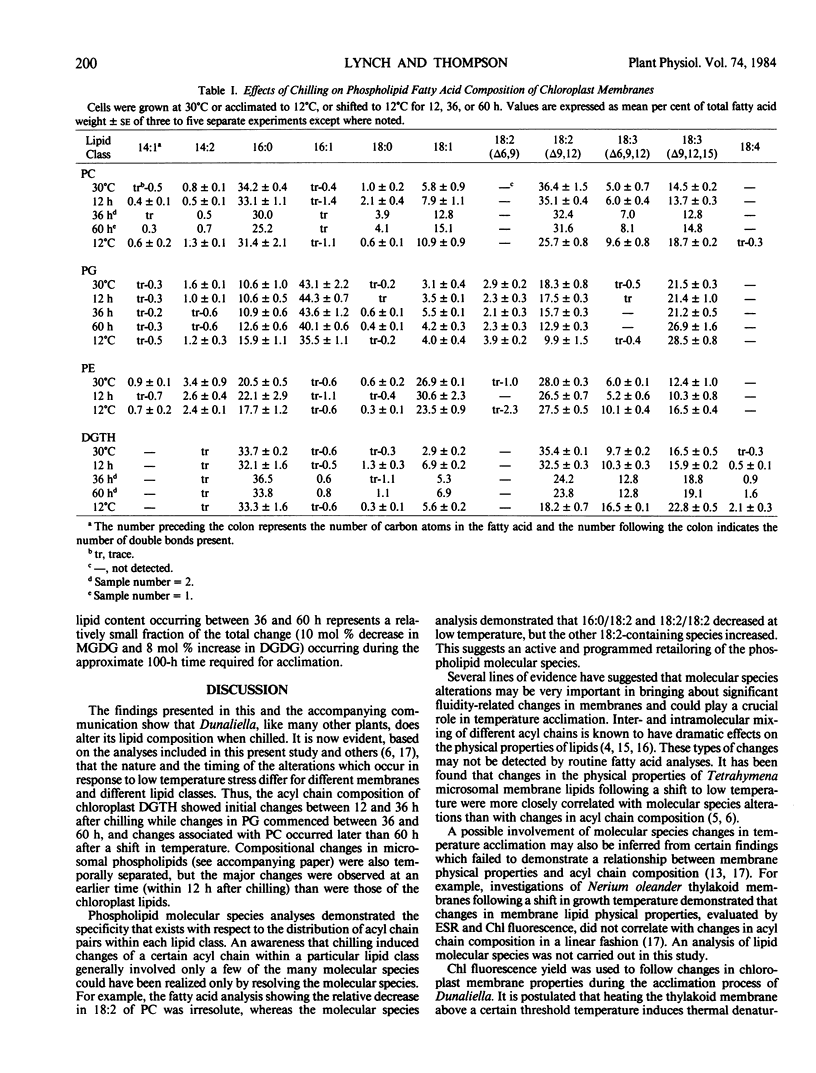
Minor alterations in phospholipid acyl chain composition were evident within 36 hours of shifting the cells from 30 to 12°C. Between 36 and 60 hours, pronounced changes in the acyl chain composition of phosphatidylglycerol (PG) were observed. Changes in the acyl chain composition of phosphatidylcholine (PC) did not occur until sometime after 60 hours.
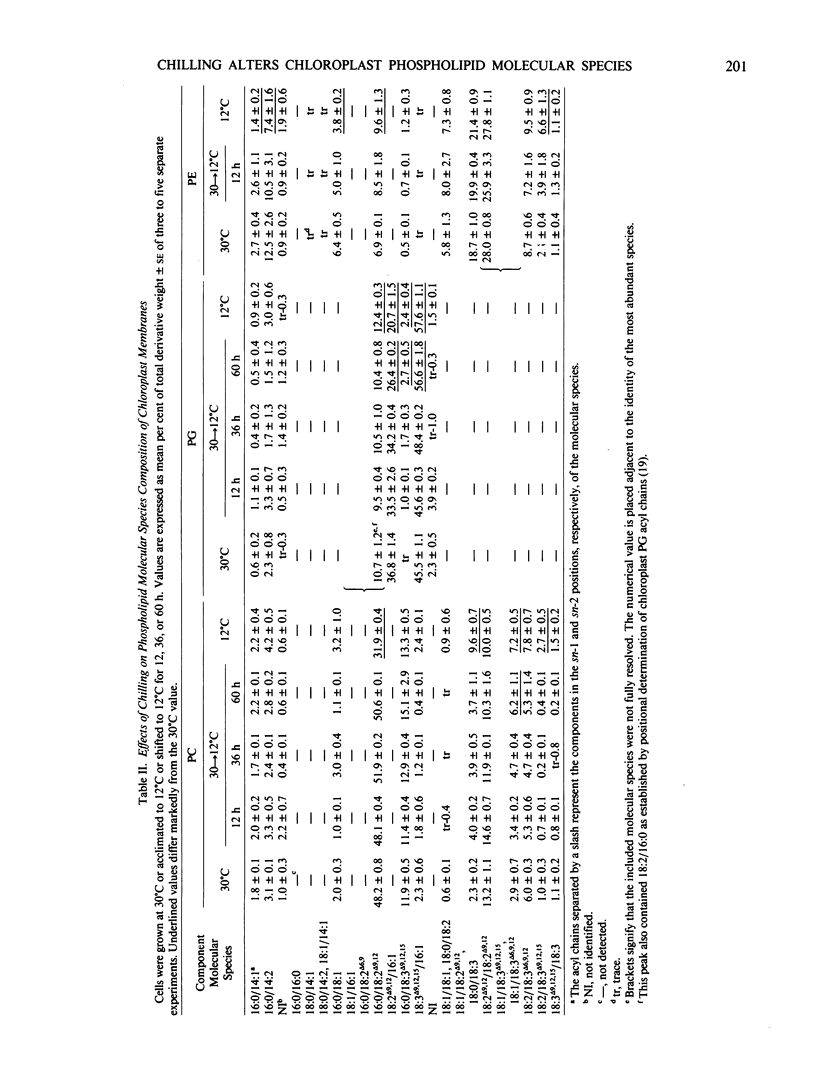
Alterations in the phospholipid molecular species during acclimation were also examined. The pattern of change observed in PC molecular species, namely a decrease in species having one saturated chain (16:0) paired with a C18 acyl chain and a concomitant increase in species having two unsaturated C18 acyl chains, suggests that molecular species changes augment fatty acid compositional changes as a mean of adapting to low temperature. The molecular species of PG were found to change abruptly between 36 and 60 hours following a shift to low temperature. During this time, a dramatic alteration in the threshold temperature of thermal denaturation of the photosynthetic apparatus, as measured by chlorophyll fluorescence, also occurred. Lipid compositional changes other than those associated with PG were negligible during this time. This strongly suggests that a correlation exists between the molecular species composition of PG and the thermal stability of the photosynthetic membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Björkman O., Staehelin L. A. Dissociation of supramolecular complexes in chloroplast membranes. A manifestation of heat damage to the photosynthetic apparatus. Biochim Biophys Acta. 1980 Oct 2;601(3):433–443. doi: 10.1016/0005-2736(80)90547-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Dickens B. F., Thompson G. A., Jr Phospholipid molecular species alterations in microsomal membranes as an initial key step during cellular acclimation to low temperature. Biochemistry. 1982 Jul 20;21(15):3604–3611. doi: 10.1021/bi00258a012. [DOI] [PubMed] [Google Scholar]
