Abstract
Enzymes that are used as animal feed supplements should be able to withstand temperatures of 60 to 90°C, which may be reached during the feed pelleting process. The thermostability properties of three histidine acid phosphatases, Aspergillus fumigatus phytase, Aspergillus niger phytase, and A. niger optimum pH 2.5 acid phosphatase, were investigated by measuring circular dichroism, fluorescence, and enzymatic activity. The phytases of A. fumigatus and A. niger were both denatured at temperatures between 50 and 70°C. After heat denaturation at temperatures up to 90°C, A. fumigatus phytase refolded completely into a nativelike, fully active conformation, while in the case of A. niger phytase exposure to 55 to 90°C was associated with an irreversible conformational change and with losses in enzymatic activity of 70 to 80%. In contrast to these two phytases, A. niger pH 2.5 acid phosphatase displayed considerably higher thermostability; denaturation, conformational changes, and irreversible inactivation were observed only at temperatures of ≥80°C. In feed pelleting experiments performed at 75°C, the recoveries of the enzymatic activities of the three acid phosphatases were similar (63 to 73%). At 85°C, however, the recovery of enzymatic activity was considerably higher for A. fumigatus phytase (51%) than for A. niger phytase (31%) or pH 2.5 acid phosphatase (14%). These findings confirm that A. niger pH 2.5 acid phosphatase is irreversibly inactivated at temperatures above 80°C and that the capacity of A. fumigatus phytase to refold properly after heat denaturation may favorably affect its pelleting stability.
The industrial importance of thermostable enzymes is increasing. Therefore, it comes as no surprise that isolation, characterization, and engineering of thermostable enzymes, as well as the search for the determinants of thermostability, are hot spots of current research (2, 3, 9–11). Different strategies can be used to obtain a thermostable enzyme with the desired catalytic activity. These strategies include (i) screening of thermophilic and hyperthermophilic organisms for the catalytic activity and substrate specificity of interest; (ii) mutagenesis of a mesophilic enzyme in order to increase its thermostability; and (iii) mutagenesis of a known thermostable enzyme that catalyzes a closely related reaction, with the aim of modifying its substrate specificity.
Phytases (EC 3.1.3.8) belong to the family of histidine acid phosphatases (4, 7) and are found primarily in microorganisms and plants. These enzymes catalyze the release of phosphate from phytic acid (myo-inositol hexakisphosphate), the major phosphorus storage form in plants. Since monogastric animals like poultry and pigs have very low or no phytase activities in their digestive tracts, inorganic phosphorus has to be added to the feed in order to meet the phosphorus requirements of these animals. Supplementation with inorganic phosphate, however, imposes ecological problems (eutrophication), which makes partial substitution of inorganic phosphate by phytase desirable. Because poultry and pig feed commonly is pelleted, a commercially attractive phytase should be able to withstand the temperatures that may be reached temporarily during the pelleting process (60 to 90°C).
As a first step towards the development of a thermostable phytase, a number of phytases from various molds have been cloned and overexpressed (4–6). In the present investigation, we analyzed the thermostabilities of selected wild-type phytases and a pH 2.5 acid phosphatase by measuring circular dichroism (CD), fluorescence, and activity and by performing feed pelleting experiments. Aspergillus niger phytase is the only phytase that is commercially available at present for feed application. Aspergillus fumigatus phytase performed better in animal experiments (16) and, at least in a purified form in test tubes, resisted heat treatment at temperatures up to 100°C (5). A. niger pH 2.5 acid phosphatase was included since it was reported to have a higher intrinsic thermostability than A. niger phytase (13, 14). The results which we obtained may facilitate the design and development of improved phytases.
MATERIALS AND METHODS
Materials.
Phytic acid (dodecasodium salt) was purchased from Sigma, and 4-nitrophenyl phosphate (disodium salt) was purchased from Merck. All of the other chemicals used were at least analytical grade and were products of Fluka or Bio-Rad. The phytases of A. niger T213 and A. fumigatus ATCC 34625, as well as the pH 2.5 acid phosphatase of A. niger T213, were overexpressed in A. niger and purified to apparent homogeneity (17, 18).
The sequence of the A. niger T213 phytase used in the present investigation differs from the sequence of the previously described A. niger NRRL3135 phytase (15) in 12 amino acids: S14A, S30T, E66D, D89E, A106V, V155I, K171E, V236A, N292H, Q297R, S345N, and V438I (4). In addition, it differs in two amino acids (Q297R and S466F) from the sequence of the A. niger var. awamori ATCC 38854 phytase (7).
Fluorescence spectroscopy.
