Abstract
[3H]Zeatin riboside was supplied to intact pea (Pisum sativum) plants either onto the leaves or onto the root nodules. When applied directly to nodules, approximately 70% of recovered radioactivity remained in the nodules, approximately 15% was detected in the root system, and 15% was in the shoot. However, when supplied to the leaves, little 3H was transported, with approximately 0.05% of recovered radioactivity being found in the root system and nodules. On a fresh weight basis, nodules accumulated more 3H than the parent root. In both types of studies, metabolites with an intact zeatin moiety were detected in root nodules.
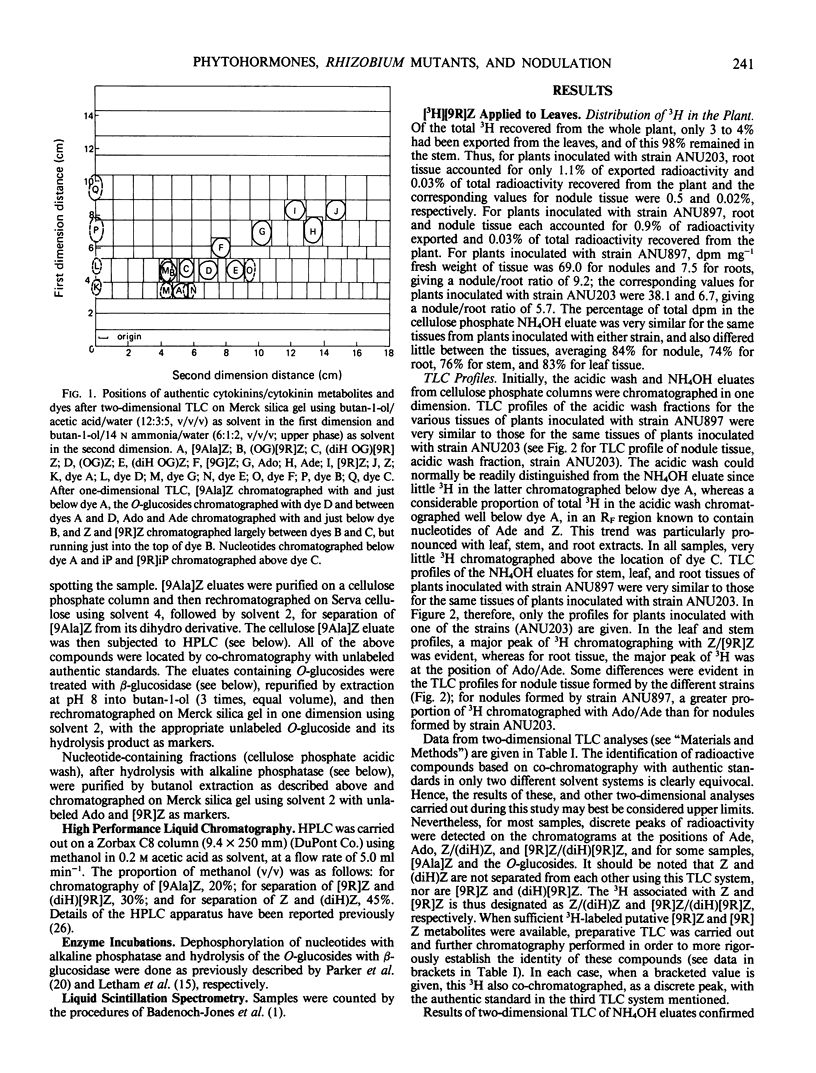
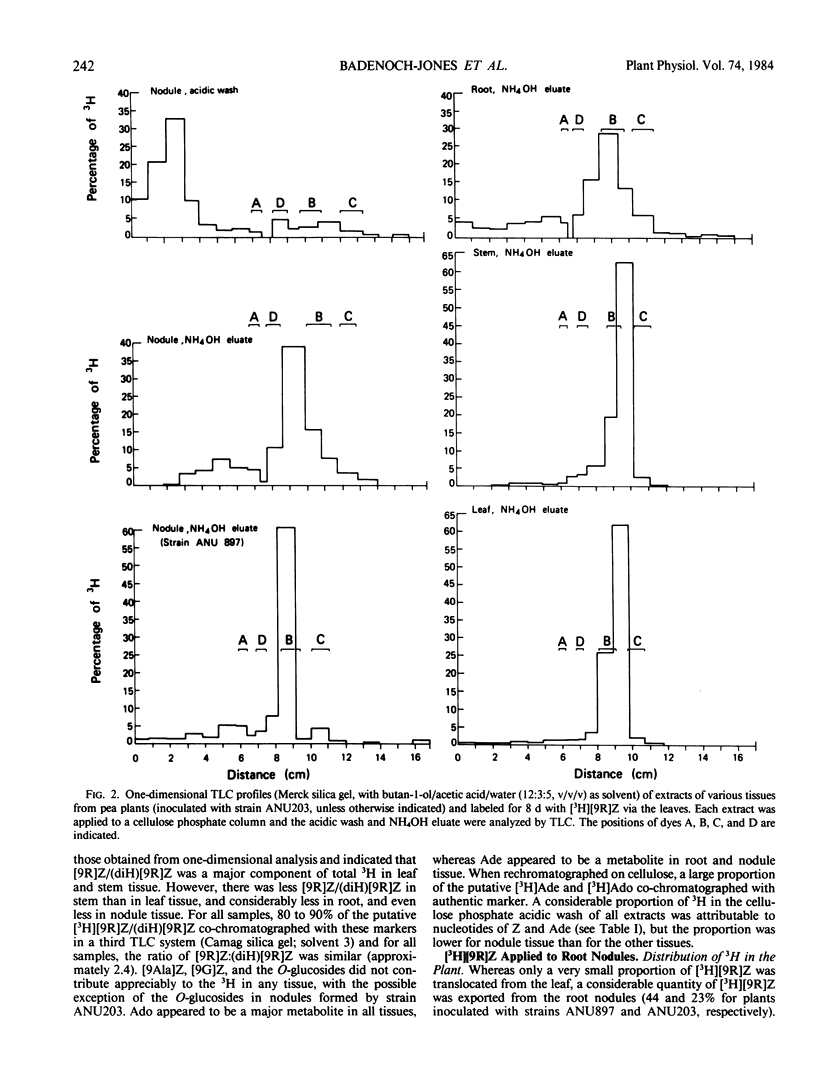
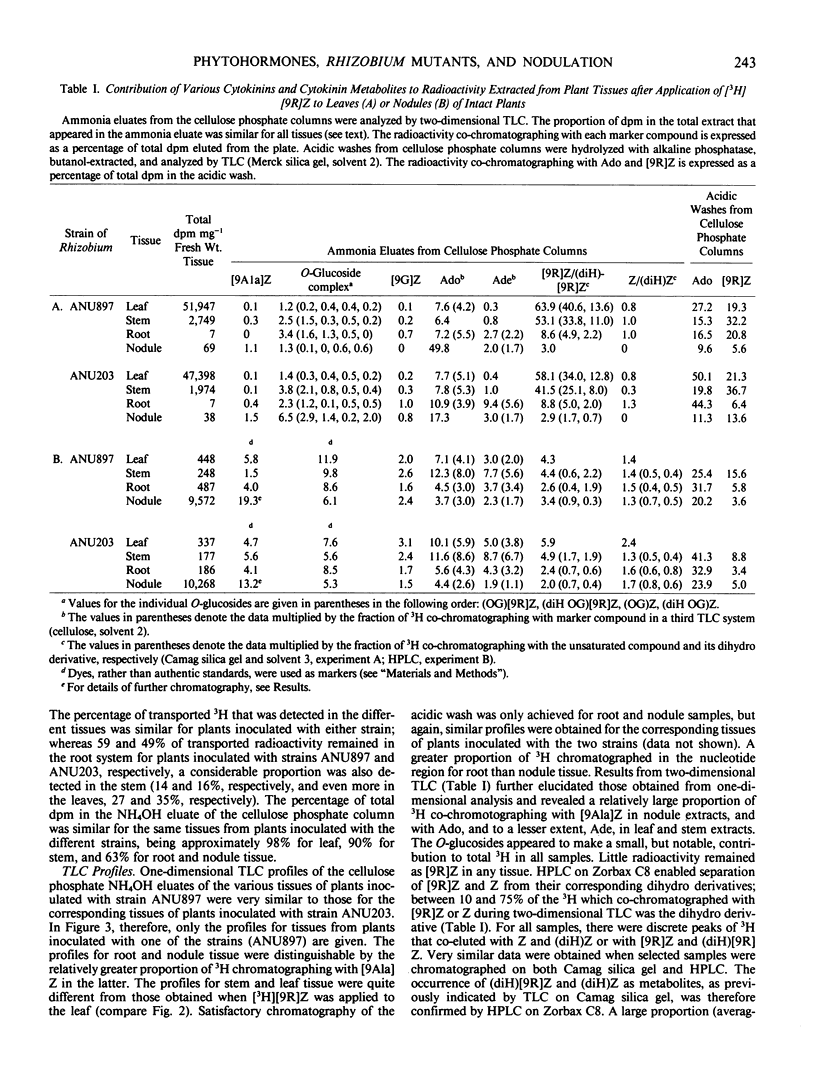
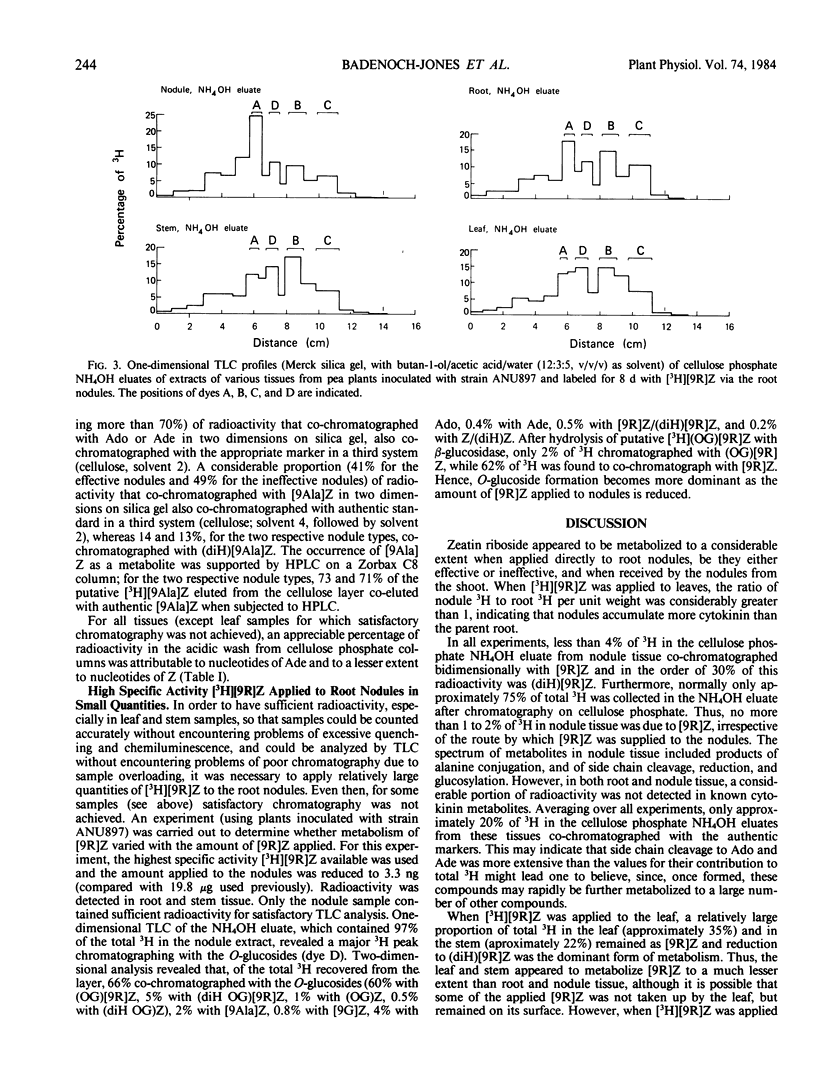
In all experiments, two-dimensional thin layer chromatography revealed that little 3H remained as zeatin riboside in root or nodule tissue at the end of the labeling period. Nodules metabolized [3H]zeatin riboside