Abstract
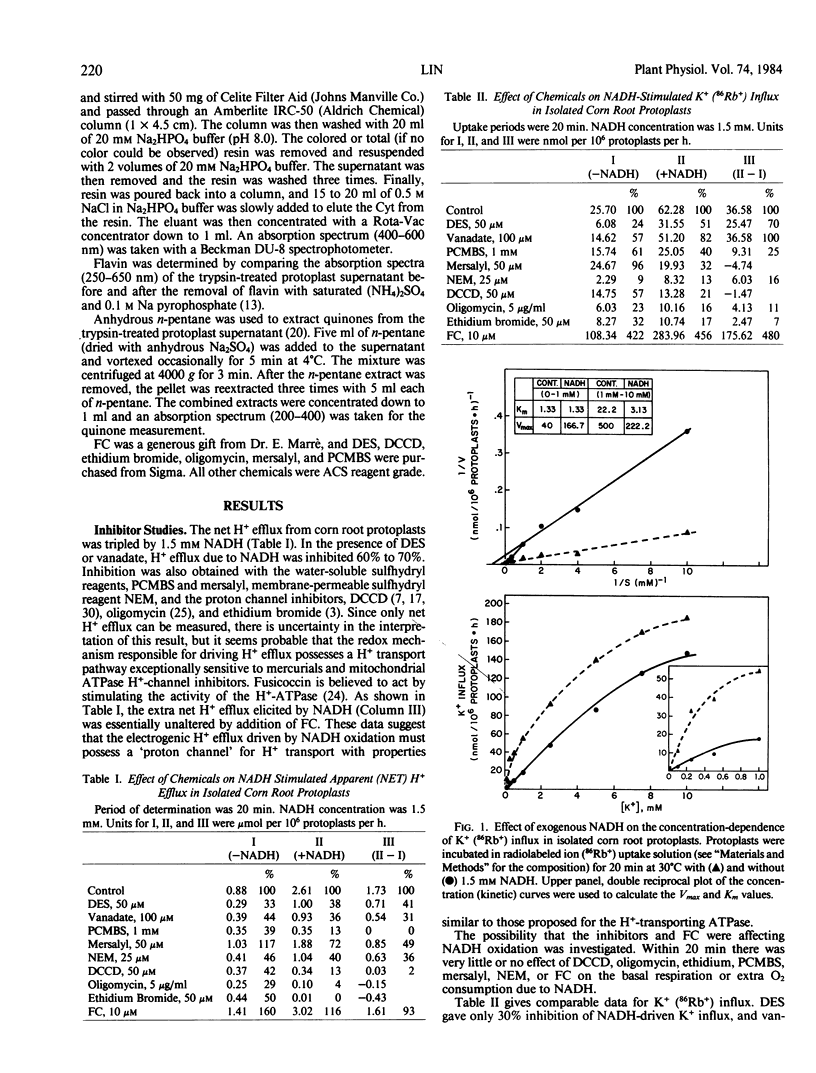
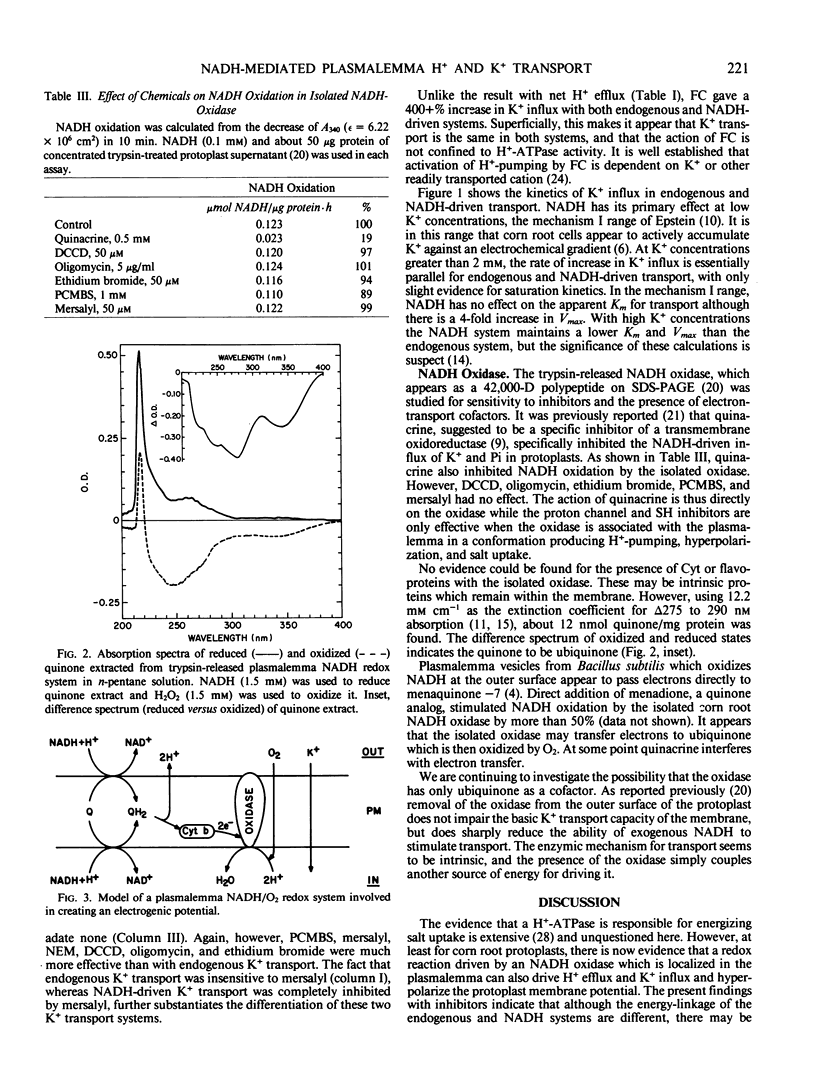
Recent experiments show that exogenous NADH increases the O2 consumption and uptake of inorganic ions into isolated corn (Zea mays L. Pioneer Hybrid 3320) root protoplasts (Lin 1982, Proc Natl Acad Sci USA 79: 3773-3776). A mild treatment of protoplasts with trypsin released most of the NADH oxidation system from the plasmalemma (Lin 1982 Plant Physiol 70: 326-328). Further studies on this system showed that exogenous NADH (1.5 millimolar) tripled the proton efflux from the protoplasts thus generating a greater electrochemical proton gradient across the plasmalemma. Trypsin also released ubiquinone (11.95 nanomoles per milligrams protein) but not flavin or cytochrome from the system. Kinetic analyses showed that 1.5 millimolar NADH quadrupled Vmax of the mechanism I (saturable) component of K+ uptake, while Km was not affected. Diethylstibestrol and vanadate inhibited basal (ATPase-mediated) K+ influx and H+ efflux, while NADH-stimulated K+ uptake was not or only slightly inhibited. p-Chloromercuribenzene-sulfonic acid, N,N′-dicyclohexylcarbodiimide, ethidium bromide, and oligomycin inhibited both ATPase- and NADH-mediated H+ and K+ fluxes. A combination of 10 millimolar fusicoccin and 1.5 millimolar NADH gave an 11-fold increase of K+ influx and a more than 3-fold increase of H+ efflux. It is concluded that a plasmalemma ATPase is not involved in the NADH-mediated ion transport mechanism. NADH oxidase is a -SH containing enzyme (protein) and the proton channel is an important element in this transport system. Fusicoccin synergistically stimulates the effect of NADH on K+ uptake.
Full text
PDF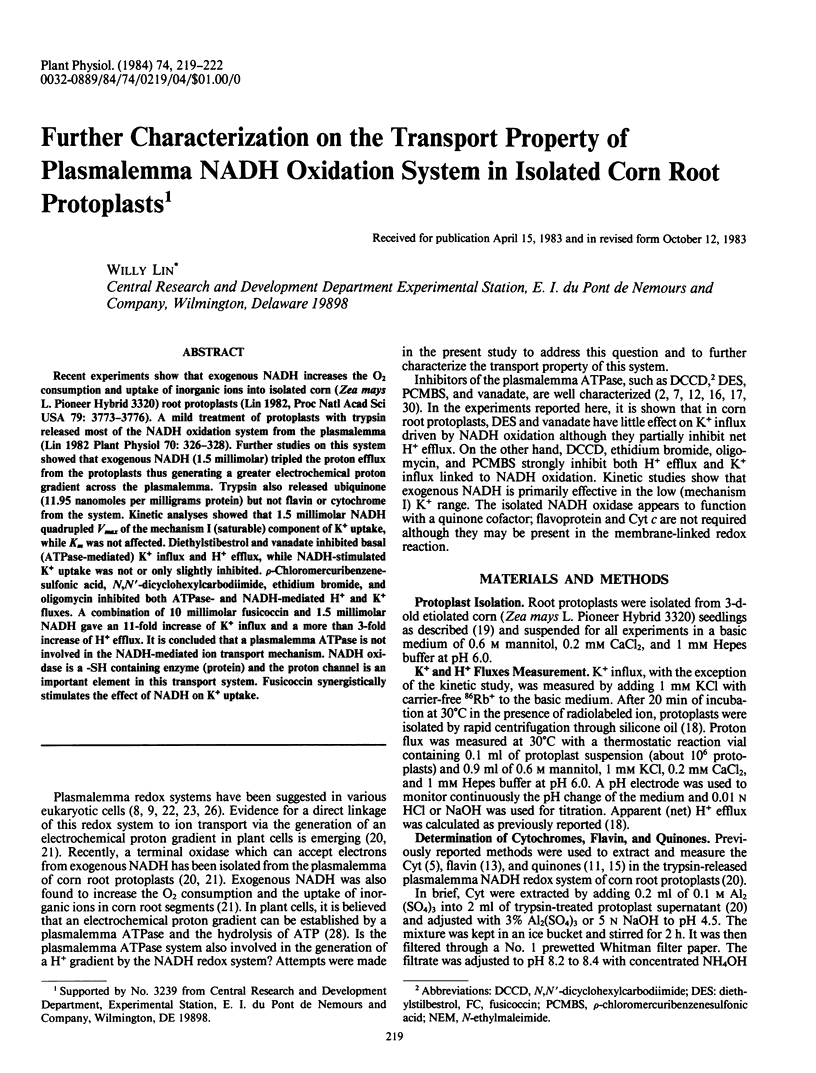

