Abstract
Empyema is a common complication of pneumonia, caused by the accumulation of purulent exudate due to pathogenic bacteria invading the pleural cavity. Parvimonas micra and Streptococcus constellatus are pathogens that rarely cause pneumonia with empyema. Herein, a case of severe empyema caused by these two pathogens, confirmed by metagenomic next-generation sequencing (mNGS) of pleural effusion cultures, is reported. A male Chinese patient in his late sixties presented with wheezing, cough, sputum expectoration, and fever. Blood and sputum cultures were negative for pathogens, but the pleural effusion culture was positive for S. constellatus, and was also found to contain P. micra, confirmed by mNGS. The patient’s symptoms improved after treatment with cefoperazone/sulbactam and moxifloxacin. Pneumonia caused by P. micra and S. constellatus is rare; however, coinfection with these pathogens may cause severe pneumonia, with or without empyema.
Keywords: Empyema, metagenomic next-generation sequencing, Parvimonas micra, severe pneumonia, Streptococcus constellatus
Introduction
Empyema is a common complication of pneumonia, resulting from the accumulation of purulent exudate caused by pathogenic bacteria invading the pleural cavity. The incidence of pneumonia with empyema has increased globally in recent years, 1 with aerobic (Streptococcus pneumoniae, Staphylococcus aureus, and Klebsiella pneumoniae) and anaerobic (Bacteroides and Peptostreptococcus spp.) pathogens reported to be common causes of pneumonia with empyema. 2 Parvimonas micra, found within the normal microflora of the human oral cavity, rarely causes pneumonia or empyema, and is challenging to identify in laboratory testing. In contrast, Streptococcus constellatus has emerged in recent years as a primary cause of purulent infections. S. constellatus can cause abscesses of the teeth, tonsils, and sinuses and can also cause empyema.2,3 To the best of our knowledge, no cases of pneumonia with empyema caused by coinfection with P. micra and S. constellatus have previously been reported. Herein, a case of pneumonia with empyema caused by P. micra and S. constellatus coinfection, confirmed by metagenomic next-generation sequencing (mNGS), is reported, with the aim of improving clinical understanding of these pathogens.
Case report
Patient characteristics
The patient’s data were deidentified for this case report. Ethical approval was not required for this study, in accordance with national guidelines, however, written informed consent was obtained from the patient for case report publication. The reporting of this study conforms to CARE guidelines. 4
A male Chinese patient in his late sixties was admitted to Beijing Tsinghua Changgung Hospital, Changping District, Beijing, China in January 2022, with the primary complaint of wheezing with cough and sputum expectoration for 2 weeks. The patient had developed wheezing without a prior obvious trigger, and the severity had gradually increased. He had experienced a gradual decrease in activity tolerance and worsening wheezing at night while supine. The wheezing was accompanied by coughing with expectoration of white mucoid sputum. Subsequently, he developed increasing pain in the left hypochondrium, which was aggravated by coughing. These symptoms were accompanied by fatigue, poor appetite, and intermittent nausea. On admission, the patient had not experienced fever, vomiting, diarrhoea, abdominal pain, abdominal distension, or urinary symptoms.
Clinical findings
Physical examination on admission revealed a body temperature of 36.5°C, pulse of 101 beats/min, respiratory rate of 18 breaths/min, blood pressure of 155/86 mmHg, and oxygen saturation (SpO2) of 98% (nasal cannula oxygen flow rate: 5 L/min). The patient was conscious, with a slightly pale conjunctiva and no yellowing of the sclera. The superficial lymph nodes were not palpably enlarged. His breath sounds were coarse in the basal area of the right lung and reduced in the basal region of the left lung, with no dry or wet rales. No abnormalities were observed in the heart and abdomen, and he had no peripheral oedema. He denied any recent periodontal disease or a history of tooth extraction; however, he rarely brushed his teeth and had poor oral hygiene.