Fluorescence measurements were performed with an SLM model 4048S instrument. The slit width was set at 4 nm, the gain was set at 200, and the excitation and emission wavelengths were set at 280 and 340 nm, respectively. The effects of temperature on the fluorescence properties of acid phosphatases were investigated in the following experiment. Samples (in 10 mM sodium acetate, pH 5.0) were diluted to an optical density at 280 nm of 0.1. Each acid phosphatase was measured in a thermostatted quartz cuvette consecutively at 30, 50, 70, 80, 90, 80, 70, 50, and 30°C. Individual measurements were obtained at 10-min intervals, the time needed to heat or cool the sample and to allow for adequate preincubation at the subsequent temperature. The enzymatic activities of the samples were measured both at the beginning and at the end of the experiment.
CD spectroscopy.
CD spectra were recorded with a Jobin Yvon model CD 6 dichrograph. The instrument was calibrated with epiandrosterone (Δɛ = 3.3 at 304 nm [2a]) and camphorsulfonic acid (Δɛ = −4.72 at 188 nm and Δɛ = 2.37 at 289 nm [1]). Far-UV CD spectra were acquired over a wavelength range of 190 to 260 nm by using 1-nm increments. The baseline obtained with solvent alone was subtracted from the sample spectra. Temperature was controlled with a Haake model D8 thermostat and with a thermostatted cell holder supplied by Jobin Yvon. Residual molar ellipticities (in degrees per square centimeter per decimole) were plotted against wavelength. The proportions of secondary-structure elements (α-helix and β-sheet) were calculated from the CD spectra with the program Provencher (8).
The samples (in 10 mM sodium acetate, pH 5.0) used for the CD measurements were filtered through 0.22-μm-pore-size membranes to remove particles and aggregates; the protein concentrations in these samples were 0.27 to 0.41 mg/ml. The samples were incubated for 20 min at 30, 50, 70, 80, or 90°C. Subsequently, either the CD spectra were measured directly at the same temperatures, or the samples were first allowed to renature for 1 h at 30°C and then the CD spectra were measured at 30°C.
Measurements of enzymatic activity.
Phytase activity was measured in an assay mixture containing 0.5% phytic acid (∼5 mM) and 200 mM sodium acetate (pH 5.0). The enzymatic activity of the pH 2.5 acid phosphatase was measured in an assay mixture containing 4-nitrophenyl phosphate (0.65 mg/ml) and 50 mM glycine (pH 2.5). In feed samples (see Fig. 7), the phytase activity was measured in an assay mixture containing 5 mM phytic acid and 200 mM sodium acetate (pH 5.5), and the acid phosphatase activity was measured in an assay mixture containing 5 mM 4-nitrophenyl phosphate and 200 mM sodium acetate (pH 5.0).
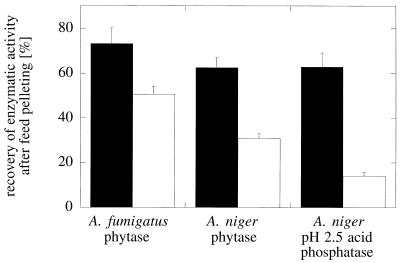
FIG. 7.
Recovery of enzymatic activity after feed pelleting. The three acid phosphatases were added in liquid form to extruded mash feed which was then pelleted at 75°C (solid bars) or 85°C (open bars). Phytase and pH 2.5 acid phosphatase activities were measured in the feed samples before and after pelleting.
After 15 min of incubation at 37°C (or at 40, 45, 50, 55, 60, 65, 70, 80, and 90°C for the experiment shown in Fig. 6), the reaction was stopped by adding an equal volume of 15% trichloroacetic acid. The liberated phosphate ions were quantified by mixing 100 μl of the assay mixture with 900 μl of H2O and 1 ml of 0.6 M H2SO4–2% ascorbic acid–0.5% ammonium molybdate. After incubation for 20 min at 50°C, the absorbance at 820 nm was measured. Standard solutions of potassium phosphate were used as references. In one experiment (see Fig. 6), the enzyme and substrate were preincubated separately for 10 min at the appropriate temperatures and then mixed to start the reaction.
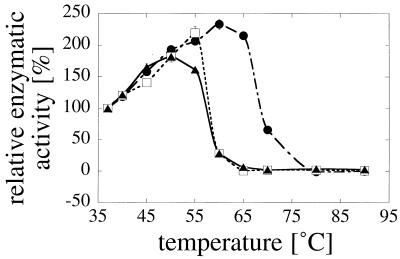
FIG. 6.
Temperature dependence of enzymatic activity. Symbols: ▴, A. fumigatus phytase; □, A. niger T213 phytase; •, A. niger pH 2.5 acid phosphatase. The enzymatic activities were measured directly at the temperatures indicated. The activity at 37°C was defined as 100%.
