Abstract
Pyruvate (Pyr) and α-ketoglutarate (αKg) accumulated when cells of Pseudomonas fluorescens NCIMB 11764 were cultivated on growth-limiting amounts of ammonia or cyanide and were shown to be responsible for the nonenzymatic removal of cyanide from culture fluids as previously reported (J.-L. Chen and D. A. Kunz, FEMS Microbiol. Lett. 156:61–67, 1997). The accumulation of keto acids in the medium paralleled the increase in cyanide-removing activity, with maximal activity (760 μmol of cyanide removed min−1 ml of culture fluid−1) being recovered after 72 h of cultivation, at which time the keto acid concentration was 23 mM. The reaction products that formed between the biologically formed keto acids and cyanide were unambiguously identified as the corresponding cyanohydrins by 13C nuclear magnetic resonance spectroscopy. Both the Pyr and α-Kg cyanohydrins were further metabolized by cell extracts and served also as nitrogenous growth substrates. Radiotracer experiments showed that CO2 (and NH3) were formed as enzymatic conversion products, with the keto acid being regenerated as a coproduct. Evidence that the enzyme responsible for cyanohydrin conversion is cyanide oxygenase, which was shown previously to be required for cyanide utilization, is based on results showing that (i) conversion occurred only when extracts were induced for the enzyme, (ii) conversion was oxygen and reduced-pyridine nucleotide dependent, and (iii) a mutant strain defective in the enzyme was unable to grow when it was provided with the cyanohydrins as a growth substrate. Pyr and αKg were further shown to protect cells from cyanide poisoning, and excretion of the two was directly linked to utilization of cyanide as a growth substrate. The results provide the basis for a new mechanism of cyanide detoxification and assimilation in which keto acids play an essential role.
Cyanide is a notorious poison. Its inhibitory effect on respiration has been known since the 1920s, when Warburg and Keilin first demonstrated that it combines with trivalent iron in cytochrome oxidase (38, 40, 44). Although highly toxic, it is a normal part of our environment for which mechanisms of biological formation (cyanogenesis) and detoxification exist (8, 22, 42). Cyanide also arises from various industrial practices such as steel coking, electroplating, and mining, but significant accumulations in the environment probably do not occur because of its highly reactive nature (13, 18, 41, 46). The interactions between microorganisms and cyanide, however, remain of interest, since the mechanisms of tolerance and assimilation are poorly understood. A number of reports documenting the ability of microorganisms to grow on cyanide have appeared, but the biochemical basis of these abilities has remained largely obscure. Most studies have reported its ability to serve as a nitrogen source only, since at the concentrations needed for it to serve as a carbon source, it is too toxic (15, 24). As far as is known, growth on cyanide requires that it be enzymatically converted to ammonia. Once formed it can then be readily incorporated into cellular macromolecules by established mechanisms (31). Two separate conversions have been described. They are hydrolytic and oxidative conversion, and they yield formic acid and carbon dioxide as reaction by-products, respectively. The enzyme responsible for hydrolytic conversion has variously been described as cyanidase, cyanide dihydratase, or cyanide nitrilase (CNN), and it catalyzes the reaction shown in equation 1.
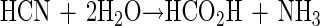 |
1 |
Mechanistically, CNNs resemble other nitrilases (e.g., EC 3.5.5.1) that catalyze the direct conversion of organic nitriles into an organic acid and ammonia but for which the substrate range appears to be limited to cyanide. The involvement of CNNs in cyanide metabolism has been reported for Alcaligenes xylosooxidans subsp. denitrificans (19, 20), Bacillus pumilus (30), and Pseudomonas sp. (45). Oxidative conversion is mediated by an enzyme described as cyanide oxygenase (CNO). This enzyme has been described for Pseudomonas fluorescens NCIMB 11764 only (15, 23–26). Recent work in our laboratory has shown that CNO functions as a monooxygenase, since a single atom of molecular oxygen was shown to be incorporated during conversion (43). Since the other atom of oxygen in CO2 was shown to be derived from water, a reaction mechanism in which cyanide undergoes initial monooxygenative attack to give an unknown intermediate (X-OH) as shown in equation 2 was proposed (43).
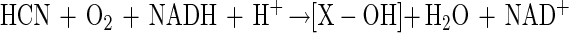 |
2 |
Further hydrolysis of X-OH is then expected to give CO2 and NH3 as shown in equation 3.
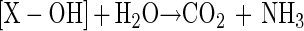 |
3 |
The nature of X-OH and whether an additional enzyme is required for its conversion are unknown. Interestingly, NCIMB 11764 also elaborates a CNN, but only CNO has been shown to be physiologically required for cyanide utilization (26). This conclusion was reached after it was found that mutants unable to grow on cyanide did not make CNO but could still elaborate CNN.
CNO is induced when cyanide (KCN) is added to nitrogen-limited cells (4, 26). This approach for obtaining cells induced for the enzyme is more convenient than growing cells on cyanide, which requires several days of fed-batch cultivation. During the course of experiments aimed at optimizing CNO induction, we discovered that the consumption of cyanide and the appearance of CNO activity in cell extracts were not concomitant (3, 4). Further experiments showed that cyanide consumption independent of that catalyzed by CNO occurred nonenzymatically and that a reaction between cyanide and a metabolite excreted into the medium was responsible for cyanide’s removal (4). Since cyanide-removing activity in culture fluids consistently copurified with iron-chelating activity, it was concluded that the responsible metabolite was a siderophore, but further identification of this siderophore was not achieved. Here we report that the compounds responsible for nonenzymatic cyanide removal are α-keto acids, namely, pyruvate (Pyr) and α-ketoglutarate (αKg). These findings help explain the earlier reported involvement of a putative siderophore, since these compounds can act as iron chelators (10, 35). However, the additional ability to serve also as effective cyanide-scavenging agents has not generally been recognized. Both Pyr and αKg were excreted into the medium when P. fluorescens NCIMB 11764 was grown on nitrogen-limiting amounts of ammonia or cyanide as a nitrogen source, and we now demonstrate that these metabolites play an essential role in the utilization of cyanide as a growth substrate.
MATERIALS AND METHODS
Strains, cultivation conditions, and cell extract preparation.