Diagnostic assessment
Arterial blood gas analysis revealed a pH of 7.43, partial pressure of oxygen (pO2) of 74 mmHg, and partial pressure of carbon dioxide (pCO2) of 31 mmHg (oxygenation index: 255 mmHg). Routine blood tests revealed a leukocyte (WBC) count of 12.89 × 109/L, haemoglobin level of 114 g/L, platelet count of 392 × 109/L, neutrophil percentage (NEUT) of 87.1%, C-reactive protein (CRP) level of 244.94 mg/L, and procalcitonin level of 0.414 ng/ml. The levels of alanine aminotransferase, aspartate aminotransferase, creatinine, and blood urea nitrogen were within normal range. Glycated haemoglobin level was 9.1%. Results of blood culture, sputum culture, and sputum acid-fast staining tests were negative.
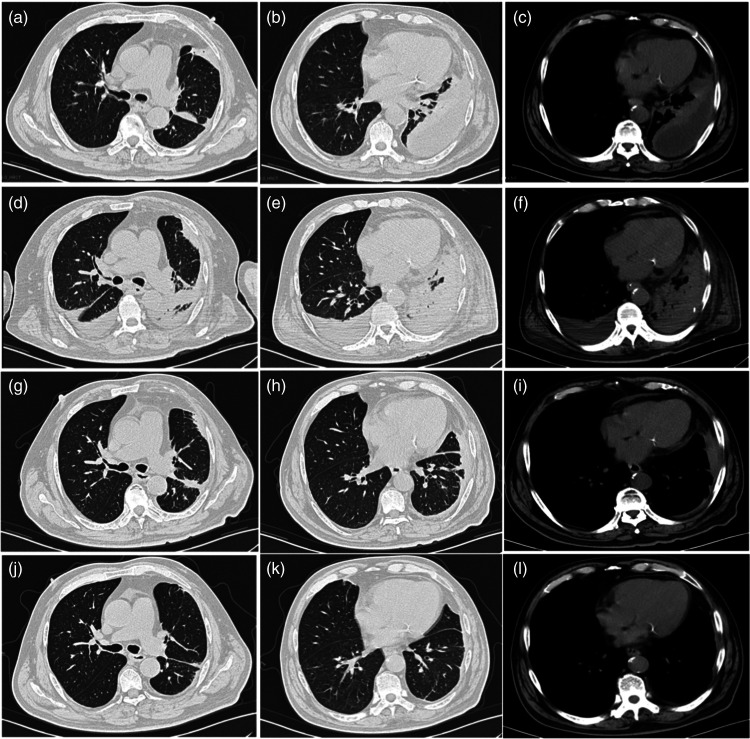
Chest computed tomography (CT) showed a left pleural effusion with pulmonary atelectasis (Figure 1a–c). An electrocardiogram revealed sinus tachycardia. Echocardiography, whole abdominal CT, and head CT showed no significant abnormalities. The initial diagnoses on admission were community-acquired pneumonia, left pleural effusion with pulmonary atelectasis, and type 2 diabetes mellitus.
Figure 1.
Chest computed tomography (CT) axial images from a Chinese male patient in his late sixties who presented with wheezing with cough and sputum expectoration for 2 weeks, showing: (a–c) left pleural effusion with pulmonary atelectasis at admission; (d–f) increased lung consolidation with partial atelectasis and increased bilateral pleural effusions on day 11 of hospitalisation; (g–i) decreased lung consolidation and pleural effusions at 1 week after discharge; and (j–l) significant improvement in bilateral pneumonia and pleural effusions at 1 month after discharge.
Therapeutic intervention
Verbal informed consent to treatment was obtained from the patient. Following admission, the patient received 400 mg moxifloxacin, by intravenous (i.v.) drip infusion, once daily. However, 3 days later, he developed a fever with a maximum temperature of 37.8°C, without chills. He experienced worsening of asthma, with a respiratory rate of up to 25–30 breaths per min, accompanied by coughing, worsening sputum production, and yellow phlegm. He also exhibited symptoms of fatigue and profuse sweating. Repeat arterial blood gas analysis revealed a pH of 7.411, pO2 of 68.2 mmHg, and pCO2 of 26.3 mmHg (oxygenation index: 170.5 mmHg). Routine blood tests revealed the following: WBC count, 16.49 × 109/L; NEUT, 88.5%; and CRP, 250 mg/L. Because the patient had developed severe pneumonia and type I respiratory failure, he was placed on non-invasive ventilator-assisted ventilation.
