Abstract
Glibenclamide (GB), oral antidiabetic sulfonylurea, is used in the management of diabetes mellitus type II. It suffers from low bioavailability due to low water solubility. This work aimed to enhance the dissolution of GB by formulating the drug as a proniosomes which then improves the pharmacological effect. GB proniosomal formulations were prepared using a slurry method with sucrose as a carrier. The formulations were characterized by particle size, zeta potential, entrapment efficiency %, flow properties of the powder, and in vitro dissolution study. The pharmacological effect was also assessed by determining and measuring the fasting blood glucose level (BGL) before and after the treatment. Formulating GB proniosomes with the slurry method produces a free-flowing powder with a particle size range from 190.050 ± 43.204 to 1369.333 ± 150.407 nm and the zeta potential was above 20 mV (-24 to −58 mV), indicating good stability. The dissolution rate for all formulations was higher than that of the pure drug, indicating the efficiency of the proniosome in enhancing the drug solubility. A significant reduction in the fasting blood glucose level (73 %) was observed in animals treated with proniosomal formulation with no sign of liver damage. In contrast, the pharmacodynamics results show a significant reduction in fasting blood glucose level for animals treated with proniosomes compared to a 17.6 % reduction in BGL after treatment with pure drug. Moreover, the histopathological results showed no sign of liver damage that occurred with proniosomal treatment. GB proniosomal formulations is a promising drug delivery system with good therapeutic efficacy and stability.
Keywords: Glibenclamide, Proniosomes, UPLC method, Pharmacodynamics study, Dissolution enhancement
1. Introduction
Diabetes mellitus (DM) is a major health problem worldwide. Globally, the burden of DM type 2 has risen. 6.28 % of the population was diagnosed with DM type 2 in 2017 and may raise worldwide (Onyango and Onyango, 2018).
Glibenclamide (GB) (Glyburide) is a second-generation sulfonylurea used in the first line for the management of type II diabetes mellitus (FDA, 2016). GB is a weak acid with a pKa of 5.3, its solubility strongly depends on the pH of the test medium and particle size. According to the Biopharmaceutical Classification system, it considers a Class II drug that is characterized by poor solubility. The bioavailability of the GB is 45 %, which is considered low, and this is attributed to the low solubility of the drug. Several attempts were made to enhance the oral bioavailability of the GB such as molecular dispersion (Ganley et al., 1984), incorporation of surfactants, inclusion complexation with cyclodextrin (Mitrevej, Sinchaipanid, and Junyaprasert, 1996) crystal modification (Hassan et al., 1997, Dora et al., 2010) and coprecipitation (Iwata and Ueda, 1996, Dora et al., 2010). Also, novel lipid nanocrystals were developed (Kumar et al., 2014). Minitablets loaded with GB co-adsorbate with Pluronic F-68 and Laponite RD were developed and showed significant improvement in the bioavailability by 1.5-fold (Tawfeeket al., 2018).
Liposomal novel delivery systems (transferosomes, niosomes and ethosomes) are vesicular nanocarriers that gained a great consideration for their roles in improving oral drug bioavailability and enhancing transdermal permeation (Zafar et al., 2023, Ansari et al., 2022, Qumbar et al., 2017). Proniosomes or dry niosomes are vesicular drug delivery systems. Proniosomes are made up of a bilayer of a non-ionic surfactant, a hydrophilic end oriented outward, and a hydrophobic end that remains in the core. Regarding the stability issue, proniosomes are physically more stable during storage and transport than noisome (Walve et al., 2001, Najlah et al., 2015). Technically, proniosomes are promising drug carriers as they possess greater chemical stability and lack many disadvantages associated with liposomes, such as high-cost and variable purity problems of phospholipids (Walve, Rane, and Gujrathi, 2001). Proniosomes are promising delivery systems for lipophilic drugs (El-Laithy et al., 2011, Rahman et al., 2015, Shehata et al., 2015).
Proniosomes are composed of carrier material, surfactant, and cholesterol. The selected carrier should be safe, and non-toxic. Many different carriers were used in proniosomes formulation such as mannitol, sucrose, maltodextrin, glucose monohydrate, and spray-dried lactose. Surfactant is the main structural component in the proniosomes. The non-ionic surfactant is the most widely used. Cholesterol acts as a membrane stabilizer by inhibiting the aggregation by electrostatic or steric effects (Gannu and Pogaku, 2011, Akhilesh et al., 2012, Gowri et al., 2013). Proniosomes have been fabricated for many drugs to improve their dissolution rate and bioavailability (Song et al., 2015, Manasa et al., 2022, Mohanty et al., 2022).
The rationale of this work is to enhance the dissolution rate as well as the pharmacological response of GB. The absorption of GB is pH-dependent, which increased in pH 6–8 and it is classified as a poorly water-soluble drug (Salih, Ghareeb and Mohammed, 2022). Proniosomes are a free-flowing powder with good stability rather than noisome, it could be easily than compressed into tablets or filled in a capsule dosage form. Proniosomes is lamellar molecules in a nanoscales. These smallest molecules have the ability to enhance the dissolution rate of the drug, based on the Noyes Whitney equation that describes the inverse relationship between the particle size and the solubility of the material. Moreover, from the manufacturing site, proniosomes can be easily manufactured on a large scale with the simplest method. It provides a drug delivery system that is chemically and physically stable on storage (Radha, Rani, and Sarvani, 2013). This formulation will enhance the dissolution rate which will enhance the pharmacological efficacy by maintaining a constant drug level with optimum concentration.
