Abstract
Swyer syndrome is a condition where individuals with a 46XY karyotype, typically associated with males, display complete gonadal dysgenesis and lack testicular differentiation. This results from a mutation in the SRY gene, which is essential for testis development. As a consequence, affected individuals who appear phenotypically female have male chromosomes but do not develop functional testes. As a result, there is an absence of testosterone that leads to lack of masculinization and the presence of female genitalia. This article describes a 20-year-old female from Pakistan who exhibited primary amenorrhea. On examination, she possessed a typical female physique but lacked breast growth and axillary hair. She had scant pubic hair with female-type external genitalia. The pelvic imaging showed a underdeveloped uterus, along with small ovaries and fallopian tubes. Her karyotype came out to be 46XY. The examination and radiological results indicated Swyer syndrome. During laparoscopy, the patient’s uterus was found to be infantile, while the fallopian tubes were healthy. Streak gonads were also present, and due to the risk of gonadoblastoma, they were surgically removed. Hormone replacement therapy was started to induce pubertal development and optimize bone mineral accumulation.
Keywords: dysgerminomas, gonadal dysgenesis, gonadoblastoma, genotype 46XY, primary amenorrhea, Swyer syndrome
Introduction
Swyer syndrome is an uncommon genetic disorder characterized by a 46XY male karyotype, but the affected individual exhibits a female appearance, a normal vagina, an underdeveloped uterus, and streak gonads instead of functional ovaries. Individuals with this condition typically exhibit average or above-average growth, fail to develop secondary sexual characteristics, and experience primary amenorrhea. Typically, the initial signs of the condition become apparent during adolescence. The absence of hormonal functioning in underdeveloped gonads leads to the absence of menstruation and secondary sexual characteristics. 1 There are reported incidence rates of 20%–30% of the risk of tumor formation in individuals with Swyer syndrome making it noteworthy. 2 In Swyer syndrome, bilateral gonadoblastoma is the most prevalent tumor type, although dysgerminoma and embryonal carcinoma may also be present. According to studies, approximately 5% of dysgerminomas are found in phenotypic females with aberrant gonads and a 46XY karyotype.2 –4 Timely identification and surgical excision of streak gonads are crucial because the risk of malignancy rises with age. The probability of malignancy begins at 5% around the age of 15, rises to 27.5% by age 30, and can reach 50% after age 40. 5
We report the case of a 20-year-old female diagnosed with Swyer syndrome, who experienced primary amenorrhea and underwent bilateral salpingo-oophorectomy to prevent the possibility of tumor formation.
Case presentation
A 20-year-old female visited the outpatient department, reporting primary amenorrhea. According to the patient, she had no weight gain, hirsutism, hair loss, eating disorder, or familial disorder. On examination, she lacked secondary sexual characteristics with normal external genitalia and a female phenotype. Her vital signs were stable. She had a body mass index (BMI) of 15.7 and was in tanner stage 2. Her routine blood tests and thyroid profiles were normal (Table 1). Her fertility profile included increased serum FSH, LH, and testosterone (Table 1). Her family history was unremarkable for delayed menarche, primary amenorrhea, and genetic disorders. Ultrasound of the pelvis showed a hypoplastic infantile uterus (5 × 2.1 cm) and small ovaries measuring 1.9 cm (right) and 1.6 cm (left) (Figure 1). Ultrasound breast showed underdeveloped breasts with no signs of malignancy or any underlying pathology. Magnetic resonance imaging (MRI) pelvis was ordered, which displayed a small uterus (1.5 × 4.0) and ovaries. To further confirm the diagnosis, Karyotyping was done which presented a 46XY genotype (Figure 2).
Table 1.
Serum, thyroid, and fertility profile results.
