Abstract
Objectives:
Preeclampsia is one of the most frequent pregnancy disorders, with a global incidence of 2%–8%. Serum 25-hydroxyvitamin D is an essential mineral for human health; some studies suggest link between 25-hydroxyvitamin D deficiency and preeclampsia, while others offer contradictory findings. Thus, the goal of this study is to evaluate the relationships between maternal 25- hydroxyvitamin D concentrations and the risk of preeclampsia. In addition to this, our study also evaluates the effects of 25- hydroxyvitamin D supplementation on the incidence of preeclampsia. Therefore, assessing 25- hydroxyvitamin D’s potential as a possible intervention to lower the risk of preeclampsia.
Methods:
The Medline database was queried from inception until July 2021 for randomized controlled trials and observational studies without any restrictions. The studies assessing the association between 25-hydroxyvitamin D deficiency and preeclampsia and the impact of 25-hydroxyvitamin D supplementation on the incidence of preeclampsia were incorporated. The results were reported using a random-effects meta-analysis and the Mantel-Haenszel odds ratio. A p-value of <0.05 was considered significant for the analysis.
Results:
This analysis includes 34 papers, including 10 randomized controlled trials and 24 observational studies. According to our pooled analysis, 25-hydroxyvitamin D supplementation was significantly associated with a lower risk of preeclampsia in pregnant women (OR: 0.50; 95% CI: 0.40–0.63; p = 0.00001), while 25-hydroxyvitamin D deficiency was significantly associated with an increased risk of preeclampsia (OR: 4.30; 95 % CI: 2.57–7.18; p < 0.00001, OR: 1.71; 95 % Cl: 1.27–2.32; p = 0.0005, OR 1.61; 95 % Cl: 1.21–2.16; p = 0.001).
Conclusion:
Results suggest that 25-hydroxyvitamin D has a significant relationship with preeclampsia as confirmed by the findings that low maternal 25-hydroxyvitamin D concentrations cause increased risk of preeclampsia while 25-hydroxyvitamin D supplementation reduces the incidence of preeclampsia. Our findings indicate that 25-hydroxyvitamin D supplementation can be used as a possible intervention strategy in preventing one of the most common causes of maternal mortality around the world, preeclampsia.
Keywords: 25(OH)D, hypertension, incidence, meta-analysis, pregnancy, preeclampsia, treatment, vitamin D
Introduction
Preeclampsia (PE) is one of the most common disorders of pregnancy, which occurs after 20th week of pregnancy and can present with following complaints such as hypertension, proteinuria and signs of end-organ damage including kidney, lung, and liver failure. 1 PE affects 2%–8% of pregnancies globally and has long-term maternal consequences, including cerebrovascular, metabolic, and cardiovascular disorders.2,3 Moreover, PE is also associated with adverse neonatal outcomes such as preterm birth, restricted fetal growth, placental abruption, bronchopulmonary dysplasia and neurodevelopmental delay,4,5 resulting in 500,000 fetal deaths globally each year. 6
Although, the exact etiology of PE is still unknown, the prevalence of serum 25-hydroxyvitamin D (25(OH)D) deficiency is found to be very high among pregnant women. Serum 25(OH)D is an essential nutrient for maintaining human health. 7 A systematic review reported the incidence of 25(OH)D deficiency among pregnant women in America, Europe, Eastern Mediterranean, South East Asia, and Western Pacific to be 64%, 57%, 46%, 87%, and 83% respectively. 8 Some studies suggest an association between 25(OH)D deficiency and PE9,10 while other studies show no such association. 11 Due to inconsistency in the results, there is no clear agreement on whether there is an association between 25(OH)D and PE, which creates the necessity to further investigate the link between them.
Therefore, in this study, we aimed to conduct a systematic review and meta-analysis of all relevant observational studies and randomized controlled trials in which the association between 25(OH)D deficiency and PE was assessed. Another aim was to assess whether 25(OH)D supplementation can reduce the incidence of PE in pregnant women.
Methods
This meta-analysis is reported in accordance with the Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The PRISMA checklist is presented in Figure 1. The study is not registered in PROSPERO as the design and methodology were finalized before we were aware of the registration requirement; however, we are committed to transparent reporting and will ensure that all pertinent data are reported. This meta-analysis only included data from previously published studies; therefore, ethical approval was deemed unnecessary.
Figure 1.
PRISMA flow chart summarizing the process of literature search and selection of studies.
Search strategy
An electronic search of MEDLINE, TRIP and Cochrane Central was conducted from their inception to July 2021, without any language restrictions, using a search string. No filters were applied in order to retrieve all relevant studies. Moreover, the reference lists of relevant articles were also searched for any other eligible studies. Articles were first shortlisted on the basis of abstracts after which full literature was reviewed to select studies. Bibliographies of the relevant review articles were also queried. In addition to this, grey and white literature were also searched. Articles retrieved from the systemic search were exported to the EndNote Reference Library Software (v17.0.1.7212), where duplicates were searched for and removed. The remaining articles were carefully assessed by two independent authors (MHB and NS). A third investigator (HA) was then consulted to resolve any disparities with consensus.