This study aimed to formulate and evaluate novel proniosomal formulations of GB prepared by a slurry method using sucrose as a carrier. The prepared formulations were evaluated for particle size, zeta potential, entrapment efficiency, in-vitro dissolution, and for its pharmacodynamic effects by measuring the fasting blood glucose level of rats after the treatment period of 14 days.
2. Methodology
2.1. Materials
Glibenclamide (GB) raw material was supplied by SPIMACO (Qassim, KSA). Potassium dihydrogen phosphate (KH2PO4) and sodium hydroxide pellets (NaOH) were supplied by BDH Laboratory (England). Sucrose was supplied by Fisher Chemical (U.S.A.) Cholesterol pure powder (Cholesterin) was supplied by RdH Laborchemikalien GmbH & Co. KG (Seelze). Formic acid ≥ 98 % was obtained from SIGMA- ALDRICH (Steinheim, Germany). HPLC grade methanol (Riedelde Haën Laboratory Chemicals, Selzer, Germany). Deionized water was obtained from a Milli-Q water purification system (Millipore, USA).
2.2. Methods
2.2.1. Preparation of GB proniosomal formulation
GB proniosomal formulations were prepared using a slurry method. A specific amount of surfactant (Tween 80), cholesterol, and 5 mg drug were dissolved in 20 ml chloroform (Table 1). The mixture was poured into a round-bottomed flask containing sucrose as a carrier. The solvent was evaporated at 40 °C using a rotary evaporator rotated at 60 rpm. After complete evaporation, the powder residue was kept in the desiccator overnight. The residue was scratched, sieved using a 0.45 mm sieve, and stored in the desiccator pending further analysis (Khudaira et al., 2020).
Table 1.
Composition of different Glibenclamide proniosomal formulations.
| Formulation | Amount of GB (mg) | Amount of total lipid (mg) | Amount of carrier (mg) | Amount of Tween 80 (mg) | Amount of cholesterol (mg) |
|---|---|---|---|---|---|
| F1 | 5 | 250 | 500 | 157.23 | 92.77 |
| F2 | 5 | 250 | 500 | 193 | 56 |
| F3 | 5 | 250 | 500 | 208.9 | 41.1 |
| F4 | 5 | 250 | 1000 | 157.23 | 92.77 |
| F5 | 5 | 250 | 1000 | 193 | 56 |
| F6 | 5 | 250 | 1000 | 208.9 | 41.1 |
| F7 | 5 | 250 | 1500 | 157.23 | 92.77 |
| F8 | 5 | 250 | 1500 | 193 | 56 |
| F9 | 5 | 250 | 1500 | 208.9 | 41.1 |
*F is a formulation code.
2.2.2. UPLC assay procedures for GB
Analysis of GB in content and dissolution sample was carried out by using our validated stability indicating method described previously (Ibrahim et al., 2022). The analytical procedures were performed using the UPLC system (Ultimate 3000® binary solvent manager) equipped with an automatic sampler and a Photodiode Array (PDA) detector. The separation was achieved by RP- isocratic elution using a mobile phase consisting of 70 % Methanol and 30 % Water containing (0.231 %) formic acid and the column temperature was adjusted at 40 °C. The mobile phase was delivered at a 0.2 ml/min flow rate through an Acquity UPLC column HSS C18 (2.1x50 mm, 1.7 μm). The total run time is 3.0 min, and recognition was performed at 225 nm.
2.2.3. Characterization of Glibenclamide proniosomal formulations
2.2.3.1. Micromeritics properties of proniosomes' powders
Angle of repose
By using the funnel method place a funnel over a flat surface with a fixed distance of 2 cm, the powder was poured and allowed to pass the funnel slowly until the powder touches the tip of the funnel to form a pile. The diameter of the pile was then measured, and the angle of repose was calculated using the equation (Boddu et al., 2017)
Where, θ - angle of repose, h – the height of the pile, r – radius of the pile. The test was performed in triplicate.
Bulk density
Using analytical balance, 2 g of the powder was weighed and added to a 10 ml cylinder to measure the initial volume. Tapping of the cylinder containing powder was carried out on a flat surface, and after every three taps record the volume until reaching the point when no change in the volume can be noticed (Boddu et al., 2017). Bulk density was given by the equation,
Where ρb- is the bulk density of granules, M is the powder mass in g, Vb is the volume of the powder in the cylinder in mL.
True/tapped density is given by the equation,
Where, ρt- is the bulk density of the powder, M is the mass of the powder in g, Vt is the volume of the powder in the measuring cylinder after tapping in mL.
Compressibility index (Car’s index)
It is directly related to the relative flow rate cohesiveness & particle size. It is a simple fast & popular method of presiding over powder flow characters (Sammour et al., 2019).