P. fluorescens NCIMB 11764, whose origin has been described previously (15, 25), was used throughout this study. A mutant strain, JL102, unable to grow on cyanide was obtained by nitrosoguanidine mutagenesis as described previously (26). The minimal medium for cultivation of cells contained 67 mM KH2PO4 (pH 7.0), 1.6 mM MgSO4 · 7H2O, and 36 μM FeSO4 · 7H2O. The inorganic salts were added aseptically to the buffer solution after it was autoclaved, as were glucose (20 mM) and various nitrogen sources. Unless otherwise indicated below, cells were incubated on a gyratory shaker (250 rpm) at 30°C. The excretion of keto acids was routinely observed in minimal medium (described above) supplied with ammonia (NH4CI, 1 mM) as the nitrogen source (referred to herein as GA medium). The inoculum for growth was derived from a 24- to 36-h-old colony grown on L agar (Lennox medium) (28), which was cultivated in GA medium for 48 h (A540, 0.7) before the entire culture (10% inoculum) was transferred to a second flask containing the same medium. The presence of keto acids and that of cyanide-removing activity in the culture fluid were determined at various times. To induce CNO, 0.1 mM KCN was added after 24 h of cultivation and cells were harvested 3 to 5 h thereafter by centrifugation at 12,000 × g for 15 min. The cells were washed in Na2HPO4-KH2PO4 (Na-K) phosphate buffer (pH 7.0) and stored at −70°C until cell extracts (150,000 × g [high-speed supernatants]) were prepared as previously described (26). Extracts were further fractionated by ultrafiltration through Centriplus 10 and 30 concentrators (Amicon) where indicated in Results. Cyanide disappearance after its addition to cultures was determined by removing samples at periodic intervals and measuring the remaining cyanide concentration by the colorimetric assay of Lambert et al. (27). The protein content in cell extracts was determined by the modified Lowry assay with bovine serum albumin as a standard (29).
CNO-catalyzed transformations.
Routine assay of CNO activity was determined by measuring the initial rate of cyanide disappearance from reaction mixtures as described previously (26, 43). Reaction mixtures in 250 μl contained 3 to 10 mg of protein ml−1 (200 μl), 2mM KCN (5 μl), 4 mM NADH (10 μl), and Na-K phosphate buffer (pH 7.0) (35 μl), and reactions were initiated with NADH. The specific activity for CNO when it was measured in this manner was 30 nmol of cyanide consumed min−1 mg of protein−1. CNO activity towards the cyanohydrins was assayed by measuring 14CO2 or NH3 production from the 14CN-labeled substrates (Pyr-14CN and Kg-14CN). For this purpose, reaction mixtures were supplied with the same components as described above except that cyanohydrin was substituted for KCN. Reaction components were added to uncapped 2-ml high-performance liquid chromatograaphy (HPLC) vials, which were placed inside separate 15-ml-capacity crimp-seal vials (Pierce Chemical Co., Rockford, Ill.). These vials were stoppered before unlabeled (2 mM) and labeled (1 to 2 μCi) cyanohydrins were injected. Reactions were initiated by the injection of NADH, and the vial assembly was incubated with shaking at 30°C. At various times, reactions were terminated by the addition of 25 μl of 0.5 N H2SO4 to the internal vial (which served also to volatilize 14CO2 present as H14CO3−). NaOH (4 N) was then added to the outer crimp-seal vial compartment, and the vial assembly was allowed to incubate for an additional 30 min to trap 14CO2 as Na2 14CO3. Radioactivity as Ba14CO3 in both the internal HPLC and outer crimp-seal vial compartments was then recovered following fractionation with BaCl2 as described previously (26, 43), and radioactivity was counted in a liquid scintillation counter.
Ammonia production from cyanohydrins was determined colorimetrically by the indophenol method of Fawcett and Scott (11). No evidence for 14CO2 or NH3 production from the radiolabeled cyanohydrins was observed in controls incubated in the absence of cell extract.
Determination of α-keto acids.
α-Keto acids in culture fluids were analyzed by HPLC with a Rainin-Varian HPLC equipped with a Knauer UV detector. Following removal of cells in a microcentrifuge, samples were acidified with concentrated H2SO4 to pH 2.0 and 10-μl samples were injected onto a Bio-Rad Aminex HPX-87H ion-exclusion column at ambient temperature. Elution was performed isocratically in aqueous mobile phase (pH 2.0) containing 0.015 N H2SO4 and 0.00034 M ethylenediamine tetraacetic acid maintained at a flow rate of 0.6 ml min−1. The concentration of each keto acid was determined from the peak area detected at 210 nm and quantitated with a Rainin Dynamax data acquisition system.
α-Keto acids were also determined by thin-layer chromatography following derivatization as the 2,4-dinitrophenylhydrazones (12, 21). Compounds were derivatized by incubating samples in a 1:2 ratio with 2,4-dinitrophenylhydrazine (0.1% in 2 N HCl) for 10 min. Derivatized compounds were extracted into an equal volume of ethylacetate, and samples were spotted onto Silica Gel 60 F-254 sheets (Alltech, Deerfield, Ill.) and chromatographed in a solvent system containing benzene-tetrahydrofuran-acetic acid (60:34:4). Pyr and αKg were also determined with lactate (EC 1.1.1.27) and glutamate dehydrogenase (EC 1.4.1.2), respectively.
Identification of cyanohydrins as reaction products.
The cyanohydrins formed from reactions of cyanide with, respectively, Pyr and αKG were identified by 13C nuclear magnetic resonance (NMR) spectroscopy. Culture fluid from cells grown for 24 h in minimal GA medium was concentrated 50-fold by lyophilization in a Speed-Vac (Savant) and incubated with K13CN (5 mg ml−1) in a sealed 2-ml HPLC vial at 30°C for 30 min until no free cyanide could be detected. The reaction contents were transferred to an NMR tube and analyzed on a Varian GEM 200 instrument at 50 MHz. 13C resonances (broad-band proton decoupled) were compared to that of tetramethylsilane (0 ppm) as an external reference. To obtain spectra in which protons and carbon are coupled, samples were analyzed in the gated decoupled mode by using software available with the spectrometer. In this mode, the proton decoupler is off at the time of the initial observed pulse in the 13C observation channel, which affords a proton-coupled 13C-NMR spectrum with the signal intensity gain resulting from nuclear Overhauser enhancement (17).
