Abstract
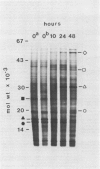
Upon initiation of ripening in avocado fruit (Persea americana Mill. cv Hass) with 10 microliters/liter ethylene, polysome prevalence and associated poly(A)+ mRNA increase approximately 3-fold early in the respiratory climacteric and drop off to preclimacteric levels at the peak of the respiratory climacteric. The increase in poly(A)+ mRNA on polysomes early in the respiratory climacteric constitutes a generic increase in constitutive mRNAs. New gene expression associated with ripening is minimal but evident after 10 hours of ethylene treatment and continues to increase relative to constitutive gene expression throughout the climacteric. The respiratory climacteric can be temporally separated into two phases. The first phase is associated with a general increase in protein synthesis, whereas the second phase reflects new gene expression and accumulation of corresponding proteins which may be responsible for softening and other ripening characteristics. A major new message on polysomes that arises concomitantly with the respiratory climacteric codes for an in vitro translation product of 53 kilodaltons which is immunoprecipitated by antiserum against avocado fruit cellulase.
Cyanide at 500 microliters/liter fails to affect the change in polysome prevalance or new gene expression associated with the ethylene-evoked climacteric in avocado fruit. Treatment of fruit with 500 microliters/liter cyanide alone initiates a respiratory increase within 4 hours, ethylene biosynthesis within 18 hours, and new gene expression akin to that educed by ethylene within 20 hours of exposure to cyanide.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awad M., Young R. E. Postharvest Variation in Cellulase, Polygalacturonase, and Pectinmethylesterase in Avocado (Persea americana Mill, cv. Fuerte) Fruits in Relation to Respiration and Ethylene Production. Plant Physiol. 1979 Aug;64(2):306–308. doi: 10.1104/pp.64.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen R. E., Laties G. G. Ethylene regulation of gene expression in carrots. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4060–4063. doi: 10.1073/pnas.79.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C., Klein I., Dilley D. R. Protein synthesis in relation to ripening of pome fruits. Plant Physiol. 1968 Jul;43(7):1146–1153. doi: 10.1104/pp.43.7.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mozer T. J. Partial purification and characterization of the mRNA for alpha-amylase from barley aleurone layers. Plant Physiol. 1980 May;65(5):834–837. doi: 10.1104/pp.65.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Richmond A., Biale J. B. Protein and nucleic acid metabolism in fruits. I. Studies of amino acid incorporation during the climacteric rise in respiration of the avocado. Plant Physiol. 1966 Oct;41(8):1247–1253. doi: 10.1104/pp.41.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Biale J. B. Protein and nucleic acid metabolism in fruits. II. RNA synthesis during the respiratory rise of the avocado. Biochim Biophys Acta. 1967 May 30;138(3):625–627. doi: 10.1016/0005-2787(67)90565-5. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R., French K. Temperature-dependent Changes in the Polysomal Population of Senescent (Ripening) Pear Fruit. Plant Physiol. 1977 Dec;60(6):930–932. doi: 10.1104/pp.60.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Effects of Cyanide and Ethylene on the Respiration of Cyanide-sensitive and Cyanide-resistant Plant Tissues. Plant Physiol. 1976 Jul;58(1):47–50. doi: 10.1104/pp.58.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Similarities between the Actions of Ethylene and Cyanide in Initiating the Climacteric and Ripening of Avocados. Plant Physiol. 1974 Oct;54(4):506–511. doi: 10.1104/pp.54.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Respiratory Contribution of the Alternate Path during Various Stages of Ripening in Avocado and Banana Fruits. Plant Physiol. 1978 Aug;62(2):249–255. doi: 10.1104/pp.62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation associated with cyanide-insensitive respiration in plant mitochondria. Biochim Biophys Acta. 1970 Dec 8;223(2):383–387. doi: 10.1016/0005-2728(70)90195-7. [DOI] [PubMed] [Google Scholar]