2.2.3.2. Determination of particle size and zeta potential
The proniosomal powders were hydrated with phosphate buffer (pH 7.4) and subjected to bath sonication for 10 min (Mohanty et al., 2022). After proper dilution, the resulting dispersion was measured for size and zeta potential using a Zeta sizer using Malvern zetasizer system version 6.02 (Nano S, Worcestershire, UK).
2.2.3.3. Determination of drug entrapment efficiency %
Proniosomal powder (100 mg) was dissolved in 10 ml phosphate buffer pH 7.4 and sonicated in a warm water bath at 50–60 °C for 10 min. A 1 ml aliquot was placed in an Eppendorf and centrifuged at 14000 rpm, for 45 min at 4 °C. The supernatant liquid was separated, diluted to 10 ml with phosphate buffer pH 7.4, filtered using a membrane filter (0.22 μm pore size) (Sammour et al., 2019), and measured using a UPLC method (Ibrahim et al., 2022). The entrapment efficiency will be calculated as follows:
2.2.3.4. Determination of drug content
The amount of proniosomal formulation was dissolved in methanol in a 10 ml volumetric flask, and, and sonicated for ten minutes followed by filtration using a syringe filter (0.22 μm pore size). The drug was measured using the UPLC method after suitable dilution.
2.3. In-vitro dissolution study
The in vitro dissolution study of GB from proniosomal powder and the pure drug was performed using USP type II (paddle) apparatus in 900 ml of phosphate buffer pH 7.4 adjusted at temperature 37 ± 0.5 °C with the rotation speed of 50 rpm (USP, 2010). An aliquot of 5 ml was collected at predetermined time intervals (5, 10, 15, 30, and 60 min), and replaced with a fresh dissolution medium to maintain constant volume after each withdrawal of the aliquot. The drug was measured using the previously mentioned UPLC method.
2.4. Differential scanning calorimetry (DSC)
Thermal analysis was performed to scan thermal behavior of GB, cholesterol, sucrose and the selected formulation of Gb proniosomal formulation, using a DSC 8000 PerkinsElmer (Waltham, MA, USA) apparatus in the temperature range of 25–250 ◦C at a heating rate of 10 ◦C/min. Pyris management software version 10.1 (Pyris Elmer, Waltham, MA, USA) was utilized for the solid-state characterization and assessment of the samples.
2.5. In-vivo pharmacodynamics study
Adult male Wistar albino rats weighed (150–250 g) were supplied by the experimental animal center of our institution maintained in an air-conditioned room (25 ± 1 °C) on a 12-h light/ dark cycle and fed on a standard chow diet and water. All animal experiments were performed per our institutional animal care guidelines. The protocol of this study was approved by the Research Ethics Committee of The Animal Care Center at the College of Pharmacy, King Saud University, Riyadh, Saudi Arabia (Approval # KSU- SE-19–57).
A single dose of STZ (55 mg/kg body weight) was injected intraperitoneally (i.p.) to rats freshly prepared in citrate buffer (PH 4.5) just before injection to induce diabetes mellitus. After 72 h, fasting blood glucose levels were monitored using a Glucometer (ACCU-CHEK®, Swiss city of Basel, Roche) and rats with a blood glucose concentration > 150 mg/dl were considered diabetic and included in this study (Ibrahim et al., 2019).
The animals were divided into four groups (G1, G2, G3, G4) with five individuals in each group, G1: (Negative control) represents healthy individuals, G2 (positive control) represents diabetic individuals not received any treatment, G3 (GB) was treated with GB active pharmaceutical ingredient (API), G4 (Study group) represent diabetic group but treated with GB proniosomal formulation by oral administration, the single daily dose equivalent to 5 mg GB was administered over fourteen days. The initial and final blood glucose level (BGL) was recorded for each individual.
2.6. Histopathology analysis
The hepatic histopathological study was done for the rat liver on the four groups of animals as described in the in vivo study. A portion of the liver was immediately fixed in 10 % neutral buffered formalin (NBF) for histological examination. After one week, the samples were dehydrated and embedded in paraffin wax using Tissue Processor Machine (Tissue-Tek, TEC, and Sakura, Japan). Then sectioned into 5–4 µm thickness using the Rotary Microtome, RM 2245 (Leica, Germany), and transferred on glass slides to dry in the oven. Next, the sections were examined under a light microscope after hematoxylin and eosin (H&E), according to Mayer's modified staining method (Titford, 2005, Lewellyn, 2009).
2.7. Statistical analysis
One way analysis of variance was performed to compare means among groups. Significance was considered at p-value < 0.05.
3. Results
3.1. Characterization of GB proniosomal formulation
3.1.1. Particle size and zeta potential
Particle size (nm) and zeta potential (mV) values for the proniosomal formulations were determined and the results are presented in Table 2. The smallest particle size with a value of (190.05 nm) and PDI (0.253) was noticed in F5, while the F2 formulation has the largest particle size with a value of (1369 nm) and PDI (0.959).
Table 2.