Gel permeation chromatography.
The three acid phosphatases (concentrations, 0.27 to 0.41 mg/ml in 10 mM sodium acetate [pH 5.0]) were incubated for 20 min at 30, 70, 80, or 90°C and then for 2 h on ice. Their molecular sizes were then determined at room temperature by gel filtration on a Superdex 200 column (fast protein liquid chromatography; Pharmacia). The elution buffer contained 50 mM sodium phosphate, 150 mM NaCl, 0.2 mM disodium EDTA, 2 mM 2-mercaptoethanol, and 1 mM sodium azide (pH 7.2).
Feed pelleting.
A commercial broiler feed containing 55% maize, 27% soya 50, 10% extruded soya, 3% fish meal, 1% soya oil, and 4% mineral premix B4245 (Agrobase, Bourgen Bresse, France) was extruded at 120°C (in order to inactivate acid phosphatases present in the feed) and crumbled. For each test, approximately 15 kg of the treated feed and 500 to 800 U of phytase or acid phosphatase (as a fermentation supernatant) per kg of feed were combined and mixed with a Forberg type F-60 mixer. Subsequently, the mixed product was pelleted with a Bühler model DFPL pellet mill at 75 or 85°C. For each batch, one sample before pelleting and three samples after pelleting were withdrawn and used to analyze the phytase or acid phosphatase activity. The feed pellets were ground with a mortar and pestle, aliquots (5 g) were suspended in 40 ml of 200 mM sodium acetate (pH 5.5), and each suspension was stirred for 30 min at 4°C. After centrifugation for 10 min at 3,000 × g and extensive dialysis of the supernatant against 200 mM sodium acetate (pH 5.5) at 4°C, phytase or acid phosphatase activity was determined as described above.
Other methods.
Protein concentrations were calculated from the optical densities at 280 nm by using theoretical absorption values calculated from the known protein sequences with the DNA* software (DNASTAR, Inc.). An absorption value of 1 A at 280 nm corresponded to 0.63 mg of pH 2.5 acid phosphatase per ml, 0.94 mg of A. fumigatus phytase per ml, and 1.04 mg of A. niger T213 phytase per ml. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed with 8 to 16% Tris-glycine gradient gels supplied by Novex. The gels were stained with colloidal Coomassie stain (Novex).
RESULTS
Temperature-dependent changes in protein fluorescence.
Fluorescence measured at excitation and emission wavelengths of 280 and 340 nm, respectively, reveals the local environments of aromatic residues in proteins. Fluorescence typically decreases as the temperature increases and when aromatic residues become exposed to a more hydrophilic environment.
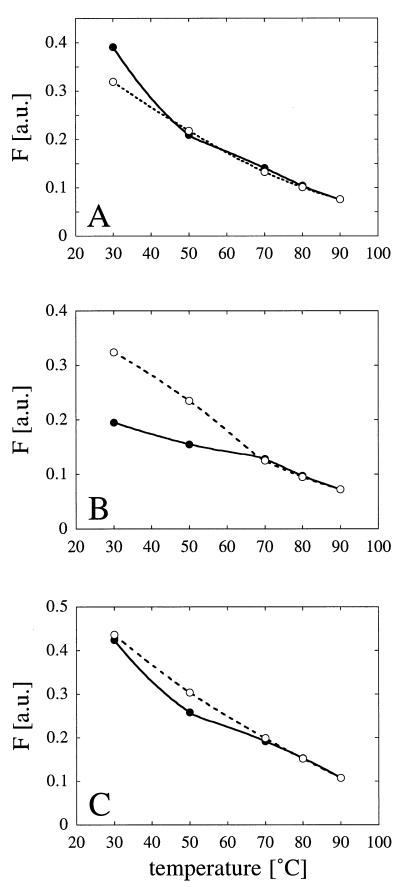
As shown in Fig. 1, protein fluorescence in fact decreased as the temperature was increased. In the case of A. niger T213 phytase (Fig. 1B), at any temperature between 70 and 90°C, the observed fluorescence was the same whether the temperature was increased from 70 to 90°C or decreased subsequently from 90 to 70°C. In contrast, the values obtained at 30 and 50°C were considerably higher after heat treatment than before heat treatment. At the end of the experiment (i.e., when the preparation was returned to 30°C), the residual enzymatic activity of A. niger T213 phytase was only 22% of the initial activity. When in a separate experiment the enzyme was incubated consecutively at 20 and 50°C and then again at 20°C, fluorescence returned to the control value (0.226 versus 0.231 arbitrary unit). After incubation at 20 and 55°C and then again at 20°C, however, a large increase in fluorescence was noticed (0.355 versus 0.226 arbitrary unit), suggesting that there was an irreversible change in the protein conformation at temperatures between 50 and 55°C that was associated with inactivation of the enzyme.
