Abstract
Surface properties of Cryptosporidium parvum oocysts were investigated by using electrophoretic mobility and hydrophobicity measurements. Oocysts purified from calf feces by several sucrose flotation steps and deionized water (DI) washes (DIS method) had an electrophoretic mobility (neutral surface charge) near 0.0 m2 V−1 s−1 over a pH range of 2 to 10. The mean electrophoretic mobility of oocysts stored in DI containing a mixture of antibiotics had a lower standard deviation (ς = 0.36) than that of oocysts stored in DI without antibiotics (ς = 0.53); their electrophoretic mobility remained unchanged up to 121 days after collection. The electrophoretic mobility of oocysts purified on a cold Percoll-sucrose gradient after the feces was defatted with ethyl acetate (EAPS method) varied linearly with pH from 0.0 m2 V−1 s−1 at pH 2.4 to −3.2 × 10−8 m2 V−1 s−1 at pH 10 (ς = 0.52), thus displaying the negative surface charge at neutral pH observed by other researchers. The hydrophobicity of oocysts and two types of polystyrene beads was measured as a function of ionic strength by adhesion to polystyrene. Oocysts were purified by the DIS method. The ionic strength of the suspending solution was varied from 0 to 95 mmol liter−1. Two-week-old oocysts exhibited strong adhesion (∼85%) at ionic strengths of 0 to 10 mmol liter−1 and moderate adhesion (∼20%) at ionic strengths of 20 to 95 mmol liter−1. Two-month-old oocysts exhibited high adhesion (∼60 to 80%) at all ionic strengths. These results show that adhesion properties governed by the electrophoretic mobility of purified C. parvum oocysts can be altered by the method of purification and that hydrophobicity can change as oocysts age.
The protozoan parasite Cryptosporidium parvum has been responsible for several recent waterborne disease outbreaks in the United States (26, 31). This gastrointestinal illness is transmitted by an environmentally durable oocyst (15). C. parvum oocysts have been identified in significant amounts in surface waters throughout the United States and Canada (27, 34, 38). Public drinking water supplies derived from filtered surface waters were implicated in all U.S. waterborne cryptosporidiosis outbreaks between 1984 and 1993 (10). Filtration is an important barrier in drinking water purification, because C. parvum oocysts are highly resistant to disinfection with chlorine (25).
Recent studies suggest that the surface properties of C. parvum oocysts may differ from those of bacteria and other microbes. Results reported by Fogel et al. (16) suggest that significant numbers of C. parvum oocysts bypassed a filtration plant that retained smaller coliform bacteria, indicating that oocysts may not adhere to filter media as readily as other microbes. In a series of microscope studies, Anguish and Ghiorse (2) reported that C. parvum oocysts seeded into soil samples and suspended in deionized water (DI), phosphate-buffered saline (PBS), or 0.1% sodium pyrophosphate did not closely associate with inorganic or organic soil particles.
The surface properties of the oocyst wall affect the interactions of the oocysts with filter media and with environmental chemicals and surfaces. Changes in the oocyst wall as they age may affect the adhesion and transport properties of oocysts in natural environments. Changes in the oocyst wall may also affect oocyst survival. For example, Robertson et al. (37) noted that when oocysts were stored in fecal material, the oocyst wall permeability of potentially viable oocysts decreased over time.
Net surface charge and hydrophobicity are important factors mediating microbial adhesion to surfaces (21, 46). Understanding the surface charge and hydrophobicity of C. parvum oocysts will aid the development of optimum filtration media and coagulants to remove oocysts from drinking water and sewage in treatment plants. Such basic knowledge will also help clarify the microscale processes involved in sorption of oocysts onto particle surfaces in natural waters. The identification of noninfective surrogates with similar surface properties will also help facilitate development of treatment strategies and laboratory transport experiments.
Surface charge measurements for C. parvum oocysts have recently been reported by Ongerth and Pecoraro (33), Drozd and Schwartzbrod (14), and Rice et al. (36). Each of these studies utilized different oocyst sources, purification methods, storage solutions, and suspending media, and the reported results varied widely. Some chemicals used for oocyst purification in these studies may damage the oocysts (9) and change oocyst surface properties, including surface charge. A survey of the literature reveals few if any studies of electrophoretic mobilities for C. parvum oocysts in which oocysts were purified and stored under controlled conditions with concern about the use of surface-active chemicals.
Microbial adhesion to hydrophobic surfaces such as polystyrene can be used as a surrogate measurement of microbial adhesion to organic material in the soil. We developed a method for estimating oocyst hydrophobicity that relied on microscopic direct counting of suspended oocyst concentrations after adhesion to a standard polystyrene surface (40, 45, 46). This method was used to measure oocyst hydrophobicity as a function of the ionic strength of the suspending solution. Polystyrene was an ideal substrate for these tests because it is very hydrophobic (1), and the percentage of particles adhering to the polystyrene substrate under the mixing action of a micropipettor provided a reliable qualitative measure of the particle-surface adhesion energy (43, 48).
The objectives of this study were to estimate the electrophoretic mobility of C. parvum oocysts and to determine the effects of purification method and presence of antibiotics on the electrophoretic mobility. We also measured the effects of solution ionic strength on the hydrophobicity of oocysts and polystyrene beads and determined how the electrophoretic mobility and hydrophobic properties of oocysts change as they age.
