Abstract
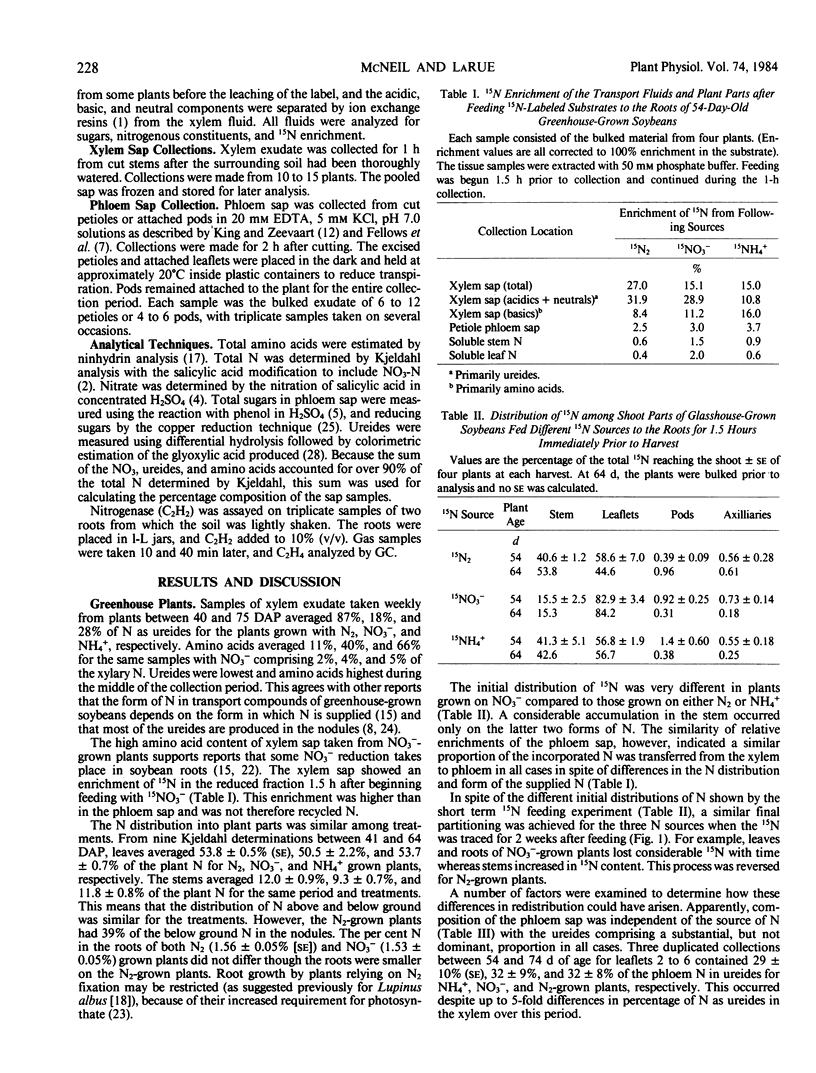
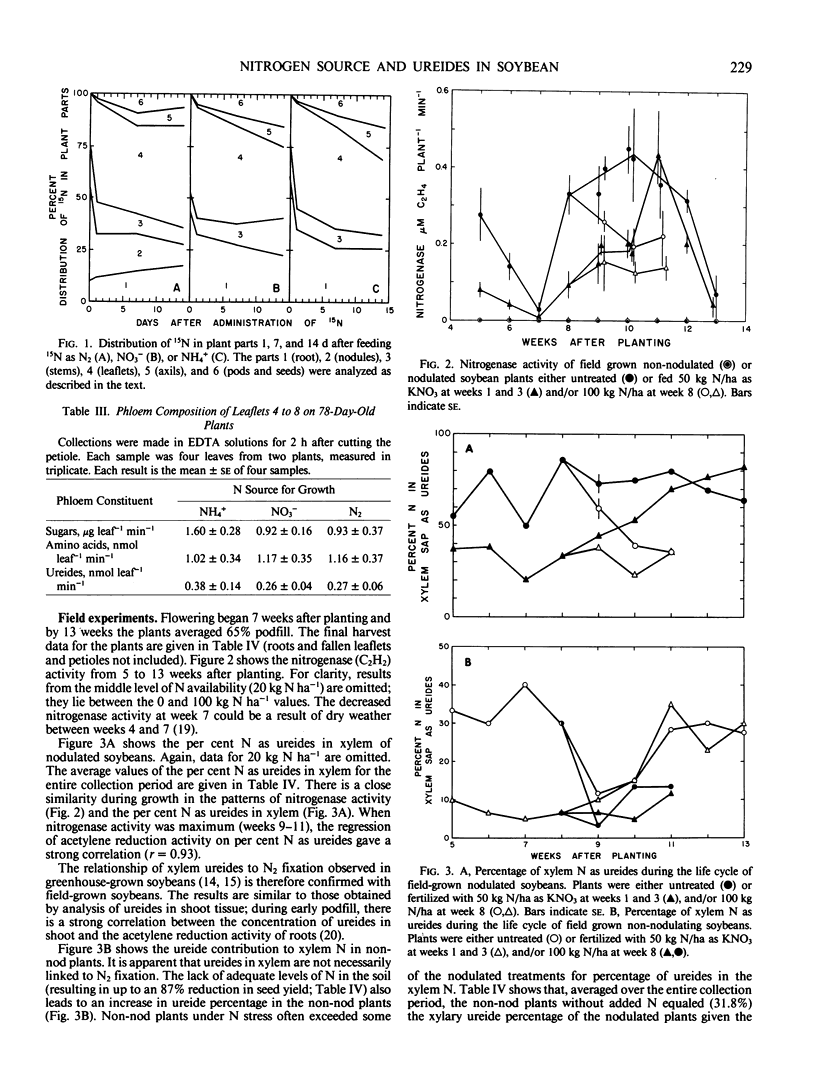
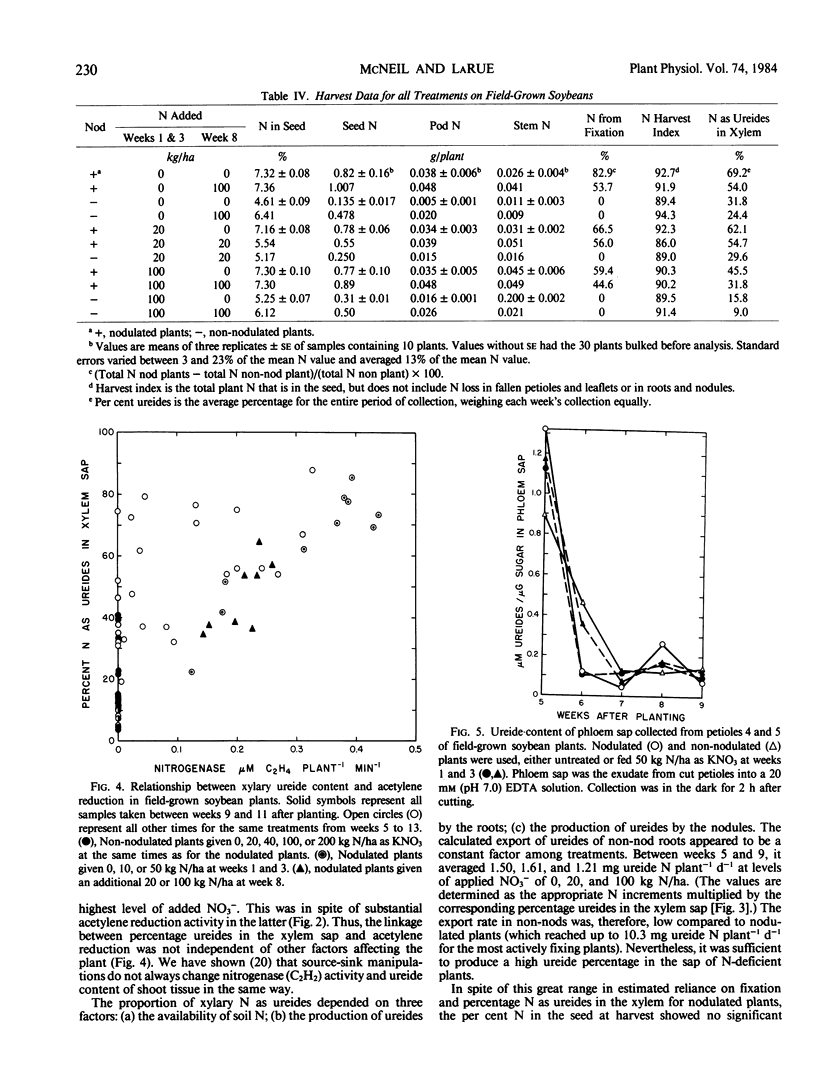
In field-grown soybeans (Glycine max L. Merr. cv Harosoy), the percentage of N in the xylem as ureides increased with increasing N2 fixation. During a 9-week collection period, the ureide content varied from 9.0 to 69.2% of the xylary N. Between 9 and 11 weeks (early pod fill), there was a good correlation (r = 0.93) between C2H2 reduction and the per cent N in xylem as ureides. The per cent N as ureides, however, does not always indicate the reliance of the plant on symbiotic N2 fixation. This ureide content also depended on the level of NO3− available to the roots. Non-nodulated soybeans given from 0 to 200 kilogram N per hectare produced xylem sap which averaged from 31.8% to 9.0% N, respectively, in the xylem as ureides over the 9-week period.
Feeding of 15N2, 15NH4, or 15NO3 to greenhouse-grown soybeans indicated substantial differences in the initial distribution of N by the xylem stream, but the ultimate distribution of N between plant parts and grain did not vary with available N or percentage of xylary N as ureides. Amino acids, not ureides, were the major source of N in