FIG. 1.
Temperature-dependent changes in protein fluorescence. (A) A. fumigatus phytase. (B) A. niger T213 phytase. (C) A. niger pH 2.5 acid phosphatase. Symbols: •, fluorescence changes associated with increases in temperature from 30 to 50, 70, 80, and 90°C; ○, fluorescence changes associated with decreases in temperature from 90 to 80, 70, 50, and 30°C. F, fluorescence; a.u., arbitrary units. For experimental details see Materials and Methods.
In contrast to A. niger T213 phytase, there was no evidence that there were irreversible changes in the conformations of A. fumigatus phytase (Fig. 1A) and A. niger pH 2.5 acid phosphatase (Fig. 1C) after they were heated to 90°C and then cooled. However, pH 2.5 acid phosphatase was completely inactivated in this experiment, while A. fumigatus phytase exhibited 87% residual activity.
Unfolding and refolding properties of acid phosphatases revealed by CD spectroscopy.
CD spectroscopy was used to determine the effects of temperature on the proportions of secondary-structure elements. When CD spectra are compared to a reference data set, they can be used to predict the α-helical and β-sheet contents of a protein. Aliquots of the individual acid phosphatases were preincubated for 20 min at 30, 50, 70, 80, or 90°C and were then directly measured at the same temperatures (Fig. 2A, C, and E). Alternatively, after the initial 20-min incubation period, the proteins were allowed to renature for 1 h at 30°C and then were measured at 30°C (Fig. 2B, D, and F).
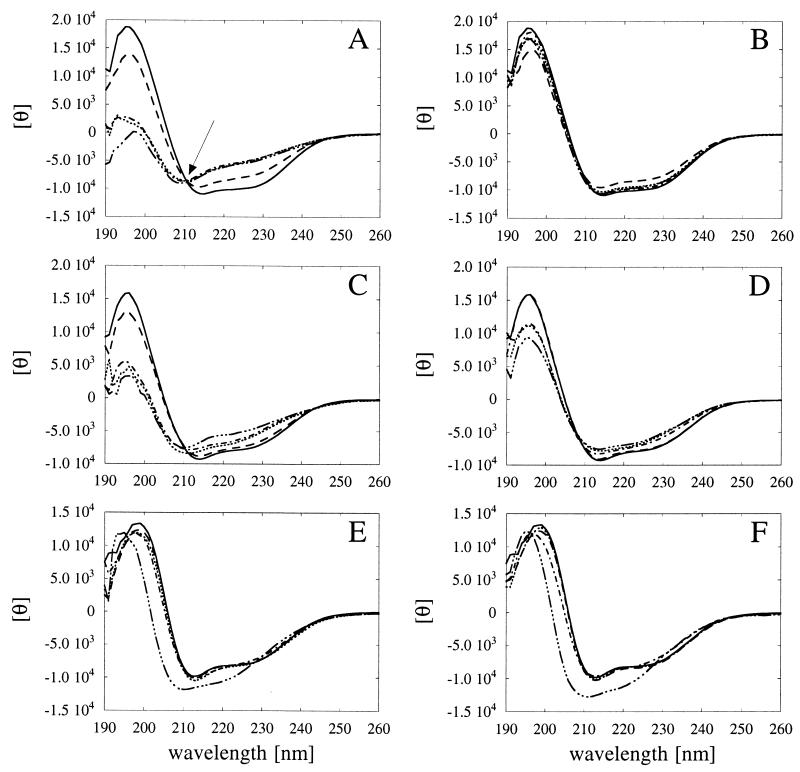
FIG. 2.
Temperature-dependent unfolding and refolding of phytases and pH 2.5 acid phosphatase as determined by CD measurements. (A and B) A. fumigatus phytase. (C and D) A. niger T213 phytase. (E and F) A. niger pH 2.5 acid phosphatase. The individual acid phosphatases were incubated for 20 min at 30°C (——), 50°C (––––), 70°C (······), 80°C (–·–·–), or 90°C (–···–···–). Then either the CD spectra were determined directly at the same temperatures (A, C, and E), or the phosphatases were allowed to renature for 1 h at 30°C, and then the spectra were determined at 30°C (B, D, and F). The isodichroitic point observed for A. fumigatus phytase is indicated by an arrow in panel A. θ, residual molar ellipticity.