MATERIALS AND METHODS
Oocyst purification.
C. parvum oocysts were purified by two methods (described below), referred to here as the DIS method and the EAPS method. All oocysts were obtained from fresh neonatal Holstein calf feces which was stored at 4°C until use. Storage time varied with treatment from 12 days to 6 months.
DIS method.
Dwight Bowman (Department of Microbiology and Immunology, Cornell University) utilized a modification of the continuous-flow flotation method of Vetterling (47) to purify oocysts from calf feces within 48 h of collection. A sample of fresh neonatal calf feces (less than 24 h old) was screened through a U.S. Standard Mesh sieve of mesh size 20, diluted approximately 1:10 with DI, and mixed with sugar solution (specific gravity [s.g.] of 1.3) to produce a mixture with an s.g. of approximately 1.12. This material was then introduced into a continuous-flow centrifuge (model V; International Equipment Corporation, Boston, Mass.) with a nonperforated basket and centrifuged at 800 × g. The outflow was collected, diluted with DI to an s.g. equal to 1.05 to 1.09, and recentrifuged. The sediment was diluted to the same s.g. and concentrated again by a third passage through the centrifuge under the same conditions. The resulting sediment was further purified by using several sucrose step gradient centrifugations around an s.g. equal to 1.08; each centrifugation was performed in an ultracentrifuge (model L8M; Beckman Instruments, Palo Alto, Calif.) with a swinging bucket rotor for 15 min at 8°C and at full speed. After the oocyst-containing layers from the step gradients were collected by aspiration, the oocysts were concentrated and washed three times by dilution in DI with centrifugation at 800 × g. After the last wash, the number of oocysts per milliliter was determined by using a hemacytometer and was adjusted to 1.0 × 107 oocysts ml−1 by the addition of DI. Samples stored with antibiotics received 100 U of penicillin G sodium ml−1, 100 μg of streptomycin sulfate ml−1, and 0.25 μg of amphoteracin B ml−1. After purification, all oocysts were stored at 4°C until use. Electrophoretic mobility measurements were conducted on DIS-purified oocysts stored with antibiotics at 4, 7, 31, 94, and 121 days after feces collection. Electrophoretic mobility measurements were conducted on DIS-purified oocysts stored without antibiotics at 4 to 7 days after feces collection. Hydrophobicity measurements were conducted on DIS-purified oocysts at 12 to 17 days and 60 to 68 days after feces collection.
EAPS method.
C. parvum oocysts were purified from calf feces by the method described by Despommier et al. (13). Briefly, this method involved sieving the fecal sample, washing with DI, fixing with formalin, extracting with ethyl acetate (EA), and floating on a cold Percoll-sucrose (PS) cushion (s.g., 1.18), followed by three or four DI washes. EAPS-purified oocysts were suspended in DI with antibiotics added as described above and stored at 4°C until use. For one fecal sample, purification was completed 35 days after collection, and electrophoretic mobility measurements were completed within 5 days of purification. The collection date for the other batch was not recorded; electrophoretic mobility measurements were completed within 3 weeks of purification and perhaps as much as 6 months after collection. These two samples were used to gauge the general effect of the EAPS method on oocyst electrophoretic mobility for comparison with literature values.
Electrophoretic mobility measurements.
The electrophoretic mobility of C. parvum oocysts was measured with a Lazer Zee meter model 501 (Pen Kem, Bedford Hills, N.Y.) at 150 V. Oocysts were suspended at approximately 106 ml−1 in 0.010 M KNO3. This solution is easily prepared, it has a conductivity low enough that the solution conductivity does not interfere with the measurement of electrophoretic mobilities, and K+ and NO3− are less likely than Na+ and Cl− to react with ionogenic compounds on the oocyst surface (17). The pH was adjusted by addition of 0.1 or 0.01 N HNO3 or KOH. Prior to measurement of oocyst electrophoretic mobility, the Lazer Zee meter was calibrated with a standard charged polymer solution.
The initial electrophoretic mobility measurement was taken at the pH of the prepared oocyst suspension (generally between 7.0 and 7.4). Each electrophoretic mobility measurement represents the average for a cloud of 10 to 20 oocysts visible in the microscopic field of view of the Lazer Zee meter chamber (18). After each measurement, the chamber was emptied and the solution pH and temperature were recorded. The pH was then adjusted downward approximately 1 pH unit by addition of HNO3 and returned to the electrophoresis chamber for the next measurement. This was continued until an electrophoretic mobility measurement was recorded at a pH of below 3.0. The pH was then raised approximately 1 pH unit at a time by addition of KOH, and measurements were continued until an electrophoretic mobility measurement was recorded at a pH of above 8 and up to 10, after which the oocyst suspension was discarded. Readings were recorded as electrophoretic mobilities, rather than zeta potentials, according to the recommendations of van Loosdrecht et al. (46). All electrophoretic mobility measurements were corrected to 20°C by the relationship μcorr = μmeas × [1 − 0.02 × (T − 20)], where μcorr = electrophoretic mobility corrected to 20°C and μmeas = electrophoretic mobility measured at T°C (35).
Hydrophobicity measurements.