| Lab investigation | Reference value | Patient value |
|---|---|---|
| Serum investigations | ||
| WBC (cells/mm3) | 4–11 | 5.4 |
| Hemoglobin (g/dL) | 11.5–17.5 | 12.9 |
| Sodium (mmol/L) | 135–50 | 137 |
| Potassium (mmol/L) | 3.5–5.1 | 4.7 |
| Total bilirubin (mg/dL) | 0.1–1.0 | 0.7 |
| ALT (U/L) | 10–50 | 33 |
| ALP (U/L) | 35–104 | 76 |
| Blood urea (mg/dL) | 10–50 | 28 |
| Creatinine (mg/dL) | 0.42–1.06 | 0.8 |
| Thyroid function tests | ||
| Free T4 (pmol/L) | 10–28 | 25 |
| Total T3 (nmol/L) | 0.6–2.0 | 1.4 |
| TSH (µIU/ml) | 0.3–4.2 | 1.7 |
| Fertility profile | ||
| FSH | 1–10 IU/L | 130.2 IU/L |
| LH | 1.6–8.3 IU/L | 90.1 IU/L |
| Prolactin | 2–26 IU/L | 13.5 IU/L |
| Testosterone | 0.4–0.45 IU/L | 0.77 IU/L |
The bold faced values show the abnormal results.
W.B.C = White blood cells; ALT= Alanine transaminase; ALP= Alkaline phosphatase; TSH= Thyroid stimulating hormone; FSH= Follicular stimulating hormone; LH= Leutinizing hormone.
Figure 1.
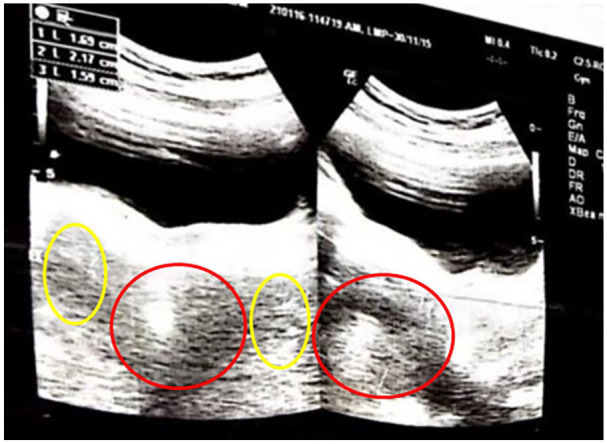
Ultrasound of the pelvis showing a hypoplastic uterus (red circle) and bilateral small ovaries (yellow circles).
Figure 2.
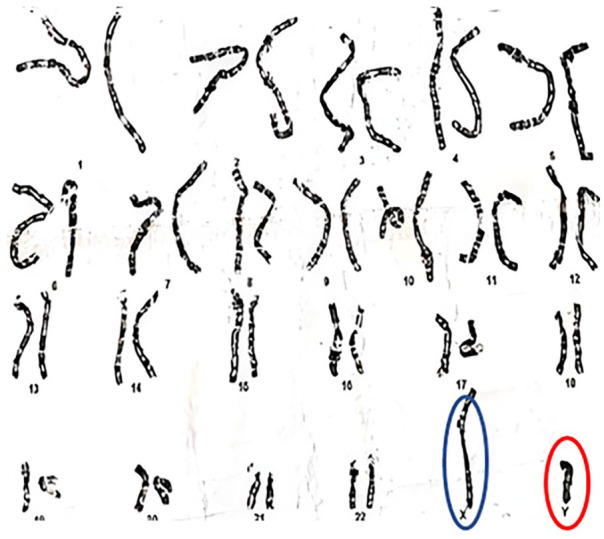
Karyotypy showing XY genotype.
The patient was prescribed tablet estradiol, folic acid 5 mg, and calcium and vitamin D supplements, all once daily. She took the same treatment for 9 months but could not achieve menarche with tablet Progylutin (estradiol + norgesterol). On diagnostic laparoscopy, a small-sized uterus, bilateral fallopian tubes, and bilateral streak ovaries were visualized (Figure 3). To rule out any chances of malignancy, she underwent a left gonadal biopsy and bilateral salpingo-oopherectomy (Figure 4). The tissue specimen of bilateral ovaries and fallopian tubes showed no evidence of dysplasia or malignancy. WT1 was positive on immunohistochemistry in the rete ovaries. She was discharged home with a prescription of estradiol and calcium supplements. A regular follow-up was advised.
Figure 3.