Cyanide antagonism by α-keto acids.
Cyanide antagonism by Pyr and αKg (or culture fluids containing the keto acids) was determined by adding these α-keto acids to minimal GA medium containing KCN as a growth inhibitor and 9 mM (NH4)2SO4 as the nitrogen source. Each keto acid was added at 1 mM to 10 ml of minimal medium in 40-ml-capacity crimp-seal culture bottles (Pierce). KCN at various concentrations (0.1 to 1.0 mM) was added by injection, the bottles were allowed to incubate for 30 min at room temperature before cells were added (1% inoculum), and the A540 was determined after 24 h of incubation. The inoculum for growth was cultivated in GA medium for 48 h before cells were harvested, washed twice in sterile Na-K phosphate buffer (pH 7.0), and suspended in the original volume of sterile minimal medium. Keto acids present in culture fluids were concentrated by lyophilization and filter sterilized before being added to a concentration estimated by HPLC to be 1 mM.
Chemicals and analytical methods.
Pyr and α-Kg cyanohydrin were prepared in solution by incubating keto acid with KCN until all of the available cyanide was consumed. The standard reaction mixture containing 80 mM keto acid and 20 mM KCN was incubated for 30 min, which yielded a solution with a 20 mM concentration of cyanohydrin. 14C-labeled pyruvate (Pyr-14CN) and ketoglutarate cyanohydrin (Kg-14CN) were prepared similarly except that reaction mixtures contained 67 mM keto acid and 18.2 mM K14CN (1 mCi ml−1). This gave 33 μCi of labeled cyanohydrin ml−1, of which 1 to 2 μCi was supplied to enzymatic reaction mixtures. KCN (97%) and K13CN (99 atom%) were obtained from Aldrich (Milwaukee, Wis.). K14CN (54 mCi mmol−1, 2.0 GBq mmol−1) was purchased from DuPont, NEN Research Products (Boston, Mass.). All other chemicals were of the highest purity available commercially. Spectrophotometric determinations were conducted either with an LKB Ultraspec II or with a Perkin-Elmer Lambda 6 UV-visible-light spectrophotometer.
RESULTS
Identification of α-keto acids as cyanide-scavenging agents.
Previous studies showed that cyanide removal from nitrogen-depleted cells of NCIMB 11764 was caused by a nonenzymatic reaction with a metabolite excreted into the medium (4). Attempts to isolate the metabolite revealed that the cyanide-removing activity consistently copurified with the iron-chelating activity, possibly suggesting the involvement of a siderophore. Strong absorption in the UV range of the partially purified metabolite was consistent with the presence of a siderophore, but attempts to demonstrate that cyanide-removing activity was associated with such compounds (e.g., pyoverdin, pyochelin, and salicylate [1]) in Pseudomonas strains were unsuccessful. Efforts to identify the metabolite by gas chromatography-mass spectrometry also met with limited success, but the presence of a small-molecular-weight organic acid that displayed both cyanide-removing and iron-chelating activity was demonstrated. Further analyses of culture fluids by ion-exclusion HPLC confirmed the presence of not one but two major organic acids. Moreover, both compounds completely disappeared when culture fluids were treated with KCN just prior to injection, suggesting strongly that the two species were responsible for cyanide removal. These findings find analogy with earlier reported observations of a similar bleaching effect at A210 when a partially purified product of unknown identity was treated with KCN (4). A comparison of the elution times for the unknown metabolites with those of various organic acid standards revealed the identities of the two as Pyr (10.26 min) and αKg (9.42 min). This was also confirmed by thin-layer chromatography of the 2,4-dinitrophenylhydrazones (Rfs of Pyr, 0.42 and 0.65 [two stereoisomers]; Rf of αKg, 0.25) and by enzymatic means. In the latter case culture filtrates (or fractions thereof) stimulated the rate of NADH oxidation in a substrate-dependent manner when they were added to reaction mixtures supplied with either lactate dehydrogenase or glutamate dehydrogenase (data not shown).
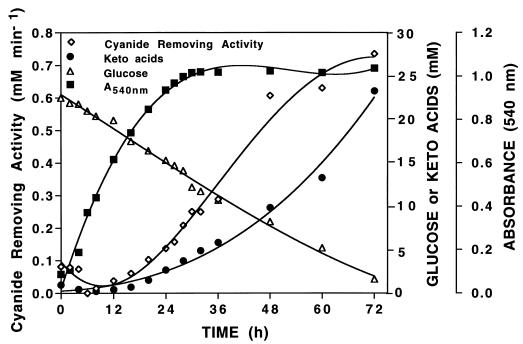
Evidence that biologically formed Pyr and αKg were responsible for the disappearance of cyanide from culture fluids of NCIMB 11764 as previously described (4) was provided by results showing a direct correlation between the two during growth (Fig. 1). Both the cyanide-removing activity and the concentration of keto acids in the culture fluid during the first 24 h of cultivation were low, but after this they both increased in a parallel fashion. The results in Fig. 1 show that the maximum cyanide-removing activity (760 μmol of cyanide removed min−1 ml of culture fluid−1) was recovered after 72 h of cultivation and was correlated with a maximum accumulated concentration of keto acid of 23 mM (20 mM Pyr and 3 mM αKg). The results further show that cells reached stationary phase after 24 h of cultivation on minimal GA medium but that glucose consumption continued long after this, presumably because glucose was converted into keto acids in the absence of available nitrogen. Control experiments showed that cyanide was also rapidly removed from aqueous solutions of commercial Pyr and αKg. Reaction rates were concentration dependent, and kinetic experiments suggest a reaction mechanism that is second order (5). Preliminary estimates of the rate coefficients for Pyr and αKg (at 2 mM) towards cyanide were, respectively, 38 and 21 μM cyanide removed min−1. Separate analyses showed also that Pyr and αKg were present in cell extracts (150,000 × g [high-speed supernatants]). Following fractionation of extracts by ultrafiltration, we observed the removal of cyanide from fractions containing molecular species of less than 10,000 Da and less than 0.1 mg · ml of protein−1. When analyzed by HPLC, both Pyr and αKG were detected at a combined concentration of about 0.5 mM (data not shown).