Adhesion experiments were performed with C. parvum oocysts and two types of polystyrene microspheres. C. parvum oocysts were purified from calf feces by the DIS method described above. Each purified sample received 100 U of penicillin G sodium ml−1, 100 μg of streptomycin sulfate ml−1, and 0.25 μg of amphotericin B ml−1. Experiments were conducted with purified oocysts at 12 to 17 days and 60 to 68 days after excretion. Stock suspensions of 4.5-μm-diameter Polybead uncharged polystyrene microspheres (catalog no. 17135; Polysciences, Warrentown, Pa.) and 6.0-μm-diameter Fluoresbrite carboxylated polystyrene microspheres (catalog no. 16392; Polysciences) were prepared by diluting to approximately 103 microspheres ml−1 in DI. All particle suspensions were stored at 4°C until use.
Particle hydrophobicity as a function of ionic strength was assessed by adhesion to polystyrene (40, 45, 46). Polystyrene microtiter plates (Falcon 3915; Becton Dickinson Labware, Franklin Lanes, N.J.) from a single production batch were used as the standard surface for adhesion measurements. Each microtiter plate contained 96 400-μl wells. Experimental treatments consisted of 20 to 40 different ionic strengths, ranging from 0 to 95 mmol liter−1, with two replicates. For each experiment, treatments were randomly distributed across a single microtiter plate.
Particle suspensions of the appropriate ionic strengths were prepared in the microtiter wells. Appropriate quantities of DI and PBS (containing 2.31 μM NaH2PO4 · H2O, 5.93 μM Na2HPO4 [anhydrous], 120.00 μM NaCl, and 12.69 μM NaN3, with the pH adjusted to 7.0 ± 0.1 with HCl) were first placed in the microtiter wells. A pipettor was used to add 20 μl of vortexed oocyst or bead suspension to each well, yielding a 240-μl suspension at the desired ionic strength. The particle concentration in the stock solution was measured with a hemacytometer (7) immediately prior to filling the wells. Due to the complex ionic makeup of the PBS solution, the ionic strength, I (moles liter−1), was calculated from solution electrical conductivity, EC (decisiemens meter−1), by using the relationship I = 0.0135 × EC (50).
Microtiter plates were covered and incubated at 4°C. After 24 h, the contents of each microtiter well were mixed with a pipettor by three rapid extractions and reinjections of 10 μl of liquid. The pipettor tip was inclined at a 45° angle, with the tip of the pipettor in the center of the well and approximately 1 mm above the bottom of the well. The suspended-particle concentration in each well was then measured with a hemacytometer, with two to four counts per microtiter well. Hydrophobicity, measured as percent adhesion, was calculated as [(C0 − Ci)/C0] × 100, where C0 is the mean number of particles measured for the control and Ci is the mean number of particles measured for a given treatment.
Due to a high degree of variability in the data set, data were transformed into Lowess plots by using Minitab (version 10.5 Xtra Power; Minitab Inc., State College, Pa.). Lowess plots use a robust locally weighted regression method to fit a smooth curve through a scatterplot, helping to reveal trends in the data (11, 12).
RESULTS
Electrophoretic mobility.
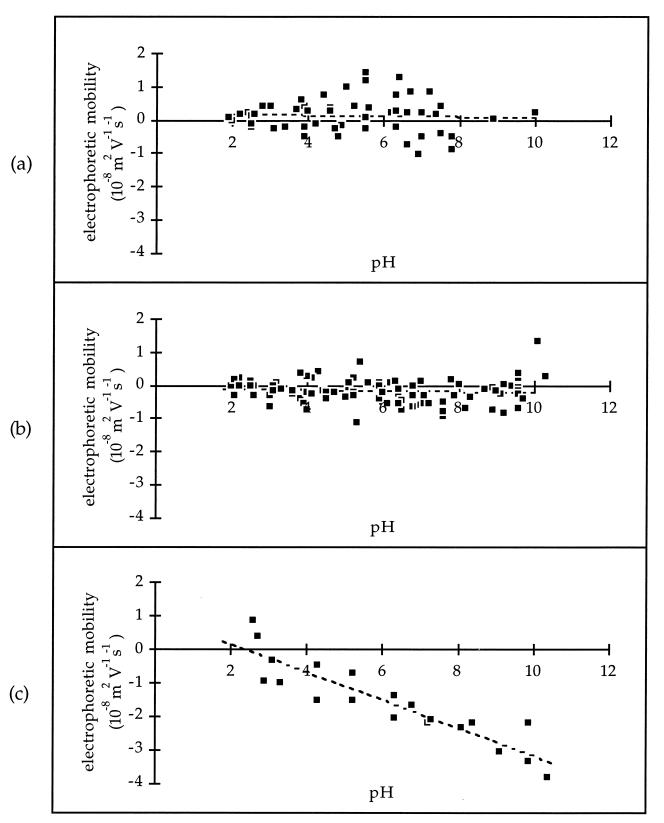
Oocysts purified by the DIS method, with and without antibiotics, exhibited an electrophoretic mobility of between approximately +10−8 and −10−8 m2 V−1 s−1 and a linear regression slope of near zero over the entire pH range investigated (Fig. 1a and 1b). Since the regression lines for these data did not cross the x axis, there are no clear isoelectric points for these measurements. For the oocyst sample stored in antibiotics, there was no difference in electrophoretic mobility between oocysts tested at 4, 7, 37, 94, and 121 days after collection. The presence of antibiotics in the DIS-purified oocyst suspensions did not significantly change the average electrophoretic mobility but did reduce the variation in electrophoretic mobility within the sample. This was reflected in a 31% drop in the standard deviation (Table 1). Again these data show no clear isoelectric point. Oocysts purified by the EAPS method exhibited a strongly linear relationship between electrophoretic mobility and pH, with a distinct isoelectric point at pH 2.37 (Fig. 1c).