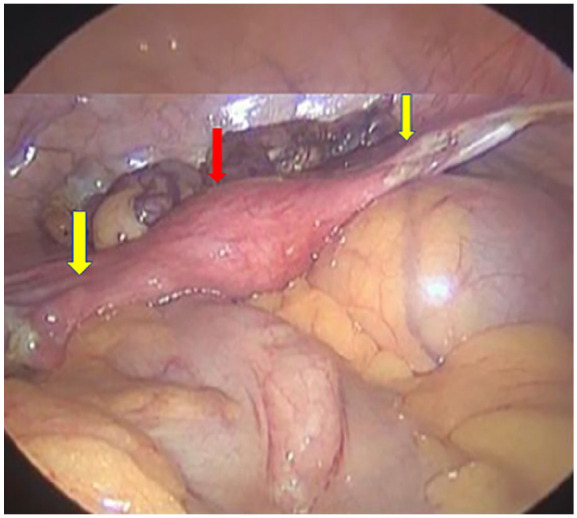
Laproscopic image showing the uterus (red arrow) and fallopian tubes (yellow arrows).
Figure 4.
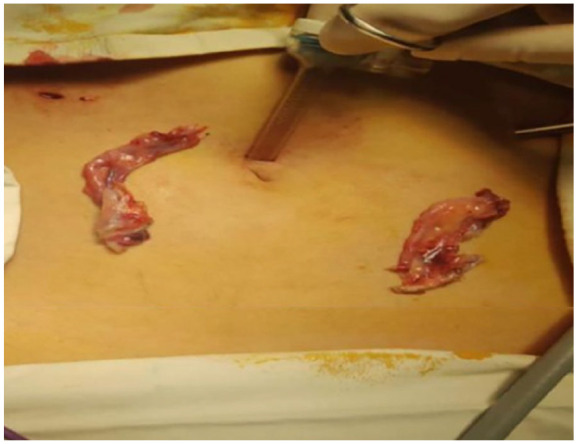
Streak gonads and fallopian tubes after salpingo-ophorectomy.
Discussion
Swyer syndrome, initially described by G. Swyer in 1955, falls under the category of “male pseudohermaphroditism.” This condition is characterized by an XY karyotype, female external genitalia, primary amenorrhea, and heightened growth.6,7 In addition, patients possess a female phenotype, vagina, a small underdeveloped uterus and non-functional gonads (either ovaries or testes) which look like underdeveloped clumps of tissue called “streak gonads.” 7 Patients lack secondary sexual characteristics and have an average or above-average growth rate. The adrenal glands are unaffected and can produce androgens. Therefore, these individuals usually possess pubic hair, although these may appear sparse. 7 Our patient exhibited all these features that helped in the diagnosis.
In a normal XY fetus, the initial phase of sexual differentiation involves the development of testes. This process occurs during the early stages of testicular formation. Several genes play a crucial role, with SRY being the most significant. 6 In cases of 46XY gonadal dysgenesis, the absence of testicular development can result from mutations in genes such as sex-determining region Y, DHH, NR5A1, or loss-of-function mutations in the testis-determining gene. In addition, duplication of DAX1 or gain-of-function mutations in MAP3K1 can also contribute to this condition. 8 In majority of the cases (80%–90%), the SRY gene remain unaffected while 10%–20% cases have mutations in the SRY gene. 6 The absence of testicular development causes lack of testosterone and antimullerian hormone (AMH) that lead to the growth of female genitalia and arrest of masculinization. In the absence of AMH, the Mullerian duct develops into the internal female organs, including the fallopian tubes, uterus, cervix, and vagina. 6
Another frequently considered condition that can be confused with Swyer syndrome is mixed gonadal dysgenesis, where the histopathological examination of the gonads shows a combination of ovarian and testicular structures, and the genotype is mosaic pattern. 6 Individuals affected by Swyer syndrome are typically reared as females and identify as females. Symptoms usually become evident during adolescence, such as lack of menstrual periods, undeveloped breasts, and broader pelvis or hips due to the hormonal inactivity of the dysgenetic gonads.
