Abstract
At ATP concentrations less than 0.2 millimolar, zinc ions cause a marked stimulation of endogenous protein phosphorylation in thylakoid membranes isolated from tobacco (Nicotiana tabacum L. cv Turkish Samsun), pea (Pisum sativum L. cv Feltham First) and spinach (Spinacia oleracea L. cv Northland). The greatest stimulatory effect was observed at Zn2+ concentrations of 1 to 2 millimolar; higher concentrations were inhibitory. The stimulatory effect of Zn2+ was independent of Mg2+ concentration from 1 to 5 millimolar and thus does not appear to be due to the formation of a Zn2+ -ATP complex. Phosphorylation of histones IIA, an exogenous protein substrate, was inhibited by 2 millimolar Zn2+. At low levels of ATP, Zn2+ not only stimulates general endogenous protein phosphorylation, but also the phosphorylation of the apoproteins of the light-harvesting chlorophyll a/b-protein complex. However, under these conditions Zn2+ inhibits the ATP-induced quenching of photosystem II fluorescence and the increase in the ratio of photosystem I to photosystem II fluorescence which are both characteristic of the State 1-State 2 transition. These results suggest that phosphorylation of the light-harvesting chlorophyll a/b-protein complex may not directly bring about the State 1-State 2 transition.
Full text
PDF
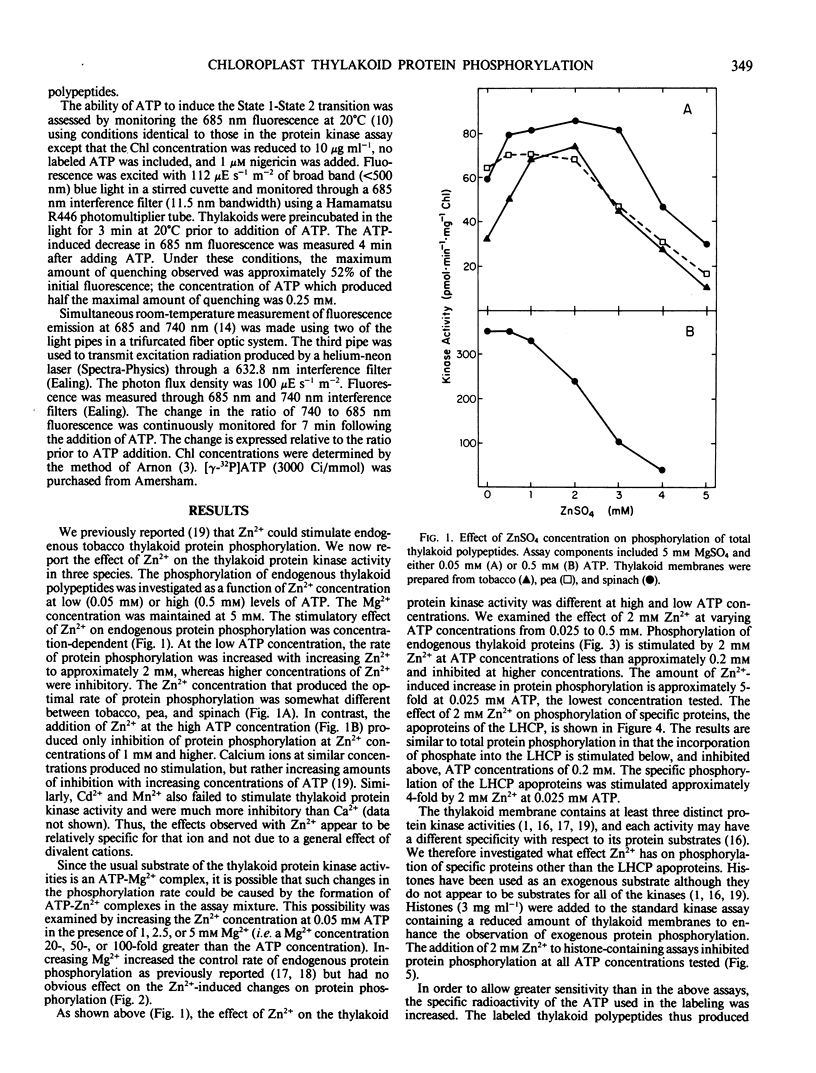
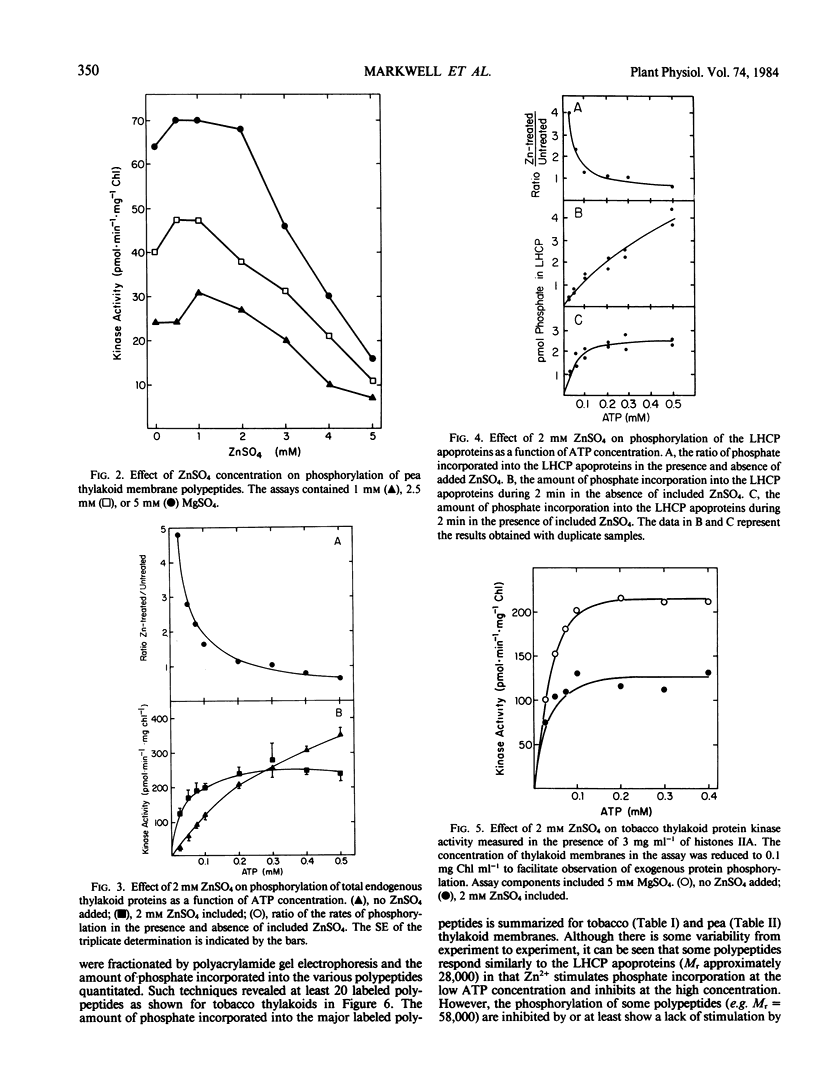