FIG. 1.
Growth profile, glucose consumption, α-keto acid excretion, and cyanide removal from culture fluid of P. fluorescens NCIMB 11764 cultivated in minimal GA medium. Cyanide-removing activity was determined by measuring the remaining cyanide concentration at periodic intervals after 2 mM KCN was injected into sealed vials containing 0.25 ml of culture fluid. Glucose was determined with glucose oxidase (EC 1.1.3.4) according to the specifications of the manufacturer (Sigma). See Materials and Methods for further analytical details. Data shown are typical results of two replicate experiments.
Identification of cyanohydrin reaction products.
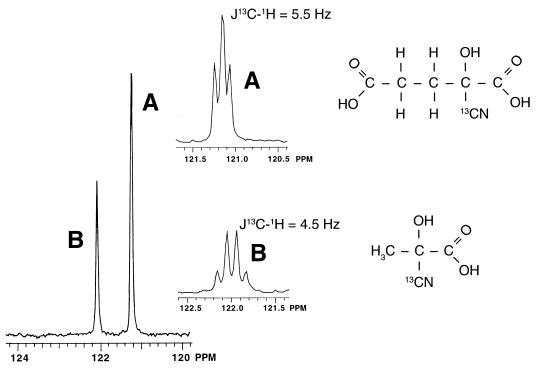
Cyanide is known to add readily to carbonyl compounds, including Pyr, for which it was reported over 40 years ago that the cyanohydrin is formed as the corresponding reaction product (14, 32). However, the description of cyanohydrins in general has received limited attention. Therefore, rigorous identification of reaction products by 13C-NMR spectroscopy was performed. Two compounds (121.24 ± 0.14 [compound A] and 122.09 ± 0.13 [compound B] ppm) were detected following the consumption of K13CN by culture fluid as shown in Fig. 2. These compounds correspond, respectively, to ketoglutarate cyanohydrin (2-cyano-2-hydroxyglutaric acid [designated Kg-CN]) and pyruvate cyanohydrin (2-cyano-2-hydroxypropanoic acid [designated Pyr-CN]), based on the fact that identical chemical species were also detected when K13CN was consumed by the authentic keto acids. Further evidence for the identity of each was provided by their carbon-proton-coupled spectra (Fig. 2 [inset]). These data show triplet and quartet patterns for the Pyr-13CN and Kg-13CN compounds, respectively, and are consistent with the spin interactions expected between the labeled carbon and adjacent protons on the β-carbon in each compound. The low magnitude of coupling constants (Fig. 2) is further indication of nonadjacent carbon-proton spin interactions (39). The cyanohydrins were further identified by HPLC. For example, the bleaching effect that cyanide had on the detection of Pyr and αKg as described above was accompanied by the appearance of two new chemical species eluting at 7.32 and 8.0 min. These were identified, respectively, as Kg-CN and Pyr-CN by comparing the elution times with those of compounds formed when authentic Pyr and αKg reacted with KCN.
FIG. 2.
13C-NMR spectra of reaction products generated after K13CN (5 mg ml−1) was added to culture fluid concentrated 50-fold from cells of P. fluorescens NCIMB 11764 grown for 24 h in GA minimal medium. Analysis was carried out at 50 MHz, and results were referenced against results with tetramethylsilane as an external standard. The inset shows 13C-1H-coupled spectra of products A and B, which correspond, respectively, to Kg-CN and Pyr-CN (whose structures are shown).
Enzymatic oxidation of the cyanohydrins by CNO.
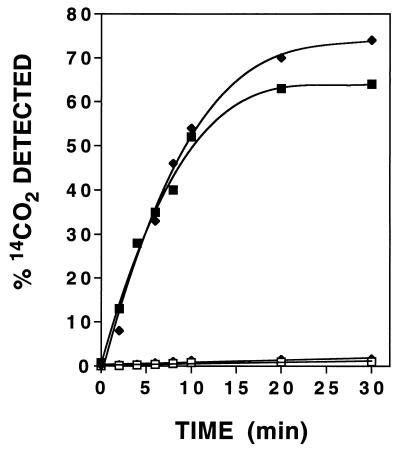
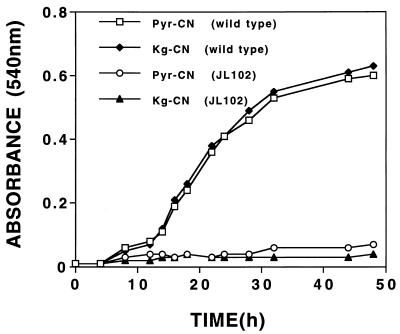
An earlier study showed that cell extracts induced for CNO transform the reaction product formed when K14CN is consumed by culture fluids into 14CO2 and NH3 (4). These results were very interesting and indicated that the reaction product, whatever its nature, was capable of being further metabolized. Since the keto acids were shown to react with cyanide, giving cyanohydrins as reaction products, it seemed logical to think that the cyanohydrins rather than free cyanide might be the true substrates for further enzymatic attack. To verify this, we performed additional experiments to show that cell extracts could oxidize the authentic cyanohydrins when they were provided as the 14CN-labeled substrates. Figure 3 shows that both Pyr-14CN and Kg-14CN were oxidized in a time-dependent manner and that 14CO2 was formed as a major reaction product. Since no conversion was observed with extracts uninduced for CNO (data not shown), or when oxygen and NADH were omitted from reaction mixtures, the involvement of CNO in catalyzing conversion was inferred. This involvement was further suggested by the growth results obtained when Pyr-CN and Kg-CN were provided as sole nitrogen sources for the wild type and a mutant strain (JL102) shown previously to be defective in CNO production (26). Figure 4 shows that both compounds supported the growth of the wild type but not the mutant strain, the interpretation being that the wild type is able to take up the cyanohydrin and convert it to ammonia but that the mutant cannot. Evidence that ammonia is indeed formed enzymatically was provided by results demonstrating its formation when the cyanohydrins were oxidized by cell extracts (Fig. 3); however, no appreciable accumulation was observed because rapid assimilation into amino acids presumably occurred in the presence of the keto acids generated as reaction coproducts. The fact that ammonia accumulation was demonstrated in an earlier study (4) in which the putative cyanohydrins were supplied as substrates in the presence of a slight excess of KCN (which inhibits further ammonia consumption) provides further evidence that indeed ammonia is enzymatically formed.