FIG. 1.
Electrophoretic mobility versus pH for DIS-purified C. parvum oocysts without antibiotics (a), DIS-purified C. parvum oocysts with antibiotics (b), and EAPS-purified C. parvum oocysts with antibiotics (c). The squares represent individual electrophoretic mobility measurements, and the dashed lines represent the least-squares regression lines through the data.
TABLE 1.
Summary of experimental measurements and literature data on electrophoretic mobility of C. parvum oocysts
| Purification method or literature citation | Slope | Isoelectric point | SD |
|---|---|---|---|
| DIS with no antibiotics (this paper) | −0.003 | 0.53 | |
| DIS with antibiotics (this paper) | −0.018 | 0.36 | |
| EAPS (this paper) | −0.416 | 2.37 | 0.52 |
| Ongerth and Pecoraro (33) | −0.471 | 3.90 | 0.39 |
| Drozd and Schwartzbrod (14)a,b | −0.224 | 2.18 | 0.40 |
| Rice et al. (36)a,c | −0.016 | 0.08 |
a Reported zeta potentials were converted to electrophoretic mobilities by using the Smoluchowski approximation (ζ = 14.1μ, where ζ = zeta potential and μ = electrophoretic mobility) and corrected to 20°C.
b Values are calculated for a pH of <8.
c No table was published; data are taken from Fig. 3 of reference 36.
Hydrophobicity.
The hydrophobicity method developed for this study utilized microtiter plates to measure adhesion to polystyrene. This method allowed a large number of adhesion tests to be conducted in a short time. For a given ionic strength, adhesion results had a high variability. Lowess plots revealed trends in the different oocyst and microsphere treatments.
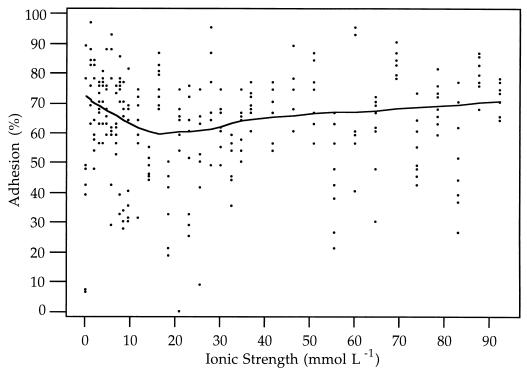
Two-week-old and 2-month-old C. parvum oocysts obtained from the same calf exhibited markedly different adhesion characteristics (Fig. 2). Adhesion characteristics of 2-week-old oocysts were strongly dependent on the ionic strength of the suspending solution. Over 80% of the 2-week-old oocysts adhered at an ionic strength of 0 mmol liter−1. The percent adhesion dropped nearly linearly as the ionic strength rose from 0 to approximately 20 mmol liter−1 and then remained between 10 and 20% as the ionic strength rose to 95 mmol liter−1.
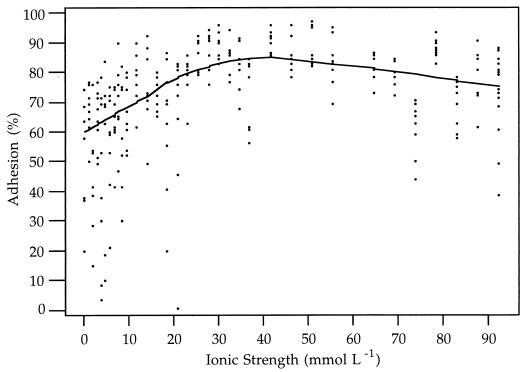
FIG. 2.
Data and Lowess plots for adhesion of 12- to 17-day-old DIS-purified C. parvum oocysts (squares and solid line) and >60-day-old DIS-purified C. parvum oocysts (circles and dashed line) to polystyrene at different ionic strengths.
The adhesion characteristics of 2-month-old C. parvum oocysts showed less dependence on the ionic strength of the suspending solution (Fig. 2). Approximately 70% of 2-month-old oocysts adhered to polystyrene at an ionic strength of 0 mmol liter−1. As the ionic strength increased, the percent adhesion dropped to approximately 62% at approximately 15 mmol liter−1 and then rose again to remain between approximately 70 and 80% at ionic strengths of 30 to 95 mmol liter−1.
The adhesion characteristics of uncharged polystyrene microspheres showed a moderate dependence on solution ionic strength (Fig. 3). Approximately 60% of the uncharged microspheres adhered to the polystyrene substrate at an ionic strength of 0 mmol liter−1. As the ionic strength increased to approximately 40 mmol liter−1, the percent adhesion rose to approximately 85%. As the ionic strength increased further, adhesion decreased nearly linearly to approximately 75% at an ionic strength of 95 mmol liter−1.
FIG. 3.