Characterization of GB proniosomal formulations.
| Particle size (nm) | PDI | Zeta potential (mV) | Entrapment efficiency(%) | Dissolution Efficiency (%) | |
|---|---|---|---|---|---|
| F1 | 821.900 ± 34.507 | 0.595 ± 0.176 | −37.80 ± 18.30 | 64.895 ± 4.479 | 45.461 |
| F2 | 1369.333 ± 150.407 | 0.959 ± 0.071 | −44.90 ± 5.58 | 66.522 ± 5.941 | 96.031 |
| F3 | 218.800 ± 55.832 | 0.476 ± 0.106 | −30.80 ± 5.95 | 75.013 ± 6.515 | 75.04 |
| F4 | 277.100 ± 36.06 | 0.512 ± 0.02 | −51.50 ± 7.35 | 73.999 ± 7.107 | 74.456 |
| F5 | 190.050 ± 43.204 | 0.253 ± 0.017 | −39.70 ± 4.48 | 68.973 ± 2.472 | 51.704 |
| F6 | 435.500 ± 16.122 | 0.464 ± 0.004 | −41.80 ± 12.90 | 93.591 ± 0.505 | 60.155 |
| F7 | 627.600 ± 17.253 | 0.604 ± 0.006 | −24.10 ± 47.80 | 73.780 ± 6.180 | 57.118 |
| F8 | 327.750 ± 18.314 | 0.594 ± 0.155 | –32.30 ± 20.80 | 60.358 ± 1.324 | 57.349 |
| F9 | 677.600 ± 1.556 | 0.646 ± 0.014 | −58.00 ± 13.30 | 81.063 ± 2.599 | 87.566 |
*F is a formulation code.
Zeta potential is a good indication of stability, an absolute value greater than 20 indicates that the dispersion system is stable. The zeta potential for all formulations was screened and ranged from 24 to 58 mV. The minimum zeta potential (-24.10 ± 47.80) was observed with F7 compared with other formulations, while F9 had the maximum zeta potential with a value of (-58.00 mV).
3.1.2. Entrapment efficiency (%)
Entrapment efficiency is one of the important values in determining the drug load of the drug delivery system, which, in turn, affects the size of the presented dose. The EE % for all proniosomal formulations ranged between 60 and 93 % as shown in Table 2 and Fig. 1.
Fig. 1.
Histogram of the dissolution efficiency DE (%) and EE% of GB-proniosomal formulations. The error bars in the Histogram (primary Axis) is the SD for DE%. The error r bars in the Square pullets (secondary Axis) is the SD for EE%.
3.1.3. Micromeritics properties of proniosomes' powders
Table 3 represents the flow properties results of all formulations. The flow properties of F1, F2, and F3 were not determined because the nature of the powder was very sticky and non-flowable. Other formulations showed poor flowability with an angle of repose ranging between 35.23 and 43.50, Carr's index from 29.09 to 43.75 %, and Hausner's ratio from 1.41 to 1.77.
Table 3.
Flow properties of GB proniosomal formulations.
| Formulation | Yield % | Angle of Repose | Bulk density | Tapped density | Carr’s index % | Hausner's ratio |
|---|---|---|---|---|---|---|
| F1 | 97.82 % | N/A | N/A | N/A | N/A | N/A |
| F2 | 95.27 % | N/A | N/A | N/A | N/A | N/A |
| F3 | 83.72 % | N/A | N/A | N/A | N/A | N/A |
| F4 | 100 % | 38.31 ± 1.13 | 0.397 | 0.651 | 39.06 | 1.641 |
| F5 | 98.09 % | 35.23 ± 2.26 | 0.445 | 0.628 | 29.09 | 1.410 |
| F6 | 89.61 % | 36.91 ± 1.54 | 0.390 | 0.693 | 43.75 | 1.778 |
| F7 | 99.11 % | 34.29 ± 0.52 | 0.438 | 0.684 | 36.066 | 1.564 |
| F8 | 97.62 % | 42 0.10 ± 1.80 | 0.504 | 0.725 | 30.435 | 1.438 |
| F9 | 99.96 % | 43.50 ± 1.04 | 0.423 | 0.729 | 41.935 | 1.722 |
3.2. In-vitro dissolution
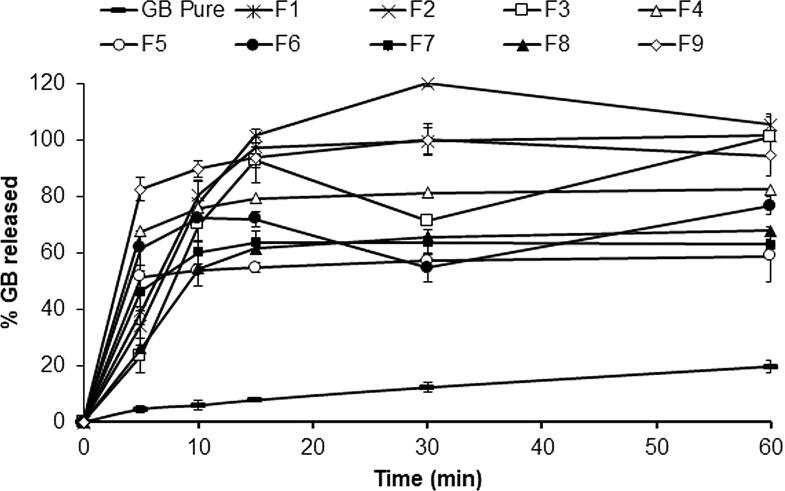
The in vitro dissolution studies were performed for GB proniosomal formulations over 60 min and were compared to the dissolution of untreated GB, Fig. 2. The dissolution profile of untreated GBN revealed very slow dissolution in which 4 % of the drug dissolved within the first 5 min, and only 19 % dissolved after 60 min. The maximum dissolution rate was attained in 30 min for all formulations followed by a steady pattern release (Fig. 2). The highest drug dissolution rats were observed in the case of the proniosomal formulae F2 and F9 in comparison to other formulations with 96.03 % and 87.57 % respectively, followed by proniosomal formulae F3 and F4 were 75.04 % and 74.46 % respectively. In contrast, the drug exhibited the lowest dissolution rate from the proniosomal formulation F1 (45.46 %). The dissolution efficiency % was calculated and presented in (Fig. 1). The DE% for proniosomes formulation ranged from 45 to 96 %. The rigidity of the proniosomal structure mainly depends on the amount of cholesterol. Moreover, the amount of lipid drug entrapped is directly related to amount of the lipid. F3 and F6 have the lowest amount of cholesterol, and here a biphasic release was observed. The highest release in the first 10 min could be attributed to the amout of free drug available at the proniosomal surface (Ismail, Faith, and Ibrahim, 2020).