FIG. 3.
Time course for 14CO2 formation from Pyr-14CN and Kg-14CN catalyzed by cell extracts from cyanide-induced cells of P. fluorescens NCIMB 11764. Reaction mixtures contained 4 mM NADH where indicated below and 2 mM (1 to 2 μCi) substrate. ■ and □, Pyr-14CN under aerobic (with NADH) and anaerobic (without NADH) conditions, respectively; ⧫ and ◊, Kg-14CN under aerobic (with NADH) and anaerobic (without NADH) conditions, respectively. Each value is the average of results from three separate experiments. Standard errors ranged from 5 to 15% but are not shown for clarity.
FIG. 4.
Growth response of the wild type and mutant strain JL102 of P. fluorescens NCIMB 11764 towards Pyr-CN and Kg-CN supplied as the sole nitrogen sources. Washed cells having previously been grown in minimal GA medium for 24 h were provided as the inoculum (1%), and each cyanohydrin was supplied at 1 mM. Data shown are typical of results of two replicate experiments.
Cyanide detoxification by excreted α-keto acids.
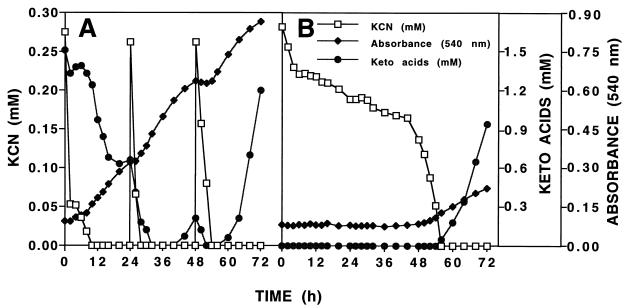
The facile reaction between cyanide and Pyr and αKg suggested that these compounds might serve as effective cyanide detoxification agents. Reports of cyanide antagonism by Pyr and αKg when they were administered to ascites tumor cells (6) or mice (7, 36) or when they were added to reaction mixtures supplied with cytochrome c oxidase (EC 1.9.3.1), which is strongly inhibited by cyanide (9), supported this hypothesis. However, to our knowledge no prior reports describing the protection of bacteria from cyanide poisoning by these compounds existed. Therefore, Pyr, αKg, or culture fluids containing these compounds were added to cells of NCIMB 11764 and the effect on growth inhibition by KCN was determined. Consistent with the results of an earlier study (37), the MIC for KCN in the absence of the keto acids was shown to be 0.3 mM. However, in the presence of 1 mM Pyr or αKg (or the equivalent amount in culture fluid), the MIC increased twofold (0.6 mM), indicating that significant protection was conferred (data not shown). These results suggested that the excretion of these compounds may protect cells from the lethal effects of cyanide and that detoxification might represent an important preliminary step in the further utilization of cyanide as a growth substrate. To further establish these possibilities, cells were cultivated on KCN and the relationship between keto acid accumulation, cyanide consumption, and growth was determined (Fig. 5). For comparative purposes, cells used for inocula were either washed or unwashed prior to being transferred to minimal medium supplied with KCN as the growth substrate. Although a number of inferences may be drawn from the data presented in Fig. 5, the fact that a 48-h lag period was observed in the culture supplied with a washed cell inoculum (Fig. 5B) suggests that in the absence of keto acids (removed during the cell-washing procedure) growth on KCN is severely impaired. Following 48 h of incubation, a moderate growth increase occurred. This increase was accompanied by the appearance of keto acids in the medium, thus indicating that growth and the accumulation of keto acids are physiologically linked. It is important to note that the keto acids are effectively titrated out when they are present at low concentrations in comparison with the concentration of available cyanide and that only when all of the cyanide has been consumed does their accumulation become evident.
FIG. 5.
Relationship between the appearance of α-keto acids in the culture medium and growth on cyanide as the sole nitrogen source during fed-batch cultivation of cells of P. fluorescens NCIMB 11764. (A) The entire culture (200 ml of cells plus culture fluid) after growth of cells for 48 h in GA medium (unwashed inoculum) was transferred to 2 liters of nitrogen-free minimal medium containing 20 mM glucose as the carbon source. (B) The cells from a 200-ml culture grown in GA medium (washed inoculum) were harvested, washed in sterile Na-K phosphate buffer (pH 7.0), and resuspended in 200 ml of sterile nitrogen-free minimal medium before being transferred. At the times indicated, KCN (0.25 mM) was added and the remaining cyanide concentration, the level of growth (A540), and the α-keto acid concentration in the medium were determined by methods described in the text. Data shown are typical of results of two replicate experiments.
The results obtained for the culture supplied with unwashed cells provides further evidence of a requirement for keto acids (Fig. 5A). In this case, cyanide disappeared immediately from the culture and, as expected, an equivalent decline in the concentration of detectable keto acid (supplied in the culture fluid of the inoculum) was also observed. Of further significance is the fact that cyanide disappearance followed a biphasic pattern; an initial rapid rate of disappearance was followed by a 2-h lag, which was succeeded by a second period of disappearance, but one in which the rate of disappearance was lower than initially observed. What inferences can be drawn from these results (Fig. 5A)? The lag observed between 2 and 4 h presumably represents the time after which the initial reaction between cyanide and available keto acid has reached completion (probably long before the 2-h sample was taken). With the reaction having reached equilibrium, no further cyanide consumption could occur unless the reaction product (cyanohydrin) was somehow removed. This removal of the reaction product, presumably, is what happens during the second phase of cyanide consumption and is concomitant with the induction of CNO, which is known to occur about 3 h after cyanide addition to cells (4, 26). Thus, once CNO is induced, any available cyanohydrin is consumed enzymatically, which in effect serves to pull the reaction in the direction of the cyanohydrin, thereby favoring further cyanide consumption. The enzymatic breakdown of cyanohydrins by CNO presumably also explains the slight rise in the keto acid concentration just before the onset of growth observed between 3 and 6 h. The growth pulse following the removal of cyanide is consistent with the expected availability of ammonia that is made possible by the apparent uptake and enzymatic breakdown of cyanohydrin substrates. Interestingly, the keto acid concentration in the medium declined significantly during growth, which we interpret to mean that these compounds can also serve as carbon sources and may indeed be preferred over glucose supplied in the medium. Once growth ceased due to nitrogen depletion (at about 24 h) a transient rise in the keto acid concentration occurred but declined instantaneously, as expected, when KCN was again pulsed into the medium. An essentially similar relationship between the pattern of growth, cyanide disappearance, and accumulation of keto acids was observed following the second (24 h) and final pulse of KCN (48 h) made to the medium.