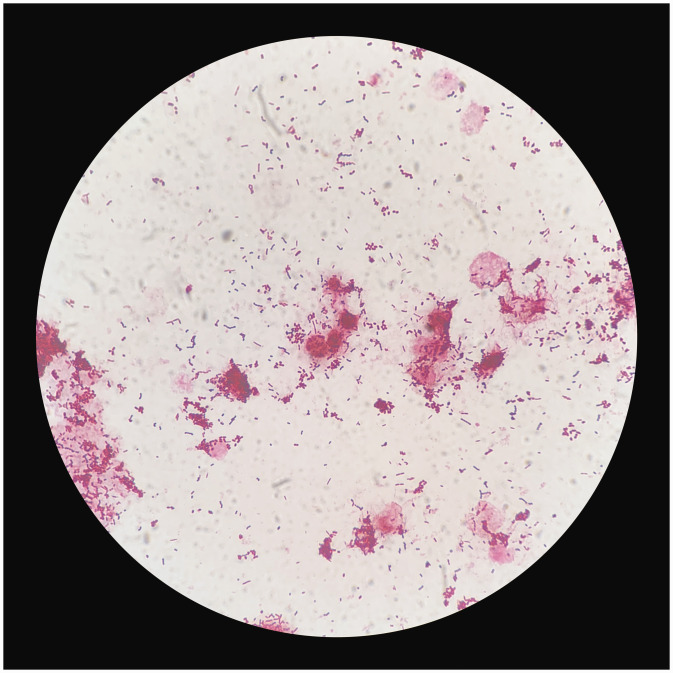
The sputum bacterial culture failed to identify a causative pathogen and moxifloxacin anti-infection therapy did not control the progression of pneumonia, so uncommon pathogens were suspected to be responsible for severe pneumonia in this patient. Consequently, the anti-infection treatment regimen was changed to 400 mg moxifloxacin, by i.v. drip infusion, once daily, combined with 3 g cefoperazone/sulbactam, by i.v. drip infusion, every 12 h. Subsequent bedside ultrasound follow-up showed a significant increase in pleural effusion. Bedside thoracentesis was performed on the left lung under ultrasound guidance, resulting in drainage of yellow, turbid, and purulent pleural effusion (Figure 2). Routine testing of the pleural effusion revealed a total leukocyte count of 7 268 cells/µl and a polymorphonuclear cell percentage of 87.5%. Biochemical testing revealed a lactate dehydrogenase level of 156 U/L, albumin level of <10 g/L, and adenosine deaminase level of >150 U/L. Results of both Sudan III and acid-fast staining tests were negative. Gram staining revealed a large number of gram-positive cocci and gram-negative bacilli in the pleural effusion (Figure 3). The pleural effusion culture was positive for S. constellatus, and subsequent drug susceptibility testing revealed that the strain was resistant to erythromycin and clindamycin (Table 1). The pleural effusion sample was then analysed for pathogens by mNGS (performed by Dinfectome Inc., Nanjing, China), revealing the presence of P. micra (4 516 reads) and S. contellatus (30 reads) (Table 2).
Figure 2.
Pleural effusion obtained during hospitalization from a Chinese male patient in his late sixties who presented with wheezing with cough and sputum expectoration for 2 weeks, showing yellow and purulent pus appearance.
Figure 3.
Photomicrograph of a gram-stained pleural effusion culture sample from a Chinese male patient in his late sixties who presented with wheezing with cough and sputum expectoration for 2 weeks, showing gram-positive cocci and gram-negative bacilli (original magnification, ×1 000).