Adhesion of 6.0-μm-diameter uncharged polystyrene microspheres (electrophoretic mobility = 0.0 m2 V−1 cm−2) to polystyrene at different ionic strengths.
The adhesion characteristics of carboxylated polystyrene microspheres also showed a moderate dependence on the ionic strength of the suspending solution (Fig. 4). Over 70% of the carboxylated microspheres adhered to the polystyrene substrate at an ionic strength of 0 mmol liter−1. As the ionic strength increased to 20 mmol liter−1, adhesion decreased to approximately 60%. As the ionic strength continued to increase to 95 mmol liter−1, adhesion to polystyrene increased nearly linearly to approximately 70%.
FIG. 4.
Adhesion of 4.5-μm-diameter carboxylated polystyrene microspheres (electrophoretic mobility = −68 m2 V−1 cm−2) to polystyrene at different ionic strengths.
DISCUSSION
Electrophoretic mobility.
Oocysts purified by the DIS method exhibited an electrophoretic mobility of very close to zero throughout the pH range examined. These oocysts are hypothesized to be similar to oocysts as they occur in natural waters, because they have had little contact with chemicals that would change their surface properties. The DIS method utilized in this study employed only sucrose and DI in the purification steps, followed by several DI washes with very large dilution ratios and storage in DI with and without antibiotics.
The presence of antibiotics had little if any effect on electrophoretic mobility. However, electrophoretic mobility measurements for oocysts stored in the presence of antibiotics had a lower standard deviation than measurements for oocysts stored without antibiotics. While the exact reasons for this effect are unclear, they could include buffering by the antibiotics, binding of the antibiotic molecules to reactive groups on the oocyst surface, or interactions between ionic species in the antibiotic solution and ionogenic groups on the oocyst surface. Further studies under controlled conditions with simpler buffering solutions are needed to clarify this effect.
Oocysts purified by the EAPS method exhibited an electrophoretic mobility strongly dependent on pH. These oocysts had come in contact with a protein cross-linking agent (formalin), a defatting agent (ethyl acetate), and a polyvinylpyrrolidone (PVP)-containing reagent (Percoll) in addition to DI and sucrose during purification. We hypothesize that the EAPS-purified oocysts had their surface chemistry, including charged ionic groups, altered to a greater degree during purification than oocysts purified by the DIS method. Our results clearly show that oocysts purified by the EAPS method were significantly different in their surface charge from oocysts purified by the DIS method.
Of the three most active ingredients used in the EAPS method (formalin, ethyl acetate, and PVP), ethyl acetate, which is commonly used to extract fats from fecal suspensions, may have caused the most significant changes in the oocyst surface chemistry. Indeed, the corrosive action of this solvent on polytetrafluoroethylene and polystyrene test tubes necessitates the use of glass test tubes. Other studies in our laboratory indicate that the permeability of the oocyst wall increased significantly after contact with ethyl acetate (3), indicating that this solvent may have removed lipids or other ethyl acetate-soluble compounds and thus modified the oocyst surface. Formalin would be expected to cross-link glycoproteins but not to have major effects on surface charge. The effects of PVP on the surface chemistry of oocysts are not known, but this reagent can be used to purify macromolecules such as DNA contaminated with humic acids (5, 51). We recommend that ethyl acetate not be used to purify oocysts for use in studies of their surface properties and that reagents such as formalin, potassium dichromate, and Percoll be avoided or used with caution.
Previous studies of oocyst surface charge were carried out by Rice et al. (36), Drozd and Schwartzbrod (14), and Ongerth and Pecoraro (33). In general, the results of these three studies are difficult to compare with ours, because each study utilized different, often not-well-described purification methods, as well as different suspending solutions and techniques for surface charge measurements. Rice et al. (36) and Drozd and Schwartzbrod (14) reported zeta potential rather than electrophoretic mobility, while Ongerth and Pecoraro (33) reported both zeta potential and electrophoretic mobility. van Loosdrecht et al. (46) recommend reporting results as electrophoretic mobilities, the measured variable, rather than converting to zeta potential, since the conversion to zeta potential relies on the unproven assumption that the oocyst surface is impermeable to ions.
Ongerth and Pecoraro (33) and Drozd and Schwartzbrod (14) used DI as their suspending solution. We decided to use 0.01 M KNO3 rather than DI as the suspending medium for electrophoretic mobility measurements for several reasons. Busscher et al. (8) found irreproducible results when they measured electrophoretic mobilities in DI but stable and reproducible results on addition of small amounts of HNO3 or NaOH. These workers reasoned that this result was probably due to low ionization of surface groups in DI. In addition, if the Smoluchowski approximation is to be used to convert electrophoretic mobility measurements to zeta potential (ζ = 14.1μ, where ζ = zeta potential and μ = electrophoretic mobility), then the ionic strength must be adjusted, since the approximation is valid only for suspending mediums with ionic strengths of greater than 0.001 mol liter−1 (24).
Rice et al. (36) used filtered natural lake water as their suspending medium. While surface charge measurements in natural waters may provide guidance for specific filtration systems, the results obtained with these media may be variable and therefore difficult to compare and interpret. For example, the complex ionic makeup, the presence of unknown chemical species and buffering capacity, or the presence of chemically reactive ionic species all can affect electrophoretic mobility measurements. Furthermore, the chemical makeup in natural waters may change dramatically over time, especially in river waters. Nonetheless, Rice et al. (36) do show a zeta potential for oocysts that is very similar to our results (Table 1).