DISCUSSION
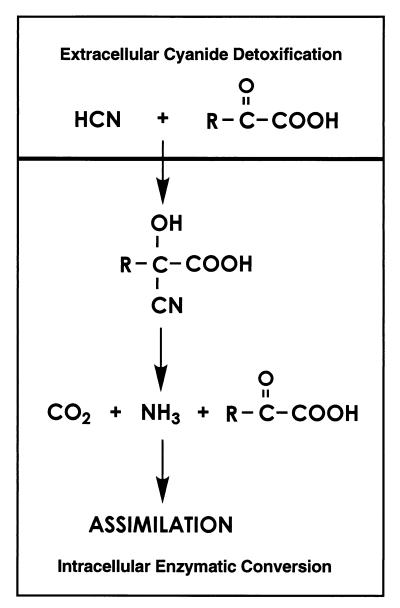
Previous studies of cyanide utilization in P. fluorescens NCIMB 11764 showed that in order to achieve growth on cyanide it was necessary to grow cells in fed-batch mode by pulsing KCN into the medium at a low concentration (less than the MIC [0.3 mM]) (15, 25). Under these conditions, growth lags somewhat behind cyanide consumption, which allows us to infer the involvement of several biochemical events. The results presented in this paper now provide an explanation for these findings and show that cyanide loss from cultures independent of that catalyzed by CNO occurs by a chemical reaction with excreted α-keto acids. Both Pyr and αKg were detected in the culture fluids of cells cultivated on limiting amounts of ammonia (Fig. 1) or KCN (Fig. 5) and reacted facilely with cyanide to give the corresponding cyanohydrins as reaction products. These were further metabolized by cell extracts to give ammonia and carbon dioxide as reaction products, thus helping to explain how cellular nitrogen is acquired from cyanide as a growth substrate. The results presented here provide the basis for a new mechanism of cyanide utilization in which α-keto acids play an essential role (Fig. 6). Accordingly, we propose that two steps are involved, one being the initial detoxification of cyanide by α-keto acids in the extracellular fluid and the second being the further metabolism of the cyanohydrin by CNO.
FIG. 6.
Proposed pathway for cyanide assimilation by P. fluorescens NCIMB 11764. R is CH3 and CH2CH2COOH for Pyr-CN and Kg-CN, respectively.
The reaction between cyanide and the keto acids is consistent with cyanide’s known tendency to attack to carbonyl compounds as a nucleophile (32). The protection these compounds conferred on NCIMB 11764 against cyanide inhibition finds further analogy with reports describing the ability of Pyr and αKg to act as cyanide antagonists (6, 7, 9, 36). Therefore, the excretion of these compounds appears to confer on cells, albeit fortuitously, a rather novel mechanism of cyanide tolerance. Further utilization as a growth substrate then appears to be dependent on the ability to transport the cyanohydrins into the cell and induce specific enzymes for their degradation. NCIMB 11764 is capable of performing both these functions, as was evidenced by its ability to oxidize (Fig. 3) and grow on (Fig. 4) the cyanohydrins as substrates. Although the complete mechanism by which the cyanohydrins are metabolized remains to be investigated, the fact that cyanide-induced extracts were required and conversion was both oxygen and reduced-pyridine nucleotide (NADH) dependent (Fig. 3) provides strong evidence that CNO is involved. This possibility is further supported by results showing that a CNO-defective mutant known to be incapable of growth on cyanide was also unable to grow when it was provided with the cyanohydrin(s) (Fig. 4). Indeed, our data suggest that the true substrate for CNO is not free cyanide but rather the cyanohydrin. The fact that the cyanohydrin is less toxic than free cyanide (CN−-HCN) and probably also significantly less inhibitory to CNO, which by analogy with other oxygenases is probably a metalloenzyme, leads to a reasonable interpretation for why the cyanohydrin may be the preferred substrate. However, an explanation for how cell extracts can catalyze the conversion of free cyanide to CO2 and NH3 in the absence of exogenously added keto acids, as was routinely demonstrated in previous studies (16, 26, 43), is required. The explanation for this, we propose, lies in the fact that small amounts of Pyr and αKg (0.5 mM) remain present when cell extracts are prepared. Therefore, when KCN is added to enzymatic reaction mixtures, it presumably reacts instantaneously with Pyr and αKg to yield the cyanohydrins. These in turn are immediately turned over by CNO, in effect, recycling the keto acids for further reaction with cyanide.
The disappearance of cyanide independent of that catalyzed by CNO was originally observed in ammonia-grown cultures to which cyanide was added as an enzyme inducer (4). We now demonstrate that this phenomenon is attributed to the accumulation of keto acids and show that the disappearance of cyanide also occurs when cells are cultivated on KCN (Fig. 5). Moreover, the fact that growth on KCN did not occur or at least was severely impeded when keto acids were absent from the medium provides strong evidence that these compounds are necessary for cyanide utilization. The key factor eliciting their accumulation, however, is not the presence of cyanide per se but nitrogen deprivation. This finding is consistent with other reports of keto acid accumulation by bacteria cultivated under related conditions of nitrogen starvation or some other form of growth limitation (10, 33, 35).