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Eckermann G., Grant M., Robertson R. N. Salt accumulation and adenosine triphosphate in carrot xylem tissue. Proc Natl Acad Sci U S A. 1966 Mar;55(3):560–564. doi: 10.1073/pnas.55.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett R. H., Selwyn M. J. Effects of triphenylsulphonium ions on mitochondria. Inhibition of adenosine triphosphatase activity. Biochem J. 1976 May 15;156(2):315–322. doi: 10.1042/bj1560315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma J., Strijker R., Alkema J. Y., Seijen H. G., Konings W. N. NADH dehydrogenase and NADH oxidation in membrane vesicle from Bacillus subtilis. Eur J Biochem. 1981 Dec;120(3):599–606. doi: 10.1111/j.1432-1033.1981.tb05742.x. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- Cheeseman J. M., Hanson J. B. Energy-linked Potassium Influx as Related to Cell Potential in Corn Roots. Plant Physiol. 1979 Nov;64(5):842–845. doi: 10.1104/pp.64.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane F. L., Löw H. NADH oxidation in liver and fat cell plasma membranes. FEBS Lett. 1976 Oct 1;68(2):153–156. doi: 10.1016/0014-5793(76)80425-5. [DOI] [PubMed] [Google Scholar]
- Ernster L., Glaser E., Norling B. Extraction and reincorporation of ubiquinone in submitochondrial particles. Methods Enzymol. 1978;53:573–579. doi: 10.1016/s0076-6879(78)53058-9. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T. Phloem loading of sucrose: involvement of membrane ATPase and proton transport. Plant Physiol. 1979 Apr;63(4):744–748. doi: 10.1104/pp.63.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Massey V. Reversible resolution of flavoproteins into apoproteins and fee flavins. Methods Enzymol. 