Inclusion and exclusion criteria
We considered RCTs which assessed risk of PE in pregnant women supplemented with 25(OH)D. We also considered observations studies which assessed incidence of PE in pregnant women with 25(OH)D deficiency. While animal studies, case reports, conference presentations, editorials, expert opinions, and unpublished studies were excluded. Any duplicate studies from the same database were excluded.
Statistical analysis
The data from the selected studies were extracted independently by two authors (MHB and NS) and verified by a third author (HA). From the RCTs, the following outcome was assessed, the association between PE and 25(OH)D supplementation. While from the finalized observational studies risk of PE with 25(OH)D deficiency in pregnant women was assessed. Revman (v5.4.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) was used for all statistical analyses. To visually assess, the results of pooling forest plots were constructed. The results were presented as odds ratios (OR) and 95% confidence intervals.
Quality assessment
A systematic quality assessment of the included trials was performed for using the Cochrane criteria risk of bias tool (Supplemental Figure 1(a) and (b)), while the Newcastle Ottawa scale (NOS) was used for the observation studies (Supplemental Tables 1 and 2). 12 The following items were used: adequacy of sequence generation, allocation concealment, blinding addressing of dropouts (incomplete outcome data), selective outcome reporting, and other probable sources of bias. Risk of bias assessment was independently performed by two authors (HA and NS); disagreements were resolved by a consensus-based discussion.
Results
Study selection
Initial search of the four electronic databases yielded 8564 potential studies. After exclusions, 34 articles were selected including 24 observational studies and 10 RCTs. The PRISMA flow chart summarizes the results of our literature search (Figure 1).
Characteristics of the studies
Twenty-four observational studies incorporating 2739 patients with <25 nmol/l (2233 Healthy Pregnant Women, 506 pre-eclamptic Women), 18,977 patients with <50 nmol/l (17,903 Healthy Pregnant Women, 1074 pre-eclamptic Women), and 4280 patients with <75 nmol/l (3412 Healthy Pregnant Women, 868 pre-eclamptic Women) met the inclusion criteria and were selected for meta-analysis. Among the included studies, all dealt with risk of PE related to different concentrations of 25(OH)D. Ten randomized controlled trials were eligible and selected. It included 3451 participants in the meta-analysis. These included 1750 subjects in the 25(OH)D treatment arm and 1701 control subjects.
The included studies were conducted in the United States of America (n = 6), Iran (n = 11), Canada (n = 4), China (n = 3), the United Kingdom (n = 1), India (n = 2), Bangladesh (n = 1), Saudi Arabia, Turkey, Qatar, Serbia, and Sweden (n = 1). The baseline characteristics of the studies are summarized in Tables 1 and 2.
Table 1.
Baseline characteristics dealing with associations between maternal 25(OH)D deficiency and increased risk of preeclampsia.
| Author | Study location | Study design | Trimester of 25(OH)D evaluation | Measurement method | Patient number | Health number | Study duration |
|---|---|---|---|---|---|---|---|
| Abedi 13 | Iran | Case control | First | ELISA kit | 59 | 59 | July 2012–November 2012 |
| Achkar 14 | Canada | Case control | NR | Automated chemiluminescence immunoassay | 169 | 1975 | NR |
| Anderson 15 | USA | Prospective study | First | Competitive radioimmunoassay | 11 | 35 | NR |
| Arisoy 16 | Turkey | Prospective cohort | Third | LC-MS | 77 | 180 | NR |
| Baker 10 | USA | Case control | Second | LC-MS | 43 | 198 | NR |
| Bener 17 | Qatar | Cohort | Third | Radioimmunoassay kits | 129 | 1744 | NR |
| Bodnar 18 | USA | Case control | NR | ELISA kits | 49 | 216 | 1997–2001 |
| Djekic-Ivankovic 19 | Serbia | Case control | Third | ELISA kits | 30 | 30 | January 2011–April 2011 |
| Flood-Nichols 20 | USA | Retrospective cohort | First | ELISA kits | 19 | 176 | 2014 (1 year) |
| Gidolf 21 | Sweden | Prospective cohort | NR | Chemiluminescent assay method | 37 | 120 | March 1994–June 1995 |
| Gupta 22 | India | Case control | First | Competitive protein binding | 50 | 50 | NR |
| Hamedanian 23 | Iran | Prospective case control | First | ELISA kits | 60 | 60 | January 2016–March 2017 |
| Mohaghegh 24 | Iran | Case control | NR | ELISA kits | 41 | 50 | November 2013–March 2014 |
| Muyayalo 25 | China | Case control | Third | Electrochemiluminescence (ECL) in an automatic luminescence apparatus | 41 | 59 | March 1–December 31, 2018 |
| Pashapour 26 | Iran | Case control | Labour | ELISA kits | 80 | 80 | January 2016–May 2016 |
| Robinson 27 | USA | Case control | Third | Double antibody radioimmunoassay | 50 | 100 | NR |
| Sadin 28 | Iran | Case control | Second | Chemiluminescent immunoassay method | 40 | 40 | NR |
| Scholl 29 | UK | Prospective cohort | Second and third | HPLC assay | 69 | 1072 | 2001–2007 |
| Shand 30 | Canada | Prospective cohort | First and second | Radioimmunoassay kits | 60 | 161 | 2004–2007 |
| Ullah 31 | Bangladesh | Case control | Second | Electrochemiluminescence immunoassay (ECLIA) | 112 | 76 | 2013 (1 year) |
| Wei 32 | Canada, Mexico | Prospective cohort | Second | Chemiluminescence immunoassay | 32 | 665 | January 2004–March 2006 |
| Wetta 33 | USA | Case control | Second | LC-MS | 89 | 177 | 2007–2008 |
| Xu 34 | USA | Retrospective cohort | Third | High sensitivity human IL-6 immunoassay kit | 100 | 100 | NR |
| Zhao 9 | China | Cohort | Second | Automated chemiluminescence immunoassay | 139 | 11012 | January 2011–December 2013 |
Table 2.