Table 1.
Drug susceptibility testing of Streptococcus constellatus isolated from the pleural effusion of a Chinese male patient in his late sixties who presented with wheezing with cough and sputum expectoration for 2 weeks.
| Antibacterial drug | Result | KB (mm) | MIC (mg/L) |
|---|---|---|---|
| Penicillin | S | ·· | ≤0.12 |
| Ceftriaxone | S | 30 | ·· |
| Erythromycin | R | 6 | ·· |
| Levofloxacin | S | 24 | ·· |
| Clindamycin | R | 6 | ·· |
| Vancomycin | S | 21 | ·· |
KB, Kirby-Bauer test for antibiotic susceptibility; MIC, minimum inhibitory concentration; R, resistant; S, sensitive.
Table 2.
List of pathogenic microorganisms detected by metagenomic next-generation sequencing of pleural effusion from a Chinese male patient in his late sixties who presented with wheezing with cough and sputum expectoration for 2 weeks.
| Bacteria | Reads |
|---|---|
| Parvimonas micra | 4516 |
| Prevotella oris | 4395 |
| Peptostreptococcus stomatis | 2573 |
| Streptococcus constellatus | 30 |
Based on bacterial culture of pleural effusion, the abovementioned anti-infection treatment was continued. Subsequently, the patient’s wheezing symptoms were alleviated, and arterial blood gas analysis on day 7 revealed a pH of 7.417, pCO2 of 35.1 mmHg, and pO2 of 105.0 mmHg (oxygenation index: 262.5 mmHg). The patient was gradually weaned from the ventilator to a high-flow oxygen mask, and given the improvement in his clinical condition, cefoperazone/sulbactam was switched to 2 g ceftriaxone, by i.v. drip infusion, once daily, with continuation of 400 mg moxifloxacin, by i.v. drip infusion, once daily. A repeat chest CT on day 11 of hospital admission revealed that bilateral pneumonia and bilateral pleural effusion had worsened with partial atelectasis (Figure 1d–f). However, the patient’s clinical condition subsequently improved, and his CRP level decreased to 28 mg/L.
Considering the improvement in the patient’s clinical condition and the lagging improvement detected on imaging, his treatment regimen was continued. As part of this regimen, the patient continued with pleural effusion drainage. In addition, his temperature normalized, and his wheezing resolved. He was provided nasal cannula oxygen at a flow rate of 2 L/min and could rest in the supine position, relieving cough and sputum production. All these findings indicated a substantial improvement in the disease course. Ceftriaxone was discontinued on day 14, but treatment with 400 mg moxifloxacin, by i.v. drip infusion, once daily, was continued. After 17 days of treatment, the patient was discharged with substantial improvement in his condition after receiving oral moxifloxacin for an additional 3 days.
Follow-up and outcomes
One week after discharge, a follow-up chest CT at the outpatient clinic revealed that pneumonia had improved and the pleural effusion had decreased (Figure 1g–i). One month after discharge, another follow-up chest CT revealed a further improvement in pneumonia and a further reduction in the pleural effusion (Figure 1j–l). A 1-year follow-up assessment indicated that the patient had fully recovered.
Discussion
To the best of our knowledge, pneumonia with empyema due to P. micra and S. constellatus co-infection has not been reported previously. P. micra, originally named Pepto-streptococcus micros, is an anaerobic gram-positive coccus, whereas S. constellatus is an aerobic gram-positive coccus belonging to the Streptococcus milleri group (with Streptococcus intermedius and Streptococcus anginosus). Both P. micra and S. constellatus are opportunistic pathogens. Here, a case of severe pneumonia with empyema caused by these two pathogens in a patient with poorly controlled diabetes is reported.
