Abstract
This study was conducted to examine protein synthesis and l-[35S] methionine incorporation into the endosperm of Zea mays L. kernels developing in vitro. Two-day-old kernels of the inbred line W64A were placed in culture on a defined medium containing 10 microCuries l-[35S] methionine per milliliter (13 milliCuries per millimole) and harvested at 10, 15, 20, 25, 30, 35, and 40 days after pollination. Cultured kernels attained a final endosperm mass of 120 milligrams compared to 175 milligrams for field-grown controls. Field and cultured kernels had similar concentrations (microgram per milligram endospern) for total protein, albumin plus globulin, zein, and glutelin fractions at most kernel ages.