Baseline characteristics dealing with associations between 25(OH)D supplementation and decreased risk of preeclampsia.
| Author | Study location | Study design | Trimester of 25(OH)D evaluation | Measurement method | Patient number | Health number | Study duration | Dosage of 25(OH)D |
|---|---|---|---|---|---|---|---|---|
| Ali 35 | Saudi Arabia | RCT | NR | 25(OH)D total assay | 83 | 81 | October 2012–Juky 2014 | 400 IU vitamin D3/tablet once daily; 4000 IU vitamin D3 (40 drops daily) |
| Azami 36 | Iran | RCT | NR | NR | 30 | 30 | NR | 1 Ferrous sulfate tablet + 1 vitamin D tablet (800 mg Ca, 200 mg Mg, 8 mg Zn and 400 IU vitamin D3) daily; 1 Ferrous sulfate tablet + 250 mg vitamin C and 55 mg vitamin E; control group: Ferrous sulfate daily |
| Sablok 11 | India | RCT | NR | Sandwich ELISA | 108 | 57 | 2010–2012 | Sufficient: 1 dose of 60,000 IU at 20 weeks, Insufficient: 2 doses of 120 000 IU at 20 and 24 weeks, deficient:4 doses of 120,000 IU cholecalciferol at 20, 24, 28 and 32 weeks; Placebo |
| Sasan 37 | Iran | RCT | NR | Liebermann-Burchard method | 70 | 72 | NR | 50,000 IU pearl vitamin D3 once every 2 weeks; Placebo |
| Mirzakhani 38 | Canada | RCT | NR | Chemiluminescence assay | 408 | 408 | October 2009–July 2011 | 4400 IU 25(OH)D; 400 IU 25(OH)D |
| Karamali 39 | Iran | RCT | NR | ELISA kit | 30 | 30 | July 2014–October 2014 | 1 oral 50,000 IU vitamin D3 every 14 days; Placebo every 14 days |
| Naghshineh 40 | Iran | RCT | NR | NR | 68 | 70 | January 2012–May 2012 | 600 IU daily of 25(OH)D; Placebo |
| Qian 41 | China | RCT | NR | Electrochemiluminescence immunoassay (ECLIA) | 30 | 30 | February 2014–October 2014 | Daily dose of 2000 IU 25(OH)D; Placebo |
| Asemi 42 | Iran | RCT | NR | ELISA kit | 23 | 23 | November 2013 – May 2014 | 25(OH)D supplement containing 800 mg calcium, 200 mg magnesium, 8 mg zinc and 400 IU Vitamin D3; Placebo |
| Rostami 43 | Iran | RCT | NR | ELISA kit | 900 | 900 | NR | Moderate Deficiency: I1 = 50,000 IU of oral D3 weekly for 6 weeks, I2 = 50,000 IU of oral D3 weekly for 6 weeks and a monthly dose of 50,000 IU of D3 until delivery, I3 = single dose of intramuscular 300,000 IU of D3, I4 = single dose of intramuscular 300,000 IU of D3 and a monthly dose of 50,000 IU of D3 until delivery. Severe Deficiency: I5 = 50,000 IU of oral D3 weekly for 12 weeks, I6 = 50,000 IU of oral D3 weekly for 12 weeks and a monthly dose of 50,000 IU of D3 until delivery. I7 = Intramuscular 300,000 IU of D3; two doses for 6 weeks, I8 = Intramuscular 300,000 IU of D3; two doses for 6 weeks, followed by a monthly dose of 50,000 IU of D3 until delivery |
Quality of assessment and publication bias
Risk of bias was assessed for both RCTs and observational studies. The Newcastle Ottawa scale was used for observational studies. While for RCTs, risk of bias was assessed through Review Manager (RevMan) 5.0 and subsequently its funnel plot. High-quality responses earn a star, totaling up to nine stars (the comparability question earns level of detail: from the answer to each question for each study (maximum detail) to a summary score equal to the number of stars earned by each study (minimum detail). Our group presented a partial score summarizing the number of stars earned by each study in each domain. Although the Cochrane Collaboration endorses the NOS, it acknowledges that researchers may want to assess study quality based not only on the quality of the analysis, covered by the NOS, but also on the quality of the reporting of the study, which is not included in this tool. 12 Overall, the quality of studies showed moderate quality.