Patients diagnosed with Swyer syndrome have an increased risk of developing germ cell tumors.3,4 The incidence of gonadoblastoma is 20%–30% in affected individuals. 5 More than 40% of gonadoblastomas are bilateral, and the most common karyotypes associated with this tumor are 46XY. Dysgerminoma often originates from a gonadoblastoma in the case of gonadal dysgenesis. 9 The likelihood of tumor formation rises with advancing age and hence, early identification and prompt prophylactic gonadectomy is crucial. 5
In addition, hormone replacement therapy (HRT) should be instituted to promote menstruation, growth of female sexual characteristics, and bone health. Individuals with the condition can lead a normal sexual life and may achieve pregnancy with donor eggs and assisted reproductive techniques. The outcome of these pregnancies is similar to those with 46XX karyotype and ovarian failure. 8 Due to the genetic nature of Swyer syndrome, it is essential to contemplate the possibility of it occurring within the same family. Testing the patient’s family members may be required to address potential long-term complications associated with the condition. 10
Conclusion
While evaluating patients with primary amenorrhea, Swyer syndrome, a rare but possible cause, must be considered. Diagnosing and managing individuals with Swyer syndrome is complex, and a vigilant workup is the key to early diagnosis. Moreover, comprehensive counseling regarding fertility options, early initiation of hormone therapy for sexual and bone maturity, constant monitoring and regular follow-up of the individual are important pillars of the management and successful outcome.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057231213270 for A rare case of Swyer syndrome from Pakistan in a young girl with primary amenorrhea and 46XY genotype by Inshal Jawed, Ayesha Azhar Javed, Syeda Alisha Johar, Daayl N Mirza, Ayesha A Abdani and Asad Ali Khan in Women’s Health
Footnotes
ORCID iDs: Inshal Jawed  https://orcid.org/0009-0000-8420-9669
https://orcid.org/0009-0000-8420-9669
Asad Ali Khan  https://orcid.org/0000-0001-8503-3036
https://orcid.org/0000-0001-8503-3036
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: Ethical approval for case report studies is not required in accordance with local/national guidelines. Written informed consent was obtained from the patient for publication of the details of this medical case and any accompanying images.
Consent for publication: Written informed consent was obtained from the patient for publication of the details of this medical case and any accompanying images.
Author contribution(s): Inshal Jawed: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing.
Ayesha Azhar Javed: Conceptualization; Data curation; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing.
Syeda Alisha Johar: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Daayl N Mirza: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing.
Ayesha A. Abdani: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Asad Ali Khan: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
References
- 1. Michala L, Goswami D, Creighton SM, et al. Swyer syndrome: presentation and outcomes. BJOG 2008; 115(6): 737–741. [DOI] [PubMed] [Google Scholar]
- 2. Morimura Y, Nishiyama H, Yanagida K, et al. Dysgerminoma with syncytiotrophoblastic giant cells arising from 46,XX pure gonadal dysgenesis. Obstet Gynecol 1998; 92: 654–656. [DOI] [PubMed] [Google Scholar]
- 3. Słowikowska-Hilczer J, Romer TE, Kula K. Neoplastic potential of germ cells in relation to disturbances of gonadal organogenesis and changes in karyotype. J Androl 2003; 24(2): 270–278. [DOI] [PubMed] [Google Scholar]
- 4. Behtash N, Karimi Zarchi M. Dysgerminoma in three patients with Swyer syndrome. World J Surg Onc 2007; 5: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Milewicz T, Mrozińska S, Szczepański W, et al. Dysgerminoma and gonadoblastoma in the course of Swyer syndrome. Pol J Pathol 2016; 67(4): 411–414. [DOI] [PubMed] [Google Scholar]
- 6. Babre V, Bendre K, Niyogi G. A rare case of Swyer’s syndrome. Int J Reprod Contracept Obstet Gynecol 2013; 2: 485–487. [Google Scholar]
- 7. Priya PK, Mishra VV, Choudhary S, et al. A case of primary amenorrhea with swyer syndrome. J Hum Reprod Sci 2017; 10(4): 310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King TFJ, Conway GS. Swyer syndrome. Curr Opin Endocrinol Diabetes Obes 2014; 21: 504–510. [DOI] [PubMed] [Google Scholar]
- 9. Arafa M, Ryiami MA, Shukri MA, et al. Bilateral gonadoblastoma overgrown by dysgerminoma of the right gonad in a patient with Swyer syndrome. Maedica (Bucur) 2021; 16(4): 734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimitri P, Cohen M, Wright N. Indications for familial screening and gonadectomy in patients with 46,XY gonadal dysgenesis. Int J Gynaecol Obstet 2006; 95(2): 167–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057231213270 for A rare case of Swyer syndrome from Pakistan in a young girl with primary amenorrhea and 46XY genotype by Inshal Jawed, Ayesha Azhar Javed, Syeda Alisha Johar, Daayl N Mirza, Ayesha A Abdani and Asad Ali Khan in Women’s Health