Parvimonas micra, which is part of the normal flora of the oral cavity and the mucosa of the gastrointestinal tract, skin, and female genital tract, 5 was previously considered an oral pathogen primarily (as P. micra is commonly a causative pathogen of chronic periodontitis). 6 Recent studies have shown that the most common site of extraoral P. micra infection is the spine, 7 followed by the joints, 8 and heart valves. 9 Moreover, P. micra has been shown to cause bacteraemia, 10 meningitis, 11 cervical and brain abscesses, 12 liver abscesses, 13 and iliopsoas abscesses. 14
Pneumonia and empyema caused by P. micra are rare,7,15 most likely because the lung is an oxygenated organ, and oxygenation is not conducive to P. micra growth. Moreover, P. micra is difficult to culture using conventional methods due to particular requirements. P. micra infections are more likely to occur in immunocompromised hosts, such as people of advanced age or those with type 2 diabetes mellitus or malignancies, and in the postoperative period.7,10,12,14,15 This suggests that primary immune dysfunction and post-operative stress may be risk factors for infection with this organism. In the present case, the patient was diagnosed with diabetes mellitus by standard testing on admission, and he did not report a previous diabetes diagnosis. Although he had no periodontal disease or history of tooth extraction, he had poor oral hygiene, which may have been the source of his P. micra infection.
Streptococcus constellatus is widely distributed in the oral cavity, nasopharynx, gastrointestinal tract, and vagina in 15–30% of healthy individuals. 16 In recent years, S. constellatus has gradually become one of the primary pathogens causing purulent infections, accounting for more purulent infections than S. intermedius or S. anginosus. 17 S. constellatus can cause empyema, liver abscess, 18 brain abscess, 19 spondylitis, 20 bacteraemia, 21 peritonitis, 22 and other diseases. A previous study showed that as many as 55.6% of pulmonary infections caused by S. constellatus manifest as a pleural effusion or empyema, making empyema more common with S. constellatus than other bacterial species. 23 This suggests that S. constellatus should be considered a possible causative pathogen in patients with lung infections presenting with empyema. The risk factors for S. constellatus infections are similar to those for P. micra infections (i.e., immunocompromised hosts with conditions such as diabetes mellitus, malignancy, or chronic obstructive pulmonary disease). 24
A previous study reported that the presence of anaerobic bacteria synergistically increases the risk of S. constellatus infection. 25 S. constellatus does not grow well in aerobic conditions but grows more readily in 5% CO2 or under anaerobic conditions. Lin et al. 23 showed that S. constellatus is often co-isolated with anaerobes in empyema. The mechanism of action is as follows: (i) anaerobic bacteria and their metabolites inhibit the ability of leukocytes to phagocytose aerobic bacteria; (ii) anaerobic bacteria can provide (i.e., synthesize) essential nutrients for bacterial growth, such as vitamin K, succinate, and various growth factors; and (iii) these bacteria can alter the local environment (including lowering oxygen tension and reducing redox potential) and produce substances that are toxic to the host, allowing various species of bacteria to reproduce concurrently.
Other bacteria identified by NGS in the present patient were Prevotella oris and Peptostreptococcus stomatis, which are specialized anaerobic bacteria that belong to the category of conditional pathogenic bacteria. A few articles have reported that P. oris can cause pleural effusion and pleuropulmonary infections.24,26 In addition, animal studies have suggested that coinfection of mice with P. intermedia and S. constellatus results in more severe pneumonia and higher mortality than infection with each pathogen separately, and may lead to lung abscesses and empyema. 27 Therefore, we believe that P. oris may also play a role as a co-source of infection. Regarding P. stomatis, dysbiosis of this microbiota may be associated with gastrointestinal tumours. 28 However, to our knowledge, there have been no reported cases of this bacterium causing empyema. Although the clinical relevance remains unclear, the present case suggests that P. stomatis can spread to the adjacent pleural cavity and may alter the surrounding microenvironment to support the progression of empyema.
In the present case, despite the initial anti-infection treatment using moxifloxacin, the patient's symptoms worsened, which may have been related to the combination of pneumonia and empyema. The patient's condition stabilized after undergoing thoracentesis and drainage, thus, source control (thoracic drainage) may be an important factor in alleviating patient symptoms.