Almost every included study in RCTs was characterized by sufficient information regarding random sequence generation, allocation concealment and personnel blinding, and outcome assessments, and showed low risk of bias because of incomplete outcome data and selective outcome reporting through Review Manager (RevMan) 5.0. Details of the quality of bias assessment for the RCTs are also shown upon visual inspection of Begg’s funnel plot asymmetry. These are shown in the Figure 2 below as well as Figure 3 shown below. Overall, the quality of studies showed moderate quality.
Figure 2.

Funnel plot for studies included in population.
Figure 3.
Forest plot displaying 25(OH)D intervention effects compared to placebo on preeclampsia (PE).
Results
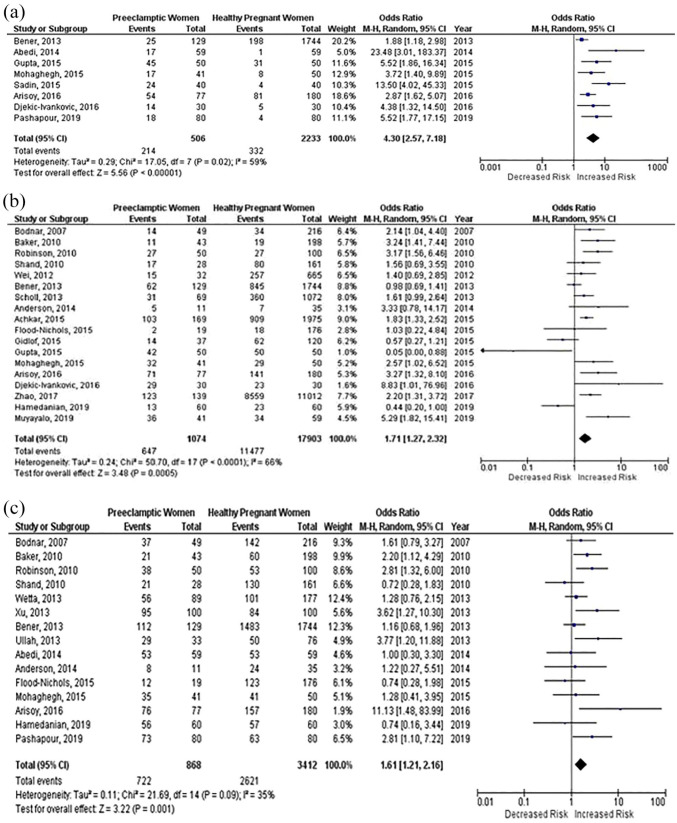
Out of the 34 selected studies, 24 studies incorporating 25,996 participants reported on association between 25(OH)D deficiency and risk of PE. In pooled analyses of these studies, revealed that 25(OH)D deficiency (<25 nmol/l, <50 nmol/l, 75 nmol/l) was associated with an increased risk of PE, (OR: 4.30; 95% CI: 2.57–7.18; p < 0.00001), (OR: 1.71; 95% Cl: 1.27–2.32; p = 0.0005) and (OR: 1.61; 95% Cl: 1.21–2.16; p = 0.001). Moderate to high heterogeneity was detected among the studies, (I 2 = 59%) for the first group, and second group, (I = 66%). Moderate heterogeneity was observed in the third group, (I = 35%). The results are summarized in Figure 4(a)–(c) for respective (<25 nmol/l, <50 nmol/l, 75 nmol/l) 25(OH)D concentrations.
Figure 4.
(a) Forest plot displaying the association between low 25(OH)D concentrations (<25 nmol/l) during pregnancy and preeclampsia (PE), (b) forest plot displaying the association between low 25(OH)D concentrations (<50 nmol/l) during pregnancy and preeclampsia (PE), and (c) forest plot displaying the association between low 25(OH)D concentrations (<75 nmol/l) during pregnancy and preeclampsia (PE).
Out of a total of 34 selected studies, 10 RCTs reported on incidence of PE and 25(OH)D supplementation. This included 3451 participants, 1750 in the 25(OH)D treatment arm and 1701 in the control (placebo) group, there was moderate to low heterogeneity observed, (I 2 = 16%). Our pooled analysis demonstrated that 25(OH)D supplementation is associated with reduce risk of PE (OR: 0.50; 95% CI: 0.40–0.63; p < 0.00001). The results are summarized in Figure 3.
Discussion
This meta-analysis focuses on two aspects: observational studies reporting the associations between 25(OH)D deficiency and the risk of PE and RCT reporting the effect of 25(OH)D supplementation on the incidence of PE. Our findings report that low maternal 25(OH)D concentrations (<25 nmol/l, <50 nmol/l, and <75 nmol/L) are associated with an increased risk of PE. Furthermore, 25(OH)D supplementation decreases the risk of PE.