The excretion of keto acids has importantant implications for understanding the mechanism of microbial cyanide tolerance and utilization in nature. Since cyanide arises naturally through plant, fungal, and bacterial cyanogenesis (2, 24, 34), the excretion of keto acids may confer on cells a natural means of acquiring cyanide tolerance. Moreover, since nitrogen (or other growth factors) is often limiting in the environment, it may be that the excretion of keto acids is a natural phenomenon that occurs irrespective of the ability to grow on cyanide. If this is so, then many bacteria may be able to acquire cyanide tolerance by keto acid excretion. Experiments to confirm this hypothesis by screening other bacteria for keto acid-linked cyanide resistance are under way in our laboratory. However, tolerance to cyanide and the ability to utilize it for growth are probably physiologically independent and only bacteria with the genetic capacity for elaborating enzymes, such as CNO, which catalyze cyanohydrin breakdown may be able to grow in its presence. The added selective advantage this ability may confer on cells is the ability to survive on cyanide under environmental conditions where it is the only available nitrogen source. Since CNO has never before been described for any other organism, efforts to isolate this enzyme from P. fluorescens NCIMB 11764 and investigate the further mechanism of cyanohydrin oxidation are under way. These and related studies of the biochemistry of cyanide utilization are sure to shed further light on the unique mechanism microorganisms have adapted for interacting with this notorious toxic natural product in the biosphere.
ACKNOWLEDGMENTS
We thank Barney J. Venables, Trac Laboratories, Inc., Denton, Tex., for gas chromatography-mass spectrometry analyses, which helped establish that the metabolite in extracellular fluids responsible for cyanide removal was an organic acid. We also thank He Huang and Michael Richmond for assistance with 13C NMR and helpful discussions.
This work was supported by the National Science Foundation (MCB 9808653) and the University of North Texas Organized Faculty Research Fund.
REFERENCES
- 1.Castignetti D. Probing Pseudomonas aeruginosa, Pseudomonas aureofaciens, Burkholderia (Pseudomonas) cepacia, Pseudomonas fluorescens, and Pseudomonas putida with the ferripyochelin receptor A gene and the synthesis of pyochelin in Pseudomonas aureofaciens, Pseudomonas fluorescens and Pseudomonas putida. Curr Microbiol. 1997;34:250–257. doi: 10.1007/s002849900178. [DOI] [PubMed] [Google Scholar]
- 2.Castric P A. The metabolism of hydrogen cyanide by bacteria. In: Vennesland B, et al., editors. Cyanide in biology. New York, N.Y: Academic Press; 1981. pp. 233–261. [Google Scholar]
- 3.Chen J-L, Kunz D A. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Cyanide degradation in Pseudomonas fluorescens can occur by an extracellular nonenzymatic mechanism, abstr. 164; p. 332. [Google Scholar]
- 4.Chen J-L, Kunz D A. Cyanide utilization in Pseudomonas fluorescens NCIMB 11764 involves a putative siderophore. FEMS Microbiol Lett. 1997;156:61–67. [Google Scholar]
- 5.Chen, J.-L., and D. A. Kunz. 1998. Unpublished results.
- 6.Cittadini A, Galeotti T, Terranova T. The effect of pyruvate on cyanide-inhibited respiration in intact ascites tumor cells. Experentia. 1971;27:633–635. doi: 10.1007/BF02136930. [DOI] [PubMed] [Google Scholar]
- 7.Cittadini A, Caprino L, Terranova T. Effect of pyruvate on the acute cyanide poisoning of mice. Experientia. 1972;28:943–944. doi: 10.1007/BF01924962. [DOI] [PubMed] [Google Scholar]
- 8.Conn E E. Introduction. Ciba Found Symp. 1988;40:1–2. [Google Scholar]
- 9.Delhumeau G, Cruz-Mendoza A M, Gomez Lojero C. Protection of cytochrome c oxidase against cyanide inhibition by pyruvate and α ketoglutarate: effect of aeration in vitro. Toxicol Appl Pharmacol. 1994;126:345–351. doi: 10.1006/taap.1994.1125. [DOI] [PubMed] [Google Scholar]
- 10.Drechsel H, Thieken A, Reissbrodt R, Jung G, Winkelmann G. α-Keto acids are novel siderophores in the genera Proteus, Providencia, and Morganella and are produced by amino acid deaminases. J Bacteriol. 1993;175:2727–2733. doi: 10.1128/jb.175.9.2727-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fawcett J K, Scott J E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedemann T E, Haugen G E. Pyruvic acid. II. The determination of keto acids in blood and urine. J Biol Chem. 1943;147:415–442. [Google Scholar]
- 13.Fuller W H. Cyanides in the environment with particular attention to the soil. In: Van Zyl D, editor. Cyanide and the environment. Geotechnical Engineering Program. Fort Collins: Colorado State University; 1988. pp. 19–46. [Google Scholar]
- 14.Green D E, Williamson S. Pyruvic and oxaloacetic cyanohydrins. Biochem J. 1937;31:617–618. doi: 10.1042/bj0310617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris R E, Knowles C J. Isolation and growth of Pseudomonas species that utilizes cyanide as a source of nitrogen. J Gen Microbiol. 1983;129:1005–1011. doi: 10.1099/00221287-129-4-1005. [DOI] [PubMed] [Google Scholar]
- 16.Harris R E, Knowles C J. The conversion of cyanide to ammonia by extracts of a strain of Pseudomonas fluorescens that utilizes cyanide as a source of nitrogen for growth. FEMS Microbiol Lett. 1983;20:337–341. [Google Scholar]
- 17.Harris R K. Nuclear magnetic resonance spectroscopy: a physiochemical view. London, United Kingdom: Pitman Books Ltd.; 1983. pp. 110–113. [Google Scholar]
- 18.Homan E R. Reactions, processes and materials with potential for cyanide exposure. In: Ballantyne B, Marrs T C, editors. Clinical and experimental toxicology of cyanides. Bristol, United Kingdom: IOP Publishing Ltd. (Wright); 1987. pp. 1–21. [Google Scholar]
- 19.Ingvorsen, K., and S. E. Godtfredsen (Novo/Nordisk A/S). September 1988. Microbial cyanide converting enzymes, their production and use. European patent application EP282351.