Parvimonas micra and S. constellatus are sensitive to antimicrobial drugs, and the prognosis is good if the appropriate antibiotics are promptly administered.29,30 The patient described in the current report was initially treated with moxifloxacin, but following aggravation of his condition, he was treated with a combination of moxifloxacin and cefoperazone/sulbactam. After the patient's vital signs stabilized, the regimen shifted to antibiotic de-escalation therapy, switching from the initial broad-spectrum antibiotic (cefperazone/sulbactam) to ceftriaxone. After 18 days of intravenous medication, the patient was discharged and continued with sequential oral administration of moxifloxacin (for 7 days) and was subsequently cured. The complete treatment course spanned 21 days.
This case report may be limited by several factors. First, no bacteria (including P. micra or S. constellatus) or fungi were detected in the sputum culture. This absence may have been because the patient’s sputum was not a high-quality specimen, as it contained squamous cells at a concentration of > 25/LP. In addition, the patient could not undergo bronchoscopy as he was administered non-invasive ventilator-assisted ventilation due to the severity of his condition. Consequently, no bronchoalveolar lavage fluid was cultured for mNGS.
In conclusion, timely and accurate identification of causative pathogens and thoracentesis may be critical in effectively treating pneumonia with empyema. In addition to routine culture, mNGS may play an essential role in rapid and accurate diagnosis. Moreover, immunocompromised individuals are susceptible to P. micra and S. constellatus infections. As coinfection is likely to exacerbate severe pneumonia and empyema, this problem is worthy of mention.
Author contributions: YD was responsible for the patient’s management, data collection, and manuscript drafting. YD, WF, YS, YL, and NL reviewed and edited the manuscript. XC was responsible for the language review. YW was responsible for the interpretation of data and supervised this manuscript. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Yao Duan https://orcid.org/0000-0001-8560-5743
Data availability statement
Restrictions apply to the availability of the data used to support the findings of this study, which were used under license for the current study, and thus are not publicly available. Data are available from the authors upon reasonable request, with the corresponding author's permission.
References
- 1.Addala DN, Bedawi EO, Rahman NM. Parapneumonic effusion and empyema. Clin Chest Med 2021; 42: 637–647. [DOI] [PubMed] [Google Scholar]
- 2.Kuryłek A, Stasiak M, Kern-Zdanowicz I. Virulence factors of Streptococcus anginosus – a molecular perspective. Front Microbiol 2022; 13: 1025136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, Xia L, Zhou H, et al. Empyema caused by Streptococcus constellatus: a case report and literature review. BMC Infect Dis 2021; 21: 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 5.Yu Q, Sun L, Xu Z, et al. Severe pneumonia caused by Parvimonas micra: a case report. BMC Infect Dis 2021; 21: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu K, Horinishi Y, Sano C, et al. Infection route of Parvimonas micra: a case report and systematic review. Healthcare (Basel) 2022; 10: 1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higashi Y, Nakamura S, Niimi H, et al. Spondylodiscitis due to Parvimonas micra diagnosed by the melting temperature mapping method: a case report. BMC Infect Dis 2017; 17: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baghban A, Gupta S. Parvimonas micra: a rare cause of native joint septic arthritis. Anaerobe 2016; 39:26–27. [DOI] [PubMed] [Google Scholar]
- 9.Ho D, Ang G, Er C, et al. An unusual presentation of Parvimonas micra infective endocarditis. Cureus 2018; 10: e3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Hara Y, Yoshimi Y, et al. Clinical characteristics of bloodstream infection by Parvimonas micra: retrospective case series and literature review. BMC Infect Dis 2020; 20: 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko JH, Baek JY, Kang CI, et al. Bacteremic meningitis caused by Parvimonas micra in an immunocompetent host. Anaerobe 2015; 34: 161–163. [DOI] [PubMed] [Google Scholar]