the phloem. The soybeans maintained a similar composition in phloem irrespective of the xylem sap constituents, with N derived from N2, NH4, or NO3 being equally accessible to the phloem stream.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Fellows R. J., Egli D. B., Leggett J. E. A Pod Leakage Technique for Phloem Translocation Studies in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1978 Nov;62(5):812–814. doi: 10.1104/pp.62.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S., Yamaguchi M. Effects of Allopurinol [4-Hydroxypyrazolo(3,4-d)Pyrimidine] on the Metabolism of Allantoin in Soybean Plants. Plant Physiol. 1978 Jul;62(1):134–138. doi: 10.1104/pp.62.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F. Relative abundance of ureides and nitrate in plant tissues of soybean as a quantitative assay of nitrogen fixation. Plant Physiol. 1982 Jul;70(1):1–6. doi: 10.1104/pp.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F. Use of the ureide technique to describe the nitrogen economy of field-grown soybeans. Plant Physiol. 1982 Jul;70(1):7–11. doi: 10.1104/pp.70.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley T. L., Peterson D. M., Schrader L. E. Long distance translocation of sucrose, serine, leucine, lysine, and carbon dioxide assimilates: I. Soybean. Plant Physiol. 1977 Feb;59(2):217–220. doi: 10.1104/pp.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil D. L., Atkins C. A., Pate J. S. Uptake and Utilization of Xylem-borne Amino Compounds by Shoot Organs of a Legume. Plant Physiol. 1979 Jun;63(6):1076–1081. doi: 10.1104/pp.63.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Layzell D. B., McNeil D. L. Modeling the transport and utilization of carbon and nitrogen in a nodulated legume. Plant Physiol. 1979 Apr;63(4):730–737. doi: 10.1104/pp.63.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Volk R. J., McClure P. R., Israel D. W., Raper C. D. Relative Content of NO(3) and Reduced N in Xylem Exudate as an Indicator of Root Reduction of Concurrently Absorbed NO(3). Plant Physiol. 1982 Jan;69(1):166–170. doi: 10.1104/pp.69.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Schubert K. R. Enzymes of Purine Biosynthesis and Catabolism in Glycine max: I. COMPARISON OF ACTIVITIES WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING NODULE DEVELOPMENT. Plant Physiol. 1981 Nov;68(5):1115–1122. doi: 10.1104/pp.68.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]