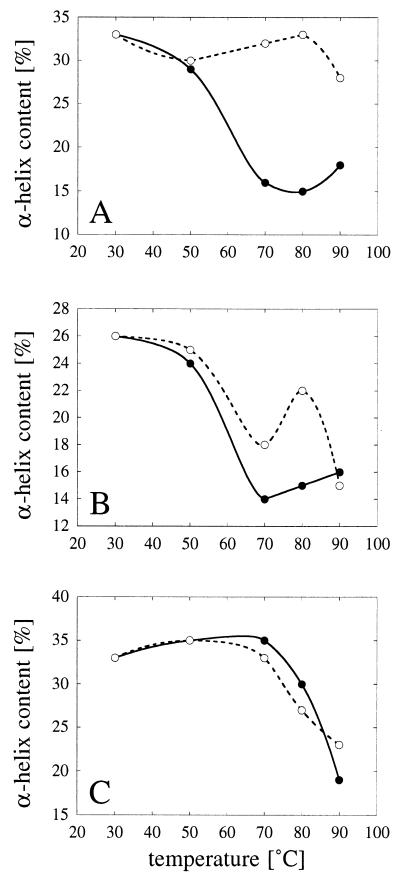
A. fumigatus phytase (Fig. 2A) and A. niger T213 phytase (Fig. 2C) seemed to be equally sensitive to heat and were denatured at temperatures between 50 and 70°C. When heat-denatured A. fumigatus phytase was returned to 30°C, it refolded into a nativelike conformation (Fig. 2B). Proper refolding of A. niger T213 phytase, on the other hand, was observed only after exposure to temperatures up to 50°C (Fig. 2D). Consistent with the hypothesis that there was an irreversible conformational change at temperatures between 50 and 55°C (see above), the CD spectra of refolded A. niger T213 phytase after exposure to 70, 80, and 90°C were significantly different from the control spectrum of the native enzyme. The different behaviors of the two phytases are also reflected in the calculated α-helical contents shown in Fig. 3 and by the fact that the CD curves for A. fumigatus phytase have an isodichroitic point (Fig. 2A, arrow), while those of A. niger T213 phytase do not (Fig. 2C). An isodichroitic point is a strong indication that one defined species is transformed into another.
FIG. 3.
Temperature-dependent changes in calculated α-helical contents. (A) A. fumigatus phytase. (B) A. niger T213 phytase. (C) A. niger pH 2.5 acid phosphatase. The α-helical contents were calculated from the CD spectra shown in Fig. 2. Symbols: •, α-helical contents after 20 min of incubation at 30, 50, 70, 80, or 90°C; ○, α-helical contents after a subsequent 1-h renaturation period at 30°C.
Almost no change in the CD spectral characteristics of pH 2.5 acid phosphatase was observed at temperatures up to 80°C (Fig. 2E), suggesting that this enzyme is in fact more thermostable than the two phytases which we investigated. Only at 90°C was a clear shift in the CD spectrum noticed, and this shift apparently was irreversible (Fig. 2E and F).
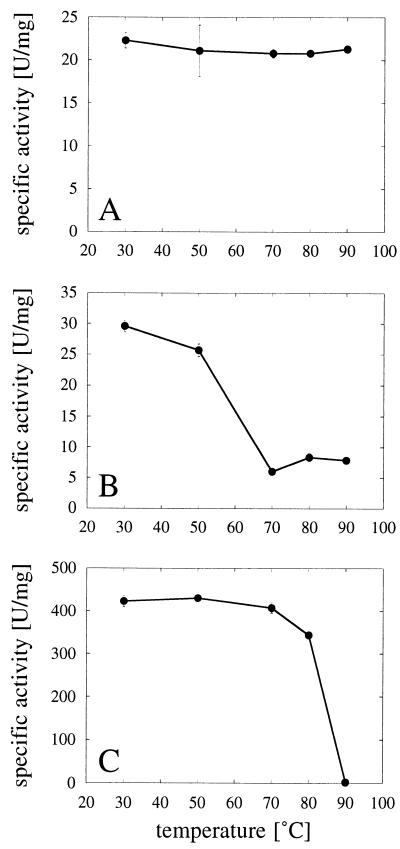
In order to correlate the CD spectral changes with enzyme inactivation, aliquots of the individual incubation mixtures were withdrawn at the end of the 20-min heating period and immediately put on ice. Since the samples were on ice for at least 1 h prior to analysis, the data in Fig. 4 reflect the enzymatic activities of the refolded proteins. Evidently, there was a perfect correlation among activity, CD, and fluorescence measurements. Only two points deserve further attention. First, although A. niger pH 2.5 acid phosphatase is more thermostable than the two phytases, it is in fact completely and irreversibly inactivated once the conformational transition occurs (at 90°C and, to a small extent, at 80°C). Second, sodium dodecyl sulfate-polyacrylamide gel electrophoresis control experiments confirmed that interference by protein degradation, even at 90°C, could not account for any of the findings presented in this paper (data not shown).