The latest guideline by the World Health Organization suggests recommending 25(OH)D supplementation for women with 25(OH)D deficiency during pre-gestational age, as it is preferred for preventing pre-eclampsia (PE). 44 The U.S. Institute of Medicine guidelines recommend a supplementation of 600 IU/day of vitamin D3 for pregnant women. 45 However, the U.S. Endocrine Society recommends maintaining serum concentrations of 25(OH)D above 30 ng/ml, with pregnant women requiring at least 600 IU/day supplementation. It’s worth noting that 1500–2000 IU/day of 25(OH)D may be necessary to maintain the serum 25(OH)D concentrations. 46
A study conducted by Hollis et al. 47 also demonstrated that higher supplementation of 4000 IU/day, as opposed to 2000 IU/day and 400 IU/day, decreased the risk of PE without causing hypercalcemia. This finding was consistent with an RCT conducted by Ali et al., 37 which indicated a lower incidence of PE with a higher dosage of 4000 IU of 25(OH)D compared to a dosage of 400 IU. Hence, supplementation with a higher dosage of 25(OH)D may yield benefits during pregnancy.
Maternal nutrient 25(OH)D deficiency might lead to a pro-inflammatory reaction, increase oxidative pressure and lead to endothelial dysfunction and vascular health impairment. 48 25(OH)D functions as a recognized regulator of inflammation. The naturally occurring dietary form, vitamin D3, is considered to lack biological activity. 49 The positive impacts of 25(OH)D are believed to be primarily mediated by 1, 25(OH)2D. A common factor in severe inflammatory disorders is the reduced concentrations of 25(OH)D, which causes the disruption of endothelial stability, leading to an increased tendency for “vascular leakage.” Experimental animal models of PE clearly illustrate that this instability in endothelial function results in placental ischemia. 49 Low serum 25(OH)D concentrations have been related with vascular endothelial cell irritation, increased nuclear factor kappa B (NF-κB) signaling-related suppression of vascular endothelial function and decreased vascular endothelial 25(OH)D receptor and 1-a hydroxylase expression. 50 On the other side, satisfactory 25(OH)D admission may assist with the maintenance of the calcium homeostasis—which is inversely related to PE 51 or may lower the multiplication of the vascular smooth muscle cells. 52 Besides, 25(OH)D may be an incredible endocrine suppressor of renin biosynthesis and could direct the renin–angiotensin system, which plays a crucial role in blood pressure control.52,53 Moreover, 25(OH)D could likewise adjust the synthesis of adipokines associated with endothelial and vascular health. 54 The defect of genes related to 25(OH)D’s effect on gene regulation dealing with the systemic inflammation and immune responses, suggests that there is a specific immune cascade of events associated with 25(OH)D deficiency that occurs early-on in pregnancy in women destined to develop PE. 55
Rostami et al. 46 study demonstrated a significant reduction in the risk of pregnancy complications, including PE, gestational diabetes mellitus (GDM), and preterm delivery, through the implementation of the screening program for the detection and treatment of 25(OH)D deficiency. The findings of this study highlighted the importance of screening program for maternal 25(OH)D deficiency, as it can significantly reduce the risk of various adverse maternal outcomes. Pregnant women included in the screening arm of the study who were taking monthly maintenance dose of 50,000 IU of Vitamin D3 had a higher likelihood of achieving a serum concentration of 25-hydroxyvitamin D (25(OH)D) greater than 20 ng/ml. This indicates that the supplementation was successful in raising their 25(OH)D concentrations. The probability of achieving these concentrations in intervention site was 53% whereas in the nonintervention site (where no screening program was implemented), it was only 0.02%. This suggests that without a screening program, majority of pregnant women with moderate or severe deficiency remained deficient at the time of pregnancy and as a result dealt with adverse maternal outcomes. The results indicated that overall, the screening program led to a 55% reduction in the risk of these adverse pregnancy outcomes. Among the women who underwent screening, only 17% experienced adverse outcomes, in contrast to the 29% of pregnancies that were complicated by these outcomes in the absence of screening. Specifically for PE, without screening 17% women developed PE compared to only 8% among the screened. The prevalence of PE remains relatively high in the low-risk pregnant population which could potentially be linked to other underlying factors such as the initially low concentrations of 25(OH)D at baseline and at the time of delivery. Given that the pathophysiological process of PE is believed to initiate early in pregnancy, the first trimester is considered a crucial period for interventions aimed at preventing this condition. 56 25(OH)D status has been associated with BMI levels and obesity,18,57 leading to severe complications including PE during pregnancy. 58 Sablok et al. 11 revealed a notable connection between BMI ⩾ 25 and low 25(OH)D concentrations (p = 0.000). The odds ratio stood at 4.6 with a 95% CI of 90.37–225.74, underscoring a robust association between higher BMI and reduced 25(OH)D concentrations. Previous literature has highlighted 25(OH)D concentration alterations among obese individuals, attributing reduced bioavailability to its deposition within the adipose tissue. 59 In the context of this study, the difference in outcomes could be attributed at least in part, to the timing of the interventions. The study also quoted that if 25(OH)D supplementation and screening was implemented early in pregnancy, it might have allowed for more optimal 25(OH)D concentrations to be established in the critical phases of placental development and caused a significant reduction in prevalence of PE. The study also highlighted that screening led to the reduction in the prevalence of secondary outcomes such as GDM by 50% and preterm delivery up to 40% in women with 25(OH)D < 20 ng/ml.