Fig. 2.
Dissolution profile for different GB-proniosomal formulations.
The selection of the best proniosomal formulation should take into consideration the particle size, proniosomal powder flowability, and drug dissolution rate. The proniosomal powder F4 exhibited a small particle size (277.100 ± 36.06 nm), highest zeta potential value (-51.50 ± 7.35 mV), rapid drug dissolution rate in terms of %DE (74.456 %), and good flow characteristics. Therefore, proniosomal formula F4 was selected for in vivo studies.
3.3. Differential scanning calorimetry (DSC)
The DSC scans of GB, CH, SU and the optimized proniosomal formula are showed in Fig. 3. Untreated GB showed an endothermic peak at 176.0 oC with a heat of fusion; ΔH, of − 81.99 J/g joule/g at a scanning rate of 10 oC/min. Chloesterol (CH) exhibited an endothermic peak at 148.39 °C with ΔH of − 65.30 J/g, while sucrose (SU) broad endothermic peak appeared at 189.13 °C with a heat of fusion of −126.3877 J/g. The DSC scans of GB proniosmal formula showed a combined endothermic peak near to sucrose melting peak (at 191.03 °C).
Fig. 3.
DSC scan of pure GB, cholesterol, sucrose, and the selected proniosomal formulation.
3.4. In-vivo pharmacodynamics study
The data of the in-vitro release study of the optimum novel GB proniosomal formulation (F4) using the newly developed UPLC green method of analysis was subsequently supported by studying the in-vivo release of the same formulation on four different groups of rats segregated into five rats in each group. The study compared the therapeutic effect (Hypoglycemic effect) by measuring the blood glucose level (BGL in mg/dl) of each rat before and after the 14-days of oral treatment of each of the GB pure reference drug (G3) and the effect of the optimum novel GB proniosomal formulation (F4) (G4) versus the control groups; negative control (Healthy individuals; G1) and positive control (diabetic individuals without treatment intervention; G2) and the results were analyzed statistically using one-way ANOVA and presented in (Fig. 4). The results showed the promising of GB proniosomal formulation in blood glucodse reduction compared to the pure drug. The blood glucose level decreased by 73.7 % after the administration of GB proniosomal formulation, while it decrease only by 17.6 % after taking the pure GB.
Fig. 4.
Histogram of the initial and final blood glucose level (BGL mg/dl) of in-vivo study groups. G1: Negative control (Healthy), G2: Positive control (Diabetic untreated), G3: pure reference drug (Diabetic treated with the pure drug), G4: optimum GB-proniosomal formulation (Diabetic treated with the optimum GB-proniosomal formulation). *statistically significant (P < 0.05).
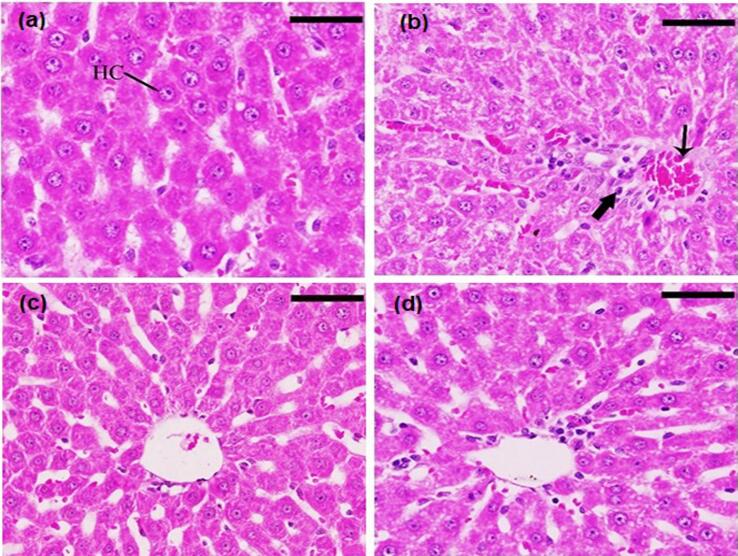
3.5. Histopathological study
The histopathological examination for G1 (healthy rats) showed a normal and ideal strand of hepatocytes, spread typically with Kupffer cells, and liver sinusoids. (Fig. 5a). In contrast, G2 (untreated diabetics rats) exhibited some pathological alterations as scattered inflammatory cells (bold arrow) and some large foci of inflammatory cells surrounding the congested vein with edema (thin arrow) were observed. Additionally, degeneration of the hepatocytes was also spotted (Fig. 5b). Fig. 4c and 4d show the histopathological features for G3 and G4 (diabetic rats treated with GB pure drug and GB proniosomal formulation, respectively). The photos show the liver with normal histological architecture. Fig. 5c shows a little extent of edema surrounding the vein, while in Fig. 5d, no edema was observed.