FIG. 4.
Temperature-dependent enzyme inactivation. (A) A. fumigatus phytase. (B) A. niger T213 phytase. (C) A. niger pH 2.5 acid phosphatase. The individual acid phosphatases were incubated for 20 min at 30, 50, 70, 80, or 90°C and then immediately put on ice. The results represent means ± standard deviations (n = 3). Enzymatic activities were measured as described in Materials and Methods.
Heat-induced aggregation of pH 2.5 acid phosphatase.
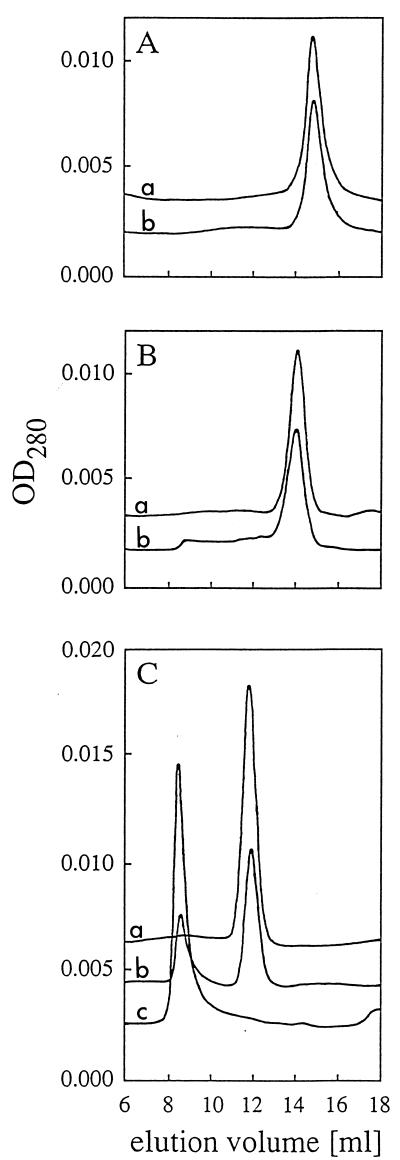
The phytases of A. niger T213 and A. fumigatus are monomeric proteins, while A. niger pH 2.5 acid phosphatase is an oligomer that is most likely composed of four identical protomers (Fig. 5) (3a). It might be anticipated that heat denaturation disrupts the tetrameric structure of pH 2.5 acid phosphatase and that, once dissociated, the individual protomers are unable to refold and reassociate properly into an active tetramer. Gel permeation chromatography, however, demonstrated that exposure to 90°C and, to a lesser extent, exposure to 80°C were associated with the formation of high-Mr aggregates (Fig. 5). No such aggregates were observed for the phytases of A. niger T213 and A. fumigatus despite the fact that A. niger T213 phytase is also inactivated significantly at 90°C.
FIG. 5.
Effect of heat denaturation on the molecular sizes of phytases and pH 2.5 acid phosphatase. (A) A. fumigatus phytase. (B) A. niger T213 phytase. (C) A. niger pH 2.5 acid phosphatase. The individual acid phosphatases were incubated for 20 min at 30°C (curve a in panels A and B), 70°C (curve a in panel C), 80°C (curve b in panel C), and 90°C (curve b in panels A and B and curve c in panel C), allowed to refold for 2 h on ice, and then analyzed by gel permeation chromatography. The reference proteins blue dextran 2000 (Mr, 2,000,000), ferritin (Mr, 440,000), and albumin (Mr, 67,000) eluted at 8.5, 11.3, and 14.4 ml, respectively. OD280, optical density at 280 nm.
Temperature dependence of enzymatic activity.
To assess specifically heat denaturation of the active site, activity was measured directly at a series of temperatures between 37 and 90°C (Fig. 6). Both phytases were inactivated at temperatures above 55°C, while loss of activity of pH 2.5 acid phosphatase was observed only at temperatures above 60 to 65°C. Since pH 2.5 acid phosphatase activity had to be measured at pH 2.5 (this enzyme exhibits virtually no activity at pH 5.0), this experiment suffered from the limitation of not corresponding strictly with the other investigations. Nevertheless, in qualitative terms, its results are consistent with the previous conclusions and confirm that pH 2.5 acid phosphatase is more thermostable than the A. niger T213 and A. fumigatus phytases.
Thermostability in feed pelleting experiments.