Electrophoretic mobilities for oocysts purified by the DIS method in this study were different from those reported by Ongerth and Pecoraro (33), who reported a regression slope of 0.471 and an isoelectric point of 3.90 (Table 1). The purification method used by Ongerth and Pecoraro (33) differs from our DIS method primarily in that they used Percoll in their purification protocol. Also, their washing and flotation steps were carried out in centrifuge tubes (20, 34), whereas we used a continuous-flow centrifuge, which may have provided purer oocyst suspensions. We are currently testing these parameters to determine their effects on oocyst electrophoretic mobility.
Electrophoretic mobilities for the oocysts purified by our EAPS method were similar to those reported by Drozd and Schwartzbrod (14), who also used formalin-treated oocysts. Their oocysts also exhibited a strong inverse relationship between surface charge and pH, with an isoelectric point of 2.18 (Table 1).
Hydrophobicity.
The data for adhesion to polystyrene indicate that except at extremely low ionic strengths, 2-week-old oocysts are less strongly attracted to negatively charged surfaces than 2-month-old oocysts (Fig. 2). For ionic strengths of over 20 mol liter−1, approximately 20% of the 2-week-old oocysts adhered to the substrate, compared to over 60% of the 2-month-old oocysts. These results suggest that the hydrophobicity of the oocyst surface changes as the oocysts age after they are excreted. The reasons for such changes are not known, but in general they may be attributed to changes in the physical and chemical properties of the oocyst surface.
As physical objects, like viruses and bacteria (6, 46), oocysts can be considered colloidal particles and modeled as such. The theory of Derjaguin, Landau, Verwey, and Overbeek (DLVO theory) provides a good description of the long-range forces involved in temporary particle-surface adhesion (42) and has been successfully used to describe the adhesion properties of many bacteria and viruses (see, e.g., references 6, 23, 39, 41, and 45). According to the DLVO theory, the net interaction energy (VT) between a spherical particle (e.g., an oocyst or polystyrene microsphere) and a charged plate (e.g., polystyrene) is the sum of the attractive van der Waals energy (VA) and the repulsive electrostatic energy (VE) (23, 41, 42):
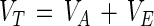 |
1 |
The value of the long-range energy balance VT determines whether temporary adhesion occurs and whether the opportunity for permanent adhesion exists. Hogg et al. (22) and Visser (48) describe the van der Waals and electrostatic energies of interaction between a plate and spherical particle as follows:
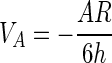 |
2 |
 |
3 |
where C1 = 1 + exp(−κh), C2 = 1 − exp(−κh), C3 = 1 − exp(−2κh), A is the system Hamaker coefficient (ergs), R is the particle radius (meters), h is the distance between the plate and particle (meters), ɛ is the dielectric constant of the suspending medium, and ψP and ψS (volts) are the surface charges of the plate and sphere, respectively. κ (meters−1), referred to as the double-layer thickness, is a function of the ionic strength of the suspending medium (49).
For C. parvum oocysts interacting with a flat polystyrene surface, many of the variables in equations 2 and 3 are fairly easy to estimate. Our electrophoretic mobility measurements showed that ψS for both 2-week-old and 2-month-old oocysts was near 0.0 mV (Table 1). The electrostatic repulsion term (equation 3) then reduces to VE = ɛRψPln[1 − exp(−2κh)]/4. For a given plate surface potential and solution ionic strength, VE reduces to a function of the separation distance h. For oocysts, the value of A in equation 2 is the only remaining unknown.
Hamaker (19) devised a continuum approach using the coefficient A to approximate the total van der Waals interaction between two bodies, where the value of A depends on the chemical compositions of their surfaces. While their complex chemical makeup makes it difficult to determine an exact A for biological particles, the value of A may be approximated from the composition of the particle surface (32). Unfortunately the lack of knowledge regarding the surface composition of C. parvum oocysts (4, 30, 44) precludes estimation of A for oocysts at this time. However, something can be said about how a change in the oocyst surface composition would affect oocyst hydrophobicity. For example, the replacement of lipids in the surface coat with sugars or glycoproteins would result in a significant increase in A (32). This rise in A would cause a corresponding rise in the attractive energy VA (equation 2). Since the surface charge (and thus VE) remained constant as the oocysts aged, we hypothesize that the observed increase in adhesion over time resulted from changes in the chemical makeup of the oocyst surface as they aged.
Oocysts stored in feces or in another environment may behave differently than the oocysts used in this experiment, which underwent some processing and were stored at 4°C in DI containing antibiotics. McEldowney and Fletcher (29) noted that both pH and temperature affected bacterial adhesion to polystyrene. Loeb et al. (28) also suggested that the ionic makeup of the suspending solution will affect the values of ψP and ψS. It is unclear how specific ions (e.g., Na+ and Cl−) in the suspending solution interact with ionogenic compounds on the oocyst surface, influencing the oocyst surface charge. Future studies should explore the sensitivity of oocyst hydrophobicity to changes in temperature and in the ionic makeup and pH of the suspending solution and how storage in fecal suspensions, river water, and other environments affects oocyst hydrophobicity.