- 20.Ingvorsen K, Hojer-Pedersen B, Godtfredsen S E. Novel cyanide-hydrolyzing enzyme from Alcaligenes xylosoxidans subsp. denitrificans. Appl Environ Microbiol. 1991;57:1783–1789. doi: 10.1128/aem.57.6.1783-1789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchner J G. Carbonyl compounds. In: Perry E, Weissberger A, editors. Technique of organic chemistry. XII. Thin-layer chromatography. New York, N.Y: John Wiley & Sons; 1967. pp. 373–391. [Google Scholar]
- 22.Knowles C J. Microorganisms and cyanide. Bacteriol Rev. 1976;40:652–680. doi: 10.1128/br.40.3.652-680.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles C J. Cyanide utilization and degradation by microorganisms. Ciba Found Symp. 1988;40:3–15. doi: 10.1002/9780470513712.ch2. [DOI] [PubMed] [Google Scholar]
- 24.Knowles C J, Bunch A W. Microbial cyanide metabolism. Adv Microb Physiol. 1986;27:73–111. doi: 10.1016/s0065-2911(08)60304-5. [DOI] [PubMed] [Google Scholar]
- 25.Kunz D A, Nagappan O, Silva-Avalos J, Delong G T. Utilization of cyanide as a nitrogenous substrate by Pseudomonas fluorescens NCIMB 11764: evidence for multiple pathways of metabolic conversion. Appl Environ Microbiol. 1992;58:2022–2029. doi: 10.1128/aem.58.6.2022-2029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunz D A, Wang C-S, Chen J-L. Alternative routes of enzymic cyanide metabolism in Pseudornonas fluorescens NCIMB 11764. Microbiology. 1994;140:1705–1712. doi: 10.1099/13500872-140-7-1705. [DOI] [PubMed] [Google Scholar]
- 27.Lambert J L, Ramasamy J, Paukstells J V. Stable reagents for the colorimetric determination of cyanide by modified König reactions. Anal Chem. 1975;47:916–918. [Google Scholar]
- 28.Lennox E S. Transduction of linked genetic characters of the host bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 29.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Meyers P R, Rawlings D E, Woods D R, Lindsey G G. Isolation and characterization of a cyanide dihydratase from Bacillus pumilus C1. J Bacteriol. 1993;175:6105–6112. doi: 10.1128/jb.175.19.6105-6112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neidhardt F C, Ingraham J L, Schaechter M. Physiology of the bacterial cell, a molecular approach. Sunderland, Mass: Sinauer Associates Inc.; 1990. [Google Scholar]
- 32.Pichat L. Syntheses and uses of isotopically labeled cyanide. In: Rappoport Z, editor. The chemistry of the cyano group. London, United Kingdom: Interscience Publishers, John Wiley & Sons; 1970. pp. 743–790. [Google Scholar]
- 33.Raunio R. Accumulation of keto acids during the growth cycle of Escherichia coli. Acta Chem Scand. 1966;20:11–16. doi: 10.3891/acta.chem.scand.20-0011. [DOI] [PubMed] [Google Scholar]
- 34.Reed R E. Cyanide compounds in plants and their effects on animals. In: Van Zyl D, editor. Cyanide and the environment. Geotechnical Engineering Program. Fort Collins: Colorado State University; 1988. pp. 47–50. [Google Scholar]
- 35.Reissbrodt R, Kingsley R, Rabsch W, Beer W, Roberts M, Williams P H. Iron-regulated excretion of α-keto acids by Salmonella typhimurium. J Bacteriol. 1997;179:4538–4544. doi: 10.1128/jb.179.14.4538-4544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz C, Morgan R L, Way L M, Way J L. Antagonism of cyanide intoxication with sodium pyruvate. Toxicol Appl Pharmacol. 1979;50:437–441. doi: 10.1016/0041-008x(79)90396-x. [DOI] [PubMed] [Google Scholar]
- 37.Silva-Avalos J, Richmond M G, Nagappan O, Kunz D A. Degradation of the metal-cyano complex tetracyanonickelate (II) by cyanide-utilizing bacterial isolates. Appl Environ Microbiol. 1990;56:3664–3670. doi: 10.1128/aem.56.12.3664-3670.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomonson L P. Cyanide as a metabolic inhibitor. In: Vennesland B, et al., editors. Cyanide in biology. New York, N.Y: Academic Press; 1981. pp. 11–28. [Google Scholar]
- 39.Stothers J B. Carbon-13 NMR spectroscopy. 1972. pp. 295–308. and 348–362. In A. T. Blomquist and H. Wasserman (ed.), Organic chemistry, vol. 24. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 40.Sykes A H. Early studies on the toxicology of cyanide. In: Vennesland B, et al., editors. Cyanide in biology. New York, N.Y: Academic Press; 1981. pp. 1–9. [Google Scholar]
- 41.Towill L, Drury J S, Whitfield B L, Lewis E B, Galyan E L, Hammons A S. Reviews of the environmental effects of pollutants. V. Cyanide. U.S. Environmental Protection Agency publication 600/1-78-027. U.S. Washington, D.C: Environmental Protection Agency; 1978. [Google Scholar]
- 42.Vennesland B, Castric P A, Conn E E, Solomonson L P, Volini M, Westley J. Cyanide metabolism. Fed Proc. 1982;41:2639–2648. [PubMed] [Google Scholar]
- 43.Wang C-S, Kunz D A, Venables B J. Incorporation of molecular oxygen and water during enzymatic oxidation of cyanide by Pseudomonas fluorescens NCIMB 11764. Appl Environ Microbiol. 1996;62:2195–2197. doi: 10.1128/aem.62.6.2195-2197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warburg O, Negelein E, Haas E. Spectroscopic demonstration of the O-transferring enzyme in the presence of cytochrome. Biochem Z. 1933;266:1–8. [Google Scholar]
- 45.White J M, Jones D D, Huang D, Gauthier J J. Conversion of cyanide to formate and ammonia by a pseudomonad obtained from industrial wastewater. J Ind Microbiol. 1988;3:263–272. [Google Scholar]
- 46.Wild S R, Rudd T, Neller A. Fate and effects of cyanide during wastewater treatment processes. Sci Total Environ. 1994;156:93–107. [Google Scholar]