Experiments performed in test tubes may not properly reflect the conditions in the feed pelleting process. Therefore, the three acid phosphatases were added in small volumes of liquid to extruded mash feed which subsequently was pelleted at either 75 or 85°C. No significant differences in the recovery of enzymatic activities were observed after feed pelleting at 75°C (Fig. 7) despite rather pronounced differences in the thermostability properties of the purified proteins in dilute, buffered aqueous solutions (see above). The low recovery of A. niger pH 2.5 acid phosphatase activity after pelleting at 85°C (14.1% ± 1.7%) may have been due to the fact that this enzyme undergoes an irreversible conformational change that is associated with enzyme inactivation at temperatures above 80°C (see above). The recovery of more A. fumigatus phytase activity than of A. niger phytase activity at 85°C may be a reflection of the capacity of A. fumigatus phytase to refold properly into an active conformation after heat denaturation.
DISCUSSION
In the present investigation, we analyzed the temperature sensitivity of acid phosphatases at three different levels of molecular organization, as follows: (i) fluorescence spectroscopy probed structural alterations in the immediate vicinity of aromatic residues; (ii) the temperature dependence of enzymatic activity revealed at which temperature the active site was denatured or structurally affected in such a way that catalysis was no longer possible; and (iii) far-UV CD spectra revealed overall changes in the proportions of secondary-structure elements. The last two approaches do not necessarily provide identical results, since it has been shown previously that the active sites of diverse enzymes are more susceptible to denaturants than the global structures are (12, 19). Despite the different parameters that are measured by the techniques which we used, all of the experiments described above provided a consistent picture which showed that the three acid phosphatases have distinct thermostability properties.
A. niger T213 phytase is not thermostable, nor does it have the capacity to refold properly after heat denaturation. At temperatures between 50 and 55°C, this enzyme undergoes an irreversible conformational rearrangement that is associated with losses in enzymatic activity of 70 to 80%.
A. fumigatus phytase, like A. niger T213 phytase, is not thermostable but has the remarkable property of being able to refold completely into a nativelike, fully active conformation after heat denaturation.
Compared to the two phytases, A. niger T213 pH 2.5 acid phosphatase has higher intrinsic thermostability, a conclusion that is consistent with previous findings (13, 14). At temperatures up to 80°C, only minor changes in CD spectral characteristics and only slight (irreversible) enzyme inactivation were observed under the experimental conditions used. Exposure to 90°C, however, resulted in an irreversible conformational change that was associated with the formation of high-Mr aggregates and with complete inactivation of the enzyme. It is quite surprising that two proteins from the same organism, the phytase and the pH 2.5 acid phosphatase of A. niger T213, exhibit such a pronounced difference in thermostability. Whether this finding has physiological relevance remains to be determined.
A number of criteria must be fulfilled by a phytase if it is to be attractive for widespread use in the animal feed industry. It should be thermostable and protease resistant, and it should have broad substrate specificity and a high specific activity. Even though the recovery of enzymatic activity after feed pelleting of the nonformulated acid phosphatases at 85°C ranged from 14 to 51%, the results presented here are a step towards meeting these ambitious criteria.
ACKNOWLEDGMENTS
Kurt Vogel and Martin Lehmann are gratefully acknowledged for stimulating discussions, and Alexandra Kronenberger and Stefan Jäggli are acknowledged for expert technical assistance.
REFERENCES
- 1.Chen G C, Yang J T. Two-point calibration of circular dichrometer with d-10-camphorsulfonic acid. Anal Lett. 1977;10:1195–1207. [Google Scholar]
- 2.Delboni L F, Mande S C, Rentier-Delrue F, Mainfroid V, Turley S, Vellieux F M D, Martial J A, Hol W G J. Crystal structure of recombinant triosephosphate isomerase from Bacillus stearothermophilus. An analysis of potential thermostability factors in six isomerases with known three-dimensional structures points to the importance of hydrophobic interactions. Protein Sci. 1995;4:2594–2604. doi: 10.1002/pro.5560041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Jobin Yvon. Instrument manual. Longjumeau, France: Jobin Yvon; 1990. [Google Scholar]
- 3.Kawamura S, Kakuta Y, Tanaka I, Hikichi K, Kuhara S, Yamasaki N, Kimura M. Glycine-15 in the bend between two α-helices can explain the thermostability of DNA binding protein HU from Bacillus stearothermophilus. Biochemistry. 1996;35:1195–1200. doi: 10.1021/bi951581l. [DOI] [PubMed] [Google Scholar]
- 3a.Kostrewa, D. Unpublished data.
