Abstract
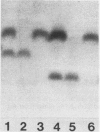
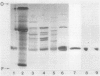
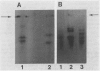
We report the first complete purifications of the cytosolic and plastid isozymes of triose phosphate isomerase (TPI; EC 5.3.1.1) from higher plants including spinach (Spinacia oleracea), lettuce (Lactuca sativa), and celery (Apium graveolens). Both isozymes are composed of two isosubunits with approximate molecular weight of 27,000; in spinach and lettuce the plastid isozyme is 200 to 400 larger than the cytosolic isozyme. The two isozymes, purified from lettuce, had closely similar amino acid compositions with the exception of methionine which was four times more prevalent in the cytosolic isozyme. Partial amino acid sequences from the N-terminus were also obtained for both lettuce TPIs. Nine of the 13 positions sequenced in the two proteins had identical amino acid residues. The partial sequences of the plant proteins showed high similarity to previously sequenced animal TPIs. Immunological studies, using antisera prepared independently against the purified plastid and cytosolic isozymes from spinach, revealed that the cytosolic isozymes from a variety of species formed an immunologically distinct group as did the plastid isozymes. However, both plastid and cytosolic TPIs shared some antigenic determinants. The overall similarity of the two isozymes and the high similarity of their partial amino acid sequences to those of several animals indicate that TPI is a very highly conserved protein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Kawasaki G. Nucleotide sequence of the triose phosphate isomerase gene of Saccharomyces cerevisiae. J Mol Appl Genet. 1982;1(5):419–434. [PubMed] [Google Scholar]
- Albery W. J., Knowles J. R. Evolution of enzyme function and the development of catalytic efficiency. Biochemistry. 1976 Dec 14;15(25):5631–5640. doi: 10.1021/bi00670a032. [DOI] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Heinrikson R. L., Nyes C. Chloroplast and cytoplasmic enzymes 1, 2, 3. Subunit structure of pea leaf aldolases. Arch Biochem Biophys. 1975 Jul;169(1):262–268. doi: 10.1016/0003-9861(75)90340-9. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Harris J. I. Primary structure of triosephosphate isomerase from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):599–611. doi: 10.1111/j.1432-1033.1980.tb04755.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cerff R., Chambers S. E. Subunit structure of higher plant glyceraldehyde-3-phosphate dehydrogenases (EC 1.2.1.12 and EC 1.2.1.13). J Biol Chem. 1979 Jul 10;254(13):6094–6098. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The amino acid sequence of rabbit muscle triose phosphate isomerase. Biochem J. 1975 Feb;145(2):335–344. doi: 10.1042/bj1450335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dorry H. A., MacGregor J. S. Rat and rabbit liver mRNAs code for fructose 1,6-bisphosphatases that differ in molecular weight. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1384–1389. doi: 10.1016/s0006-291x(82)80151-4. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Milman J. D., Priddle J. D., Offord R. E. Studies on the subunit structure and amino acid sequence of trisoe phosphate isomerase from chicken breast muscle. Biochem J. 1974 Apr;139(1):11–22. doi: 10.1042/bj1390011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb L. D. Conservation and duplication of isozymes in plants. Science. 1982 Apr 23;216(4544):373–380. doi: 10.1126/science.216.4544.373. [DOI] [PubMed] [Google Scholar]
- Gottlieb L. D. Evidence for duplication and divergence of the structural gene for phosphoglucoisomerase in diploid species of clarkia. Genetics. 1977 Jun;86(2):289–307. doi: 10.1093/genetics/86.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert M., Schnarrenberger C. Purification and subunit structure of glucosephosphate isomerase 2 from spinach leaves and immunochemical comparison with other isomerases. Arch Biochem Biophys. 1982 Sep;217(2):452–459. doi: 10.1016/0003-9861(82)90523-9. [DOI] [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Kolb E., Harris J. I., Bridgen J. Triose phosphate isomerase from the coelacanth. An approach to the rapid determination of an amino acid sequence with small amounts of material. Biochem J. 1974 Feb;137(2):185–197. doi: 10.1042/bj1370185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochloron and the theory of symbiogenesis. Ann N Y Acad Sci. 1981;361:325–329. doi: 10.1111/j.1749-6632.1981.tb46528.x. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Dennis D. T. Isozymes of the glycolytic enzymes in endosperm from developing castor oil seeds. Plant Physiol. 1982 Apr;69(4):825–828. doi: 10.1104/pp.69.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Pichersky E., Gottlieb L. D. Evidence for duplication of the structural genes coding plastid and cytosolic isozymes of triose phosphate isomerase in diploid species of clarkia. Genetics. 1983 Oct;105(2):421–436. doi: 10.1093/genetics/105.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden N. F. Genetic and biochemical implications of the endosymbiotic origin of the chloroplast. J Mol Evol. 1981;17(3):133–139. doi: 10.1007/BF01733906. [DOI] [PubMed] [Google Scholar]
- Weeden N. F., Gottlieb L. D. Dissociation, reassociation, and purification of plastid and cytosolic phosphoglucose isomerase isozymes. Plant Physiol. 1982 Mar;69(3):717–723. doi: 10.1104/pp.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden N. F., Gottlieb L. D. Isolation of cytoplasmic enzymes from pollen. Plant Physiol. 1980 Sep;66(3):400–403. doi: 10.1104/pp.66.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden N. F., Higgins R. C., Gottlieb L. D. Immunological similarity between a cyanobacterial enzyme and a nuclear DNA-encoded plastid-specific isozyme from spinach. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5953–5955. doi: 10.1073/pnas.79.19.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]