ACKNOWLEDGMENTS
This research was supported in part by grants from the New York City Department of Environmental Conservation and the Edna Bailey Sussman Fund.
We thank Dwight Bowman for supplying C. parvum oocysts and Leonard Lion for the use of his Pen Kem Lazer Zee meter. We also thank Juliet Bryant, Michael Jenkins, Charles McCulloch, Kelvin Minniefield, and Mark J. Walker for their assistance and Kathleen Buckley, Nancy Doon, Hilary Grimes, and Sharon Guest-Tagliavento for laboratory assistance.
REFERENCES
- 1.Absolom D R, Lamberti F V, Policova Z, Zingg W, van Oss C J, Neumann A W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46:90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguish L J, Ghiorse W C. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl Environ Microbiol. 1997;63:724–733. doi: 10.1128/aem.63.2.724-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anguish, L. J., and M. B. Jenkins. Unpublished data.
- 4.Anthony L C, Bowman D D, Jenkins M B, Eaglesham B S, Kachlany S C, Ghiorse W C. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Chemical composition and ultrastructure of the oocyst walls of wildtype Cryptosporidium parvum; p. 459. [Google Scholar]
- 5.Bethelet M, Whyte L G, Greer C W. Rapid, direct extraction of DNA from soils for PCR analysis using polyvinyl polypyrrolidone spin columns. FEMS Microbiol Lett. 1996;138:17–22. doi: 10.1111/j.1574-6968.1996.tb08128.x. [DOI] [PubMed] [Google Scholar]
- 6.Bitton G. Adsorption of viruses onto surfaces in soil and water. Water Res. 1975;9:473–484. [Google Scholar]
- 7.Brush C F. Surface and transport properties of Cryptosporidium parvum oocysts. Ph.D. dissertation. Ithaca, N.Y: Cornell University; 1997. [Google Scholar]
- 8.Busscher H J, Bellon-Fontaine M-N, Mozes N, van der Mai H C, Sjollema J, Leonard A J, Rouxhet P G, Cerf O. An interlaboratory comparison of physico-chemical methods for studying the surface properties of microorganisms—application to Streptococcus thermophilus and Leuconostoc mesenteroides. J Microbiol Methods. 1990;12:101–115. [Google Scholar]
- 9.Campbell A T, Robertson L J, Smith H V. Effects of preservation on viability of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1993;59:4361–4362. doi: 10.1128/aem.59.12.4361-4362.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Assessing the public health threat associated with waterborne cryptosporidiosis: report of a workshop. Morbid Mortal Weekly Rep. 1995;44(RR-6):2. [PubMed] [Google Scholar]
- 11.Cleveland W S. Robust locally weighted regression and smoothing scatterplots. J Am Statist Assoc. 1979;74:829–836. [Google Scholar]
- 12.Cressie N A C. Statistics for spatial data. New York, N.Y: John Wiley and Sons; 1993. [Google Scholar]
- 13.Despommier D D, Gwadz R W, Hotez P J. Parasitic diseases. New York, N.Y: Springer-Verlag; 1995. [Google Scholar]
- 14.Drozd C, Schwartzbrod J. Hydrophobic and electrostatic cell surface properties of Cryptosporidium parvum. Appl Environ Microbiol. 1996;62:1227–1232. doi: 10.1128/aem.62.4.1227-1232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayer R, Ungar B L P. Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev. 1986;50:458–483. doi: 10.1128/mr.50.4.458-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogel D, Isaac-Renton J, Guasparini R, Moorehead W, Ongerth J. Removing Giardia and Cryptosporidium by slow sand filtration. J Am Water Works Assoc. 1993;85:77–84. [Google Scholar]
- 17.Goetz, P. J. (Pen Kem, Inc., Bedford Hills, N.Y.). 1995. Personal communication.