All the studies included in this meta-analysis and systematic review use different 25(OH)D assays, which are used to measure the serum concentration of 25(OH)D. The need for standardization arises from the fact that different assays can yield slightly different results for the same sample. Wise et al. 60 is an intralaboratory comparison study that was conducted by the vitamin D standardization program (VDSP), focused on evaluating the degree of variability and potential bias introduced by different commonly used assay methods when measuring total serum 25(OH)D concentrations. The study assessed a total of 13 assays; among these 11 were immunoassays and one was a liquid chromatography-Tandem mass spectrometry (LC-MS/MS) assay. In total, 50 single-donor serum samples were considered. The assays were evaluated based on their precision (%CV) and accuracy (%bias) compared to the reference measurement procedures and VDSP. The results indicated that the majority of the assays met the VDSP criteria for both accuracy and precision.
Strengths and limitations
The strengths of our study include that we used a range of studies with variable ages forming a wide age gap, which would help us negate any biases. We have also taken studies from different parts of the world avoiding our results being generalized to a specific population. Moreover; our meta-analysis also has important clinical relevance as it provides evidence that 25(OH)D supplementation can reduce risk of PE, but also investigates the relationship between 25(OH)D deficiency and the risk of PE. This aspect is not addressed in the previous articles. This study also specifically points out the potential of 25(OH)D supplementation as an intervention strategy to prevent PE, which could have significant implications for reducing maternal mortality worldwide.
However, our study also encountered certain limitations. In observational studies, 25(OH)D concentrations were documented at varying gestational ages, and throughout diverse follow-up periods. Considering that female physiology undergoes significant changes during different gestational ages, there are noteworthy fluctuations in 25(OH)D concentrations in the body. These variations are crucial for sustaining heightened intestinal calcium absorption for the developing fetus. 61 In RCTs, as different dosages of supplementations had been administered in different studies, and given the small pool of studies (n = 11) we were unable to deduce a dosage effect. Another limitation may be that, all studies were not adjusted for smoking but, according to Bodnar et al. 62 there was “no absolute or relative difference in risk” after adjusting their cohort study for smoking. Further RCTs should be focused on finding the correct effective dosage and safety of different dosages for women with different ethnicities, as well as coming up with a dosage regimen by continued surveillance by doctors (i.e., daily, weekly or a single dose). More research can also be carried out to infer whether 25(OH)D given in combination with other nutrients is more effective or not and if high or low risk pregnancies both require it.
Conclusion
The findings of our meta-analysis suggest that 25(OH)D deficiency (<25 nmol/l, <50 nmol/l, <75 nmol/l) was associated with increased risk of PE. Moreover, the results also illustrated that 25(OH)D supplementation was associated with reduced risk of PE. However, more comprehensive RCTs are still required to identify the most effective dosage of 25(OH)D supplementation for women of different ethnicities.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-docx-3-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-docx-4-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-jpg-5-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-jpg-6-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Acknowledgments
The authors would like to acknowledge Research council of Pakistan for their support along with all the aspects of conducting this study.
Footnotes
Author contributions: AA generated the subject matter. NS and SSJ formulated the research design and helped in the introduction and methodology of the article. HA and MHB gathered pertinent research, assessed its quality, and extracted and examined the data. SG and EKJ provided feedback on the review and meta-analysis. AA, FQ, SA, and SS contributed to the manuscript’s preparation for publishing by assisting in all facets, encompassing results and citations. All authors have perused and endorsed the manuscript.
Availability of data and materials: All data included in this review has been cited and can be accessed online.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Not applicable.
Informed consent: Not applicable.
ORCID iD: Muhammad Hadi Baqai  https://orcid.org/0000-0002-9351-4769
https://orcid.org/0000-0002-9351-4769
Supplemental material: Supplemental material for this article is available online.