Fig. 5.
Histopathological examination of liver and hepatocyte a) negative control group, healthy animal; b) positive control group, diabetic animal untreated; c) diabetic animal treated with pure GB; and d) diabetic animals treated with GB proniosomes. Sections were stained with (H&E), and the scale bar = 25 μm, 400X.
4. Discussion
Increasing the amount of cholesterol led to increasing particle size up to the optimum ratio. This could be due to an increase in the hydrophobicity of particle surfaces, promoting aggregation and/or fusion of the individual particles (Khudaira et al., 2020). This phenomenon is well recognized with a low amount of carrier (F1-F3), by increasing the amount of carrier, interstitial bridges between particles are formed, which prevent irreversible interparticle fusion (Ismail, Fetih, and Ibrahim, 2020).
The zeta potential results showed that F9 had the maximum zeta potential with a value of (-58.00 mV). The larger value of zeta potential means the number of charges available in the dispersion system which enhances the repulsion between the particles and increase system stability (Alsarra et al., 2005, Yasam et al., 2014). Moreover, cholesterol plays an important role in vesicular stability by increasing its membrane rigidity (Sammour et al., 2019).
The determination of EE% showed that Tween: cholesterol ratio exerted an agonistic effect on the EE%. Increasing the cholesterol level in the formulation resulted in decreasing the EE%. Therefore, F2 and F9 had the highest EE% with a lower cholesterol ratio. Cholesterol is a key factor to prepare stable proniosomes. Cholesterol act as a membrane stabilizer by accommodating itself among bilayer membranes via hydrogen bonding, and its steroidal nucleus aligns itself parallel to the hydrocarbon chains of the surfactants, and the hydroxyl groups project to the adjacent ester linkages of surfactants' polar head groups. The reduction in the EE% with increasing the cholesterol amount could be explained by competing mechanisms. Cholesterol competes with the lipophilic drug for packing space in the bilayer, thus, limiting the drug from the assembly in the vesicle bilayers during the niosome formation (Ismail et al., 2020, Samy et al., 2018, Mazyed and Zakaria, 2019).
The flow properties of F1, F2, and F3 were not determined because the nature of the powder was very sticky and non-flowable. This could be attributed to the amount of carrier in relation to the amount of lipid, and as the amount of lipid was constant the amount of carrier needed to coat the lipid mixture plays an important role in the proniosomal flowability (Buddo et al., 2017). Formulations F1, F2, and F3 contain the lowest amount of carrier which makes the powder sticky and non-flowable.
The in-vitro dissolution study showed that the highest dissolution rats were observed in the case of the proniosomal formulae F2 and F9 in comparison to other formulations with 96.03 % and 87.57 % respectively. This could be attributed to the ratio of tween: cholesterol might as well as the amount of saccharide carrier impact the proniosomal powder flowability and drug dissolution rate as well. The proniosomal formulations F2 and F9 are composed of higher tween: cholesterol ratios. The presence of a high concentration of surfactant might enhance drug dissolution by minimizing the interfacial tension between drug particles and the dissolution medium, and in turn, increases particles’ wettability, which results in enhancing the drug dissolution rate (Liu and Wang, 2007).
The DSC scan of the selected GB proniosmal formula showed a combined endothermic peak near to sucrose melting peak (at 191.03 °C), indicating that GB endothermic peak was completely disappeared in the proniosmal formula. Disppearence of the drug melting endotherm might indicate homogeneous distribution of the drug particles in the proniosomal matrix, in addition to loss of its crystallinity, which might result in enhancing its dissolution rate (Mahrouset al., 2010).
The in-vivo pharmacodynamics study performed on F4 in comparison to positive and negative control as well as pure drug showed that the proniosomal formulation (F4) succeeded in reducing the blood glucose level by 73.7 % compared with only 17.6 % after treatment with a pure drug (P = 0.002). Moreover, the histopathological figures showed the absence of edema around the vein in case of group treated with GB proniosomes (F4). This indicates the efficacy of GB proniosomes in improving liver conditions and treating the side effect of diabetes on liver cells. A study done by Rambiritch, et al., showed that the reduction in blood glucose level was significant only from zero to 2.5 mg GB and the reduction was by 19 %. The reduction of the blood glucose level is not proportionally with increasing the dose of GB (Rambiritch, Maharaj, and Naidoo, 2014).
5. Conclusion
The vesicular proniosomal formulation for GB is considered one of the most successful methods in enhancing the dissolution rate of the drug. This simple and easy process produced vesicular particles with nanosize and good stability. The pharmacological activity of GB proniosomal formulation showed a significant improvement compared with pure drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research. (IFKSURC-1-0821).