- Dickens B. F., Thompson G. A., Jr Rapid membrane response during low-temperature acclimation. Correlation of early changes in the physical properties and lipid composition of Tetrahymena microsomal membranes. Biochim Biophys Acta. 1981 Jun 22;644(2):211–218. doi: 10.1016/0005-2736(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Lynch D. V., Gundersen R. E., Thompson G. A. Separation of galactolipid molecular species by high-performance liquid chromatography. Plant Physiol. 1983 Jul;72(3):903–905. doi: 10.1104/pp.72.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Thompson G. A. Low Temperature-Induced Alterations in the Chloroplast and Microsomal Membranes of Dunaliella salina. Plant Physiol. 1982 Jun;69(6):1369–1375. doi: 10.1104/pp.69.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley K. R., Thompson G. A. Lipid Composition and Metabolism of Volvox carteri. Plant Physiol. 1980 Feb;65(2):260–265. doi: 10.1104/pp.65.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myher J. J., Kuksis A. Resolution of diacylglycerol moieties of natural glycerophospholipids by gas-liquid chromatography on polar capillary columns. Can J Biochem. 1982 Jun;60(6):638–650. doi: 10.1139/o82-079. [DOI] [PubMed] [Google Scholar]
- Pearcy R. W. Effects of Growth Temperature on the Thermal Stability of the Photosynthetic Apparatus of Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1977 May;59(5):873–878. doi: 10.1104/pp.59.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Hauser H., Paltauf F. The inter- and intra-molecular mixing of hydrocarbon chains in lecithin-water systems. Chem Phys Lipids. 1972 Mar;8(2):127–133. doi: 10.1016/0009-3084(72)90024-2. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Schreiber U., Armond P. A. Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochim Biophys Acta. 1978 Apr 11;502(1):138–151. doi: 10.1016/0005-2728(78)90138-x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Fukushima H., Kasai R., Nozawa Y. Studies on thermal adaptation in Tetrahymena membrane lipids. Changes in positional distribution of fatty acids in diacyl-phospholipids and alkyl-acyl-phospholipids during temperature acclimation. Biochim Biophys Acta. 1981 Jul 24;665(1):66–73. doi: 10.1016/0005-2760(81)90233-2. [DOI] [PubMed] [Google Scholar]