Images in this article
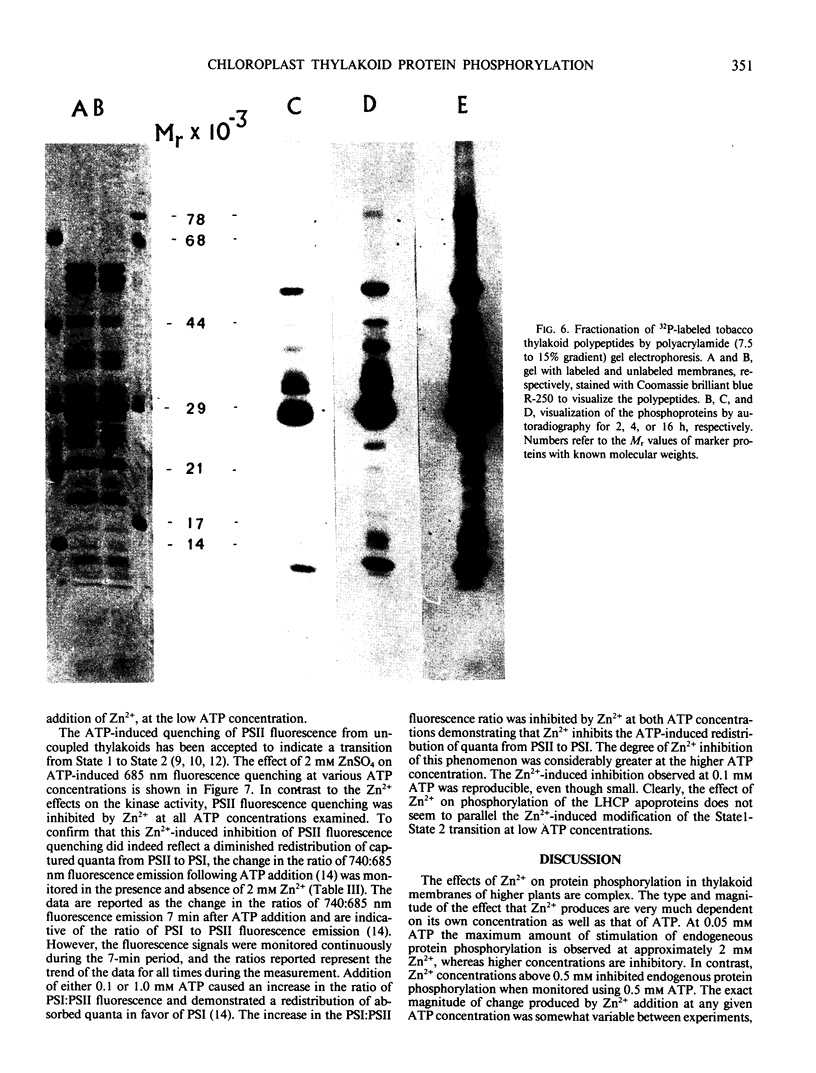
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfonzo R., Nelson N., Racker E. A Light-dependent Protein Kinase Activity of Chloroplasts. Plant Physiol. 1980 Apr;65(4):730–734. doi: 10.1104/pp.65.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur J Biochem. 1980 Feb;104(1):85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farchaus J. W., Widger W. R., Cramer W. A., Dilley R. A. Kinase-induced changes in electron transport rates of spinach chloroplasts. Arch Biochem Biophys. 1982 Aug;217(1):362–367. doi: 10.1016/0003-9861(82)90512-4. [DOI] [PubMed] [Google Scholar]
- Haworth P., Kyle D. J., Arntzen C. J. Protein phosphorylation and excitation energy distribution in normal intermittent-light-grown, and a chlorophyll b-less mutant of barley. Arch Biochem Biophys. 1982 Oct 1;218(1):199–206. doi: 10.1016/0003-9861(82)90336-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucero H. A., Lin Z. F., Racker E. Protein kinases from spinach chloroplasts. II. Protein substrate specificity and kinetic properties. J Biol Chem. 1982 Oct 25;257(20):12157–12160. [PubMed] [Google Scholar]
- Owens G. C., Ohad I. Phosphorylation of chlamydomonas reinhardi chloroplast membrane proteins in vivo and in vitro. J Cell Biol. 1982 Jun;93(3):712–718. doi: 10.1083/jcb.93.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]