References
- 1. Peraçoli JC, Borges VTM, Ramos JGL, et al. Pre-eclampsia/Eclampsia. Rev Bras Ginecol Obstet 2019; 41(5): 318–332. [DOI] [PubMed] [Google Scholar]
- 2. Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev 2013; 71(Suppl 1(0 1)): S18–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335(7627): 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Backes CH, Markham K, Moorehead P, et al. Maternal preeclampsia and neonatal outcomes. J Pregnancy 2011; 2011: 214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fondjo LA, Boamah VE, Fierti A, et al. Knowledge of preeclampsia and its associated factors among pregnant women: a possible link to reduce related adverse outcomes. BMC Pregnancy Childbirth 2019; 19(1): 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009; 113(6): 1299–1306. [DOI] [PubMed] [Google Scholar]
- 7. Samimi M, Kashi M, Foroozanfard F, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J Hum Nutr Diet 2016; 29(4): 505–515. [DOI] [PubMed] [Google Scholar]
- 8. Saraf R, Morton SMB, Camargo CA, Jr, et al. Global summary of maternal and newborn vitamin D status—a systematic review. Matern Child Nutr 2016; 12(4): 647–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao X, Fang R, Yu R, et al. Maternal vitamin D status in the late second trimester and the risk of severe preeclampsia in Southeastern China. Nutrients 2017; 9(2): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker AM, Haeri S, Camargo CA, Jr, et al. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab 2010; 95(11): 5105–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sablok A, Batra A, Thariani K, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf) 2015; 83(4): 536–541. [DOI] [PubMed] [Google Scholar]
- 12. Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol 2014; 6: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abedi P, Mohaghegh Z, Afshary P, et al. The relationship of serum vitamin D with pre-eclampsia in the Iranian women. Matern Child Nutr 2014; 10(2): 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Achkar M, Dodds L, Giguère Y, et al. Vitamin D status in early pregnancy and risk of preeclampsia. Am J Obstet Gynecol 2015; 212(4): 511.e1–511.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson CM, Ralph JL, Johnson L, et al. First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas. Life Sci 2015; 129: 10–15. [DOI] [PubMed] [Google Scholar]
- 16. Arisoy R, Bostancı E, Erdogdu E, et al. Association between maternal serum 25-hydroxyvitamin D level and pre-eclampsia. J Matern Fetal Neonatal Med 2015; 29(12):1941–1944. [DOI] [PubMed] [Google Scholar]
- 17. Bener A, Al-Hamaq AO, Saleh NM. Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. Int J Womens Health 2013; 5: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 2007; 92(9): 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Djekic-Ivankovic M, Weiler H, Jones G, et al. Vitamin D status in mothers with pre-eclampsia and their infants: a case-control study from Serbia, a country without a vitamin D fortification policy. Public Health Nutr 2017; 20(10): 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flood-Nichols SK, Tinnemore D, Huang RR, et al. Vitamin D deficiency in early pregnancy. PLoS One 2015; 10(4): e0123763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gidlöf S, Silva AT, Gustafsson S, et al. Vitamin D and the risk of preeclampsia—a nested case-control study. Acta Obstet Gynecol Scand 2015; 94(8): 904–908. [DOI] [PubMed] [Google Scholar]
- 22. Gupta T, Wahi S, Gupta N, et al. Correlation of vitamin D levels in term normotensive and pre-eclamptic patients in labor. J Obstet Gynaecol India 2016; 66(3): 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamedanian L, Badehnoosh B, Razavi-Khorasani N, et al. Evaluation of vitamin D status, parathyroid hormone, and calcium among Iranian pregnant women with preeclampsia: a case-control study. Int J Reprod Biomed 2019; 17(11): 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohaghegh Z, Abedi P, Dilgouni T, et al. The relation of preeclampsia and serum level of 25-hydroxyvitamin D in mothers and their neonates: a case control study in Iran. Horm Metab Res 2015; 47(4): 284–288. [DOI] [PubMed] [Google Scholar]
- 25. Muyayalo KP, Huang X-B, Qian Z, et al. Low circulating levels of vitamin D may contribute to the occurrence of preeclampsia through deregulation of Treg /Th17 cell ratio. Am J Reprod Immunol 2019; 82(4): e13168. [DOI] [PubMed] [Google Scholar]
- 26. Pashapour S, Golmohammadlou S, Behroozi-Lak T, et al. Relationship between low maternal vitamin D status and the risk of severe preeclampsia: a case control study. Pregnancy Hypertens 2019; 15: 161–165. [DOI] [PubMed] [Google Scholar]
- 27. Robinson CJ, Alanis MC, Wagner CL, et al. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol 2010; 203(4): 366.e1–366.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sadin B, Pourghassem Gargari B, Pourteymour Fard Tabrizi F. Vitamin D status in preeclamptic and non-preeclamptic pregnant women: a case-control study in the North West of Iran. Health Promot Perspect 2015; 5(3): 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scholl TO, Chen X, Stein TP. Vitamin D, secondary hyperparathyroidism, and preeclampsia. Am J Clin Nutr 2013; 98(3): 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shand AW, Nassar N, Von Dadelszen P, et al. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG 2010; 117(13): 1593–1598. [DOI] [PubMed] [Google Scholar]
- 31. Ullah MI, Koch CA, Tamanna S, et al. Vitamin D deficiency and the risk of preeclampsia and eclampsia in Bangladesh. Horm Metab Res 2013; 45(9): 682–687. [DOI] [PubMed] [Google Scholar]
- 32. Wei SQ, Audibert F, Hidiroglou N, et al. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG 2012; 119(7): 832–839. [DOI] [PubMed] [Google Scholar]