1978;53:429–437. doi: 10.1016/s0076-6879(78)53047-4. [DOI] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium transport in corn roots : I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982 Dec;70(6):1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A. Determination of contents and redox states of ubiquinone and menaquinone. Methods Enzymol. 1978;53:579–591. doi: 10.1016/s0076-6879(78)53059-0. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Corn Root Protoplasts: ISOLATION AND GENERAL CHARACTERIZATION OF ION TRANSPORT . Plant Physiol. 1980 Oct;66(4):550–554. doi: 10.1104/pp.66.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Isolation of NADH Oxidation System from the Plasmalemma of Corn Root Protoplasts. Plant Physiol. 1982 Jul;70(1):326–328. doi: 10.1104/pp.70.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Potassium and Phosphate Uptake in Corn Roots: Further Evidence for an Electrogenic H/K Exchanger and an OH/Pi Antiporter. Plant Physiol. 1979 May;63(5):952–955. doi: 10.1104/pp.63.5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Responses of corn root protoplasts to exogenous reduced nicotinamide adenine dinucleotide: Oxygen consumption, ion uptake, and membrane potential. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3773–3776. doi: 10.1073/pnas.79.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw H., Crane F. L. Redox function in plasma membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):141–161. doi: 10.1016/0304-4157(78)90002-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Proton-translocation phosphorylation in mitochondria, chloroplasts and bacteria: natural fuel cells and solar cells. Fed Proc. 1967 Sep;26(5):1370–1379. [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]