- 18.Goetz P J, Penniman J G., Jr A new technique for microelectrophoretic measurements. Am Lab. 1976;8(10):21–30. [Google Scholar]
- 19.Hamaker H C. The London-van der Waals attraction between spherical particles. Physica. 1937;4:1058–1072. [Google Scholar]
- 20.Hansen J S, Ongerth J E. Effects of time and watershed characteristics on the concentration of Cryptosporidium oocysts in river water. Appl Environ Microbiol. 1991;57:2790–2795. doi: 10.1128/aem.57.10.2790-2795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey R W, George L H, Smith R L, LeBlanc D R. Transport of microspheres and indigenous bacteria through a sandy aquifer: Results of natural and forged-gradient tracer experiments. Environ Sci Technol. 1989;23:51–56. [Google Scholar]
- 22.Hogg R, Healy T W, Fuerstenau D W. Mutual coagulation of colloidal dispersions. Trans Faraday Soc. 1966;62:1638–1651. [Google Scholar]
- 23.Israelachvili J N. Intermolecular and surface forces. New York, N.Y: Academic Press; 1992. [Google Scholar]
- 24.James A M. The electrical properties and topochemistry of bacterial cells. Adv Colloid Interface Sci. 1982;15:171–221. [Google Scholar]
- 25.Korich D G, Mead J R, Madore M S, Sinclair N A, Sterling C R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990;56:1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer M H, Herwaldt B L, Craun G F, Calderon R L, Juranek D D. Waterborne disease: 1993 and 1994. J Am Water Works Assoc. 1996;88:66–80. [Google Scholar]
- 27.LeChevallier M W, Norton W D, Lee R G. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl Environ Microbiol. 1991;57:2610–2616. doi: 10.1128/aem.57.9.2610-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb A L, Overbeek J T G, Wiersema P H. The electrical double layer around a spherical colloid particle. Cambridge, Mass: MIT Press; 1961. [Google Scholar]
- 29.McEldowney S, Fletcher M. Effect of pH, temperature, and growth conditions on the adhesion of a gliding bacterium and three nongliding bacteria to polystyrene. Microb Ecol. 1988;16:183–195. doi: 10.1007/BF02018913. [DOI] [PubMed] [Google Scholar]
- 30.Mitschler R R, Welti R, Upton S J. A comparative study of lipid compositions of Cryptosporidium parvum (Apicomplexa) and Madin-Darby bovine kidney cells. J Eukaryot Microbiol. 1994;41:8–12. doi: 10.1111/j.1550-7408.1994.tb05927.x. [DOI] [PubMed] [Google Scholar]
- 31.Moore A C, Herwaldt B L, Craun G F, Calderon R L, Highsmith A K, Juranek D D. Surveillance for waterborne disease outbreaks—United States, 1991–1992. Morbid Mortal Weekly Rep. 1993;42(SS-5):1–22. [PubMed] [Google Scholar]
- 32.Nir S. Van der Waals interactions between surfaces of biological interest. Prog Surf Sci. 1976;8:1–58. [Google Scholar]
- 33.Ongerth J E, Pecoraro J P. Technical report: electrophoretic mobility of Cryptosporidium oocysts and Giardia cysts. J Environ Eng. 1996;122:228–231. [Google Scholar]
- 34.Ongerth J E, Stibbs H H. Identification of Cryptosporidium oocysts in river water. Appl Environ Microbiol. 1987;53:672–676. doi: 10.1128/aem.53.4.672-676.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pen Kem, Inc. Lazer Zee model 501 operators manual. Bedford Hills, N.Y: Pen Kem, Inc.; 1976. [Google Scholar]
- 36.Rice E W, Fox K R, Miltner R J, Lytle D A, Johnson C H. Evaluating plant performance with endospores. J Am Water Works Assoc. 1996;88:122–130. [Google Scholar]
- 37.Robertson L J, Campbell A T, Smith H V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl Environ Microbiol. 1992;58:3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose J B, Gerba C P, Jakubowski W. Survey of potable water supplies for Cryptosporidium and Giardia. Environ Sci Technol. 1991;25:1393–1400. [Google Scholar]
- 39.Rosenberg M, Doyle R J. Microbial cell surface hydrophobicity: history, measurement, and significance. In: Doyle R J, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C: American Society for Microbiology; 1990. pp. 1–37. [Google Scholar]
- 40.Rosenberg M. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl Environ Microbiol. 1981;42:375–377. doi: 10.1128/aem.42.2.375-377.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutter P R, Vincent B. Physiochemical interactions of the substratum, microorganisms, and the fluid phase. In: Marshall K C, editor. Microbial adhesion and aggregation. New York, N.Y: Springer-Verlag; 1984. pp. 21–38. [Google Scholar]
- 42.Shaw D J. Colloid and surface chemistry. Oxford, United Kingdom: Butterworth-Heinemann; 1992. [Google Scholar]
- 43.Taylor A C. Adhesion of cells to surfaces. In: Manly R S, editor. Adhesion in biological systems. New York, N.Y: Academic Press; 1970. pp. 51–71. [Google Scholar]
- 44.Tilley M, Upton S J, Blagburn B L, Anderson B C. Identification on outer oocyst wall proteins of three Cryptosporidium (Apicomplexa: Cryptosporidiidae) species by 125I surface labeling. Infect Immun. 1990;58:252–253. doi: 10.1128/iai.58.1.252-253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Loosdrecht M C M, Lyklema J, Norde W, Zehnder A J B. Bacterial adhesion: a physicochemical approach. Microb Ecol. 1989;17:1–15. doi: 10.1007/BF02025589. [DOI] [PubMed] [Google Scholar]
- 46.van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987;53:1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vetterling J M. Continuous-flow differential density flotation of coccidial oocysts and a comparison with other methods. J Parasitol. 1969;55:412–417. [PubMed] [Google Scholar]
- 48.Visser J. Adhesion of colloidal particles. Surf Coll Sci. 1976;8:3–84. [Google Scholar]
- 49.Weiss L. Biophysical aspects of initial cell interactions with solid surfaces. Fed Proc. 1971;30:1649–1657. [PubMed] [Google Scholar]
- 50.Wolt J. Soil solution chemistry: applications to environmental science and agriculture. New York, N.Y: John Wiley & Sons; 1994. [Google Scholar]
- 51.Young C C, Burghoff R L, Kiem L G, Minak-Bernero V, Lute J R, Hinton S M. Polyvinylpyrrolidone-agarose gel electrophoresis purification of polymerase chain reaction-amplifiable DNA from soils. Appl Environ Microbiol. 1993;59:1972–1974. doi: 10.1128/aem.59.6.1972-1974.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]