- 33. Wetta LA, Biggio JR, Cliver S, et al. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am J Perinatol 2014; 31(6): 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu L, Lee M, Jeyabalan A, et al. The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am J Obstet Gynecol 2014; 210(2): 149.e1–149.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali AM, Alobaid A, Malhis TN, et al. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia—randomized controlled trial. Clin Nutr 2019; 38(2): 557–563. [DOI] [PubMed] [Google Scholar]
- 36. Azami M, Azadi T, Farhang S, et al. The effects of multi mineral-vitamin D and vitamins (C+E) supplementation in the prevention of preeclampsia: an RCT. Int J Reprod Biomed 2017; 15(5): 273–278. [PMC free article] [PubMed] [Google Scholar]
- 37. Behjat Sasan S, Zandvakili F, Soufizadeh N, et al. The effects of vitamin D supplement on prevention of recurrence of preeclampsia in pregnant women with a history of preeclampsia. Obstet Gynecol Int 2017; 2017: 8249264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mirzakhani H, Litonjua AA, McElrath TF, et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest 2016; 126(12): 4702–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karamali M, Beihaghi E, Mohammadi AA, et al. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm Metab Res 2015; 47(12): 867–872. [DOI] [PubMed] [Google Scholar]
- 40. Naghshineh E, Sheikhaliyan S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Advan Biomed Res 2016; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qian L, Wang H, Wu F, et al. Vitamin D3 alters Toll-like receptor 4 signaling in monocytes of pregnant women at risk for preeclampsia. Int J Clin Exp Med 2015; 8(10): 18041–18049. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Asemi Z, Esmaillzadeh A. The effect of multi mineral-vitamin D supplementation on pregnancy outcomes in pregnant women at risk for pre-eclampsia. Int J Prev Med 2015; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rostami M, Tehrani FR, Simbar M, et al. Effectiveness of prenatal vitamin D deficiency screening and treatment program: a stratified randomized field trial. J Clin Endocrinol Metab 2018; 103(8): 2936–2948. [DOI] [PubMed] [Google Scholar]
- 44. Guideline: Vitamin D supplementation in pregnant women . Geneva: World Health Organization, https://www.ncbi.nlm.nih.gov/books/NBK310616/ (2012). [PubMed] [Google Scholar]
- 45. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Ross AC, Taylor CL, Yaktine AL, et al., editors. Washington, DC: National Academies Press (US), 2011. [PubMed] [Google Scholar]
- 46. Mazzoleni S, Magni G, Toderini D. Effect of vitamin D3 seasonal supplementation with 1500 IU/day in north Italian children (DINOS study). Ital J Pediatr 2019; 45(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011; 26(10): 2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cardús A, Parisi E, Gallego C, et al. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int 2006; 69(8): 1377–1384. [DOI] [PubMed] [Google Scholar]
- 49. Hollis BW, Wagner CL. New insights into the vitamin D requirements during pregnancy. Bone Res 2017; 5: 17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jablonski KL, Chonchol M, Pierce GL, et al. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011; 57(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Evans KN, Bulmer JN, Kilby MD, et al. Vitamin D and placental-decidual function. J Soc Gynecol Investig 2004; 11(5): 263–271. [DOI] [PubMed] [Google Scholar]
- 52. Dinca M, Serban MC, Sahebkar A, et al. Does vitamin D supplementation alter plasma adipokines concentrations? A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2016; 107: 360–371. [DOI] [PubMed] [Google Scholar]
- 53. Mirhosseini N, Vatanparast H, Kimball SM. The association between serum 25(OH)D status and blood pressure in participants of a community-based program taking vitamin D supplements. Nutrients 2017; 9(11): 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yakoob MY, Salam RA, Khan FR, et al. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst Rev 2016; 11(11): CD008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wagner CL, Hollis BW. The implications of vitamin D status during pregnancy on mother and her developing child. Front Endocrinol 2018; 9: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karras SN, Anagnostis P, Naughton D, et al. Vitamin D during pregnancy: why observational studies suggest deficiency and interventional studies show no improvement in clinical outcomes? A narrative review. J Endocrinol Invest 2015; 38(12): 1265–1275. [DOI] [PubMed] [Google Scholar]
- 57. Andersen LB, Abrahamsen B, Dalgård C, et al. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: a population-based cohort study. Clin Endocrinol 2013; 79(3): 333–341. [DOI] [PubMed] [Google Scholar]
- 58. Karlsson T, Andersson L, Hussain A, et al. Lower vitamin D status in obese compared with normal-weight women despite higher vitamin D intake in early pregnancy. Clin Nutr 2015; 34(5): 892–898. [DOI] [PubMed] [Google Scholar]
- 59. Arunabh S, Pollack S, Yeh J, et al. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 2003; 88(1): 157–161. [DOI] [PubMed] [Google Scholar]
- 60. Wise SA, Camara JE, Sempos CT, et al. Vitamin D Standardization Program (VDSP) intralaboratory study for the assessment of 25-hydroxyvitamin D assay variability and bias. J Steroid Biochem Mol Biol 2021; 212: 105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soma-Pillay P, Nelson-Piercy C, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovasc J Afr 2016; 27(2): 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bodnar LM, Simhan HN, Catov JM, et al. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014; 25(2): 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-docx-3-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-docx-4-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-jpg-5-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine
Supplemental material, sj-jpg-6-smo-10.1177_20503121231212093 for Vitamin D and preeclampsia: A systematic review and meta-analysis by Abdulla AlSubai, Muhammad Hadi Baqai, Hifza Agha, Neha Shankarlal, Syed Sarmad Javaid, Eshika Kumari Jesrani, Shalni Golani, Abdullah Akram, Faiza Qureshi, Shaheer Ahmed and Simran Saran in SAGE Open Medicine