- 12.Shtaya A, Schuster H, Riley P, et al. Oesophageal pleural fistula presenting with Parvimonas micra infection causing cervical and brain abscesses. Anaerobe 2017; 47: 233–237. [DOI] [PubMed] [Google Scholar]
- 13.Kim EY, Baek YH, Jung DS, et al. Concomitant liver and brain abscesses caused by Parvimonas micra. Korean J Gastroenterol 2019; 73: 230–234. [DOI] [PubMed] [Google Scholar]
- 14.Sawai T, Koga S, Ide S, et al. An iliopsoas abscess caused by Parvimonas micra: a case report. J Med Case Rep 2019; 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobo F, Borrego J, Rojo MD, et al. Polymicrobial anaerobic bacteremia due to Atopobium rimae and Parvimonas micra in a patient with cancer. Anaerobe 2018; 54: 260–263. [DOI] [PubMed] [Google Scholar]
- 16.Pilarczyk-Zurek M, Sitkiewicz I, Koziel J. The clinical view on Streptococcus anginosus group - opportunistic pathogens coming out of hiding. Front Microbiol 2022; 13: 956677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carretero RG. Cerebellar abscesses, infective endocarditis and bacteraemia due to a rare pathogen: Streptococcus constellatus. BMJ Case Rep 2017; 2017: bcr2017221374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dsouza R, Roopavathana B, Chase S, et al. Streptococcus constellatus: a rare causative agent of pyogenic liver abscess. BMJ Case Rep 2019; 12: e229738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao K, Zhao D, Luo Y, et al. Streptococcus constellatus causing brain abscess identified by metagenomics next-generation sequencing. Surg Infect (Larchmt) 2022; 23: 207–208. [DOI] [PubMed] [Google Scholar]
- 20.Lim SW, Lim HY, Kannaiah T, et al. Streptococcus constellatus spondylodiscitis in a teenager: a case report. Malays Orthop J 2017; 11: 50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sala A, Restaino S, De Carlo CD, et al. Postoperative Streptococcus constellatus bacteremia in a 75-year-old patient with pyometra: a case report. Am J Case Rep 2021; 22: e931167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tymon-Rosario J, Atrio JM, Yoon HA, et al. Streptococcus constellatus peritonitis and subsequent septic shock following intrauterine device removal. Case Rep Obstet Gynecol 2019; 2019: 6491617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Zhang Y, Bao C, et al. The clinical features and management of empyema caused by Streptococcus constellatus. Infect Drug Resist 2022; 15: 6267–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viswanath LS, Gunalan A, Jamir I, et al. Prevotella oris: a lesser known etiological agent of pleural effusion. Anaerobe 2022; 78: 102644. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi S, Yatera K, Kawanami T, et al. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm Med 2015; 15: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández Vecilla D, Roche Matheus MP, Iglesias Hidalgo G, et al. Two cases of Prevotella oris causing serious pleuropulmonary infections. Rev Esp Quimioter 2023; 36: 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinzato T, Saito A. A mechanism of pathogenicity of “Streptococcus milleri group” in pulmonary infection: synergy with an anaerobe. J Med Microbiol 1994; 40: 118–123. [DOI] [PubMed] [Google Scholar]
- 28.Shen X, Li J, Li J, et al. Fecal Enterotoxigenic Bacteroides fragilis-Peptostreptococcus stomatis-Parvimonas micra biomarker for noninvasive diagnosis and prognosis of colorectal laterally spreading tumor. Front Oncol 2021; 11: 661048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rams TE, Feik D, Mortensen JE, et al. Antibiotic susceptibility of periodontal Streptococcus constellatus and Streptococcus intermedius clinical isolates. J Periodontol 2014; 85: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 30.Carretero RG, Luna-Heredia E, Olid-Velilla M, et al. Bacteraemia due to Parvimonas micra, a commensal pathogen, in a patient with an oesophageal tumour. BMJ Case Rep 2016; 2016: bcr2016217740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of the data used to support the findings of this study, which were used under license for the current study, and thus are not publicly available. Data are available from the authors upon reasonable request, with the corresponding author's permission.