Footnotes
Peer review under responsibility of King Saud University.
References
- Akhilesh D., Faisha G., Kamath J.V. Comparative study of carriers used in proniosomes. Int. J. Pharm. Chem. Sci. 2012;1(1):164–173. [Google Scholar]
- Alsarra I.A., Bosela A.A., Ahmed S.M., Mahrous G.M. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur. J. Pharm. Biopharm. 2005;59:485–490. doi: 10.1016/j.ejpb.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Ansari A.J., Aldawsari M.F., Zafar A., et al. In vitro release and cytotoxicity study of encapsulated sulfasalazine within LTSP micellar/liposomal and TSP micellar/niosomal nano-formulations. Alex. Eng. J. 2022;61(12):9749–9756. [Google Scholar]
- Boddu M., Choppari V., Rapalli V.K., Badam M. Formulation and evaluation of proniosomes of felodipine. Drug Des. 2017;6:1–9. [Google Scholar]
- Dora C.P., Singh S.K., Kumar S., Datusalia A.K., Deep A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol. Pharma. 2010;67:283–290. [PubMed] [Google Scholar]
- El-Laithy H.M., Shoukry O., Mahran L.G. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur. J. Pharm. Biopharm. 2011;77:43–55. doi: 10.1016/j.ejpb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, FDA,. (n.d.). Diaßeta® (glyburide) Tablets USP 1.25, 2.5 and 5 mg (Revised July 2016) Retrieved June 1, 2023, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017532s038lbl.pdf.
- Ganley J.A., McEven J., Calvert R.T., Barker C.J. The effect of in-vivo dispersion and gastric emptying on glibenclamide absorption from a novel, rapidly dissolving capsule formulation. J. Pharm. Pharmacol. 1984;36:734–739. doi: 10.1111/j.2042-7158.1984.tb04861.x. [DOI] [PubMed] [Google Scholar]
- Gannu P.K., Pogaku R. Nonionic surfactant vesicular systems for effective drug delivery - an overview. Acta Pharma. Sin. B. 2011;1(4):208–209. [Google Scholar]
- Gowri S.P., Lakshmi H.V., Bhanu V.N., Brahmaiah B., Sreekanth N., Chandu B.R. Proniosome: a novel approach to vesicular drug delivery system. Pharma J. 2013;2(3):166–173. [Google Scholar]
- Hassan M.A., Sallam E., Al-Hindawi M.K. Preparation and characterization of a new polymorphic form and a solvate of glibenclamide. Acta Pharm. Hungarica. 1997;67:81–88. [PubMed] [Google Scholar]
- Ibrahim M.A., Abou El Ela A.E.F., Al-Rasheed N.M., Al-Amin M.A. Physicochemical and pharmacodynamic evaluation of pioglitazone binary systems with hydrophilic carriers. Pharm. Dev. Technol. 2019;24:883–1390. doi: 10.1080/10837450.2019.1616300. [DOI] [PubMed] [Google Scholar]
- Ibrahim M.A., Alshora D.H., Alowayid M.A., Alanazi N.A., Almutairi R.A. Development and validation of a green UPLC analytical procedure for Glibenclamide determination in pharmaceutical product using response surface methodology. Orient. J. Chem. 2022;38:865–874. [Google Scholar]
- Ismail M.S., Fetih G., Ibrahim E.A. Development, and assessment of oral nifedipine colloidal provesicles. WJPP. 2020;9:1411–1443. [Google Scholar]
- Iwata M., Ueda H. Dissolution properties of glibenclamide in combinations with polyvinylpyrrolidone. Drug Dev. Ind. Pharm. 1996;22:1161–1165. [Google Scholar]
- Khudaira N., Agounia A., Elrayessb M.A., Najlahc M., Younesd H.M., Elhissi A. Letrozole-loaded nonionic surfactant vesicles prepared via a slurry-based proniosome technology: Formulation development and characterization. JDDST. 2020;58:1–12. [Google Scholar]
- Kumar B.S., Saraswathi R., Kumar K.V., Jha S.K., Venkates D.P., Dhanaraj S.A. Development, and characterization of lecithin stabilized glibenclamide nanocrystals for enhanced solubility and drug delivery. Drug Deliv. 2014;21:173–184. doi: 10.3109/10717544.2013.840690. [DOI] [PubMed] [Google Scholar]
- Lewellyn B.D. Nuclear staining with alum hematoxylin. Biotech. Histochem. 2009;84:159–177. doi: 10.1080/10520290903052899. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang X. Improved dissolution of oleanolic acid with ternary solid dispersions. AAPS PharmSciTech. 2007;8:1–5. doi: 10.1208/pt0801016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrous G.M., Ibrahim M.A., El-Badry M., Al-Anazi F.K. Indomethacin sustained release pellets prepared by extrusion– spheronization. J. Drug Delivery Sci. Technol. 2010;20:119–125. [Google Scholar]
- Manasa B., Shanmugam V., Prakash P. Formulation and evaluation of itraconazole proniosomal gel for topical drug delivery. Acta Sci. Pharm. Sci. 2022;6:18–43. [Google Scholar]
- Mazyed E.A., Zakaria S. Enhancement of dissolution characteristics of clopidogrel bisulphate by proniosomes. Int. J. App. Pharm. 2019;11:77–85. [Google Scholar]