to the following cytokinins/cytokinin metabolites: zeatin, adenosine, adenine, the O-glucosides of zeatin and zeatin riboside, lupinic acid, nucleotides of adenine and zeatin, and the dihydro derivatives of many of these compounds.
Although a few small differences were observed, there were no major differences between root and nodule tissue in their metabolism of [3H] zeatin riboside. Furthermore, any differences between effective and ineffective nodules were generally minor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badenoch-Jones J., Rolfe B. G., Letham D. S. Phytohormones, Rhizobium Mutants, and Nodulation in Legumes : III. Auxin Metabolism in Effective and Ineffective Pea Root Nodules. Plant Physiol. 1983 Oct;73(2):347–352. doi: 10.1104/pp.73.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Rolfe B. G. Plasmids and stability of symbiotic properties of Rhizobium trifolii. J Bacteriol. 1982 Aug;151(2):560–568. doi: 10.1128/jb.151.2.560-568.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letham D. S., Wilson ?MM, Parker C. W., Jenkins I. D., Macleod J. K., Summons R. E. Regulators of cell division in plant tissue. XXIII. The identity of an unusual metabolite of 6-benzylaminopurine. Biochim Biophys Acta. 1975 Jul 14;399(1):61–70. doi: 10.1016/0304-4165(75)90211-1. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Syõno K., Torrey J. G. Identification of Cytokinins of Root Nodules of the Garden Pea, Pisum sativum L. Plant Physiol. 1976 Apr;57(4):602–606. doi: 10.1104/pp.57.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORREY J. G. Kinetin as trigger for mitosis in mature endomitotic plant cells. Exp Cell Res. 1961 Mar;23:281–299. doi: 10.1016/0014-4827(61)90038-6. [DOI] [PubMed] [Google Scholar]
- Terrine C., Laloue M. Kinetics of N-(Delta-Isopentenyl)Adenosine Degradation in Tobacco Cells: EVIDENCE OF A REGULATORY MECHANISM UNDER THE CONTROL OF CYTOKININS. Plant Physiol. 1980 Jun;65(6):1090–1095. doi: 10.1104/pp.65.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]