- 4.Mitchell D B, Vogel K, Weimann B, Pasamontes L, van Loon A P G M. The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology. 1997;143:245–252. doi: 10.1099/00221287-143-1-245. [DOI] [PubMed] [Google Scholar]
- 5.Pasamontes L, Haiker M, Wyss M, Tessier M, van Loon A P G M. Gene cloning, purification, and characterization of a heat-stable phytase from the fungus Aspergillus fumigatus. Appl Environ Microbiol. 1997;63:1696–1700. doi: 10.1128/aem.63.5.1696-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasamontes L, Haiker M, Henriquez-Huecas M, Mitchell D B, van Loon A P G M. Cloning of the phytases from Emericella nidulans and the thermophilic fungus Talaromyces thermophilus. Biochim Biophys Acta. 1997;1353:217–223. doi: 10.1016/s0167-4781(97)00107-3. [DOI] [PubMed] [Google Scholar]
- 7.Piddington C S, Houston C S, Paloheimo M, Cantrell M, Miettinen-Oinonen A, Nevalainen H, Rambosek J. The cloning and sequencing of the genes encoding phytase (phy) and pH 2.5-optimum acid phosphatase (aph) from Aspergillus niger var. awamori. Gene. 1993;133:55–62. doi: 10.1016/0378-1119(93)90224-q. [DOI] [PubMed] [Google Scholar]
- 8.Provencher S W, Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981;20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai M, Moriyama H, Onodera K, Kadono S, Numata K, Hayashi Y, Kawaguchi J, Yamagishi A, Oshima T, Tanaka N. The crystal structure of thermostable mutants of chimeric 3-isopropylmalate dehydrogenase, 2T2M6T. Protein Eng. 1995;8:763–767. doi: 10.1093/protein/8.8.763. [DOI] [PubMed] [Google Scholar]
- 10.Szilágyi A, Závodszky P. Structural basis for the extreme thermostability of d-glyceraldehyde-3-phosphate dehydrogenase from Thermotoga maritima: analysis based on homology modelling. Protein Eng. 1995;8:779–789. doi: 10.1093/protein/8.8.779. [DOI] [PubMed] [Google Scholar]
- 11.Tanner J J, Hecht R M, Krause K L. Determinants of enzyme thermostability observed in the molecular structure of Thermus aquaticusd-glyceraldehyde-3-phosphate dehydrogenase at 2.5 Å resolution. Biochemistry. 1996;35:2597–2609. doi: 10.1021/bi951988q. [DOI] [PubMed] [Google Scholar]
- 12.Tsou C-L. Conformational flexibility of enzyme active sites. Science. 1993;262:380–381. doi: 10.1126/science.8211158. [DOI] [PubMed] [Google Scholar]
- 13.Ullah A H J, Gibson D M. Extracellular phytase (E.C. 3.1.3.8) from Aspergillus ficuum NRRL 3135: purification and characterization. Prep Biochem. 1987;17:63–91. doi: 10.1080/00327488708062477. [DOI] [PubMed] [Google Scholar]
- 14.Ullah A H J, Cummins B J. Purification, N-terminal amino acid sequence and characterization of pH 2.5 optimum acid phosphatase (E.C. 3.1.3.2) from Aspergillus ficuum. Prep Biochem. 1987;17:397–422. doi: 10.1080/00327488708062504. [DOI] [PubMed] [Google Scholar]
- 15.van Hartingsveldt W, van Zeijl C M J, Harteveld G M, Gouka R J, Suykerbuyk M E G, Luiten R G M, van Paridon P A, Selten G C M, Veenstra A E, van Gorcom R F M, van den Hondel C A M J J. Cloning, characterization and overexpression of the phytase-encoding gene (phyA) of Aspergillus niger. Gene. 1993;127:87–94. doi: 10.1016/0378-1119(93)90620-i. [DOI] [PubMed] [Google Scholar]
- 16.van Loon A P G M, Simoes-Nunes C, Wyss M, Tomschy A, Hug D, Vogel K, Pasamontes L. A heat-resistant phytase of Aspergillus fumigatus with superior performance in animal experiments. Phytase optimization and natural variability. In: Rasmussen S K, Raboy V, Dalbøge H, Loewns F, editors. The biochemistry of phytate and phytases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. [Google Scholar]
- 17.Wyss, M., L. Pasamontes, A. Friedlein, R. Rémy, M. Tessier, A. Kronenberger, A. Middendorf, M. Lehmann, L. Schnoebelen, U. Röthlisberger, E. Kusznir, G. Wahl, F. Müller, H.-W. Lahm, K. Vogel, and A. P. G. M. van Loon. Biophysical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): molecular size, glycosylation pattern and engineering of proteolytic resistance. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 18.Wyss, M., R. Brugger, A. Kronenberger, R. Rémy, R. Fimbel, G. Oesterhelt, M. Lehmann, and A. P. G. M. van Loon. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 19.Yao Q-Z, Tian M, Tsou C-L. Comparison of the rates of inactivation and conformational changes of creatine kinase during urea denaturation. Biochemistry. 1984;23:2740–2744. doi: 10.1021/bi00307a032. [DOI] [PubMed] [Google Scholar]