- Mitrevej A., Sinchaipanid N., Junyaprasert V. Effect of grinding of β-cyclodextrin and glibenclamide on tablet properties. Part I. In Vitro. Drug Dev. Ind. Pharm. 1996;22:1237–1241. [Google Scholar]
- Mohanty D., Zafar A., Jafar M., Upadhyay A.K., Haque M.A., Gupta J.K., et al. Development, in-vitro characterization and preclinical evaluation of esomeprazole-encapsulated proniosomal formulation for the enhancement of anti-ulcer activity. Molecules. 2022;27:2748. doi: 10.3390/molecules27092748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najlah M., Hidayat K., Omer H.K., Mwesigwa E., Ahmed W., AlObaidy K.G., et al. A facile approach to manufacturing non-ionic surfactant nanodipsersions using proniosome technology and high-pressure homogenization. J. Liposome Res. 2015;25:32–37. doi: 10.3109/08982104.2014.924140. [DOI] [PubMed] [Google Scholar]
- Onyango E.M., Onyango B.M. The rise of noncommunicable diseases in Kenya: an examination of the time trends and contribution of the changes in diet and physical inactivity. J. Epidemiol. Glob. Health. 2018;8:1–7. doi: 10.2991/j.jegh.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qumbar M., Zafar A., Imam S.S., et al. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In-vitro characterization and in-vivo activity. Biomed. Pharmacother. 2017;93:255–266. doi: 10.1016/j.biopha.2017.06.043. [DOI] [PubMed] [Google Scholar]
- Radha G.V., Rani T.S., Sarvani B. A review on proniosomal drug delivery system for targeted drug action. J. Basic Clin. Pharm. 2013;4:42–48. doi: 10.4103/0976-0105.113609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S.A., Abdelmalak N.S., Badawi A., Elbayoumy T., Sabry N., El Ramly A. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: in-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv. 2015;22:731–739. doi: 10.3109/10717544.2014.896428. [DOI] [PubMed] [Google Scholar]
- Rambiritch V., Maharaj B., Naidoo P. Glibenclamide in patients with poorly controlled type 2 diabetes: a 12-week, prospective, single-center, open-label, dose-escalation study. Clin. Pharmacol. 2014;4(6):63–69. doi: 10.2147/CPAA.S54809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih O.S., Ghareeb M.M., Mohammed M.F. Comparison in-vitro release and ph effect among different oral antidiabetic drugs: A review. Sys. Rev. Pharm. 2022;13:366–370. [Google Scholar]
- Sammour R.M.F., Taher M., Chatterjee B., Shahiwala A., Mahmood S. Optimization of aceclofenac proniosomes by using different carriers. Part 1: Development and characterization. Pharmaceutics. 2019;11:1–19. doi: 10.3390/pharmaceutics11070350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy A.M., Ramadan A.A., Abu El-Enin A.S.M., Mortagi Y.I.M. Formulation and optimization of itraconazole proniosomes using box behnken design. Int. J. App. Pharm. 2018;10:41–51. [Google Scholar]
- Shehata T.M., Abdallah M.H., Ibrahim M.M. Proniosomal oral tablets for controlled delivery and enhanced pharmacokinetic properties of acemetacin. AAPS PharmSciTech. 2015;16:375–383. doi: 10.1208/s12249-014-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Tian B., Chen F., Zhang W., Pan Y., Zhang Q., et al. Potentials of proniosomes for improving the oral bioavailability of poorly water-soluble drugs. Drug Deve. Ind. Pharm. 2015;41(1):51–62. doi: 10.3109/03639045.2013.845841. [DOI] [PubMed] [Google Scholar]
- Tawfeek H.M., Roberts M., El Hamd M.A., et al. Glibenclamide mini-tablets with an enhanced pharmacokinetic and pharmacodynamic performance. AAPS PharmSciTech. 2018;19:2948–2960. doi: 10.1208/s12249-018-1108-y. [DOI] [PubMed] [Google Scholar]
- Titford M. The long history of hematoxylin. Biotech. Histochem. 2005;80:73–78. doi: 10.1080/10520290500138372. [DOI] [PubMed] [Google Scholar]
- USP NF revision bulletin, October 1, 2010.
- Walve J.R., Rane B.R., Gujrathi N.A. Proniosomes: A surrogate carrier for improved transdermal drug delivery system. Int. J. Res. Ayurveda Pharm. 2001;2:743–750. [Google Scholar]
- Yasam V.R., Jakki S.L., Natarajan J., Kuppusamy G. A review on novel vesicular drug delivery: Proniosomes. Drug Deliv. 2014;21:243–249. doi: 10.3109/10717544.2013.841783. [DOI] [PubMed] [Google Scholar]
- Zafar A., Yasir M., Khalid M. 2023. Development of Piperine-Loaded Soft Binary Ethosomal Gel to Improve Transdermal Delivery: Box-Bhekhen Design Optimization, Ex-Vivo Permeation, and Antimicrobial Evaluation. J Clust Sci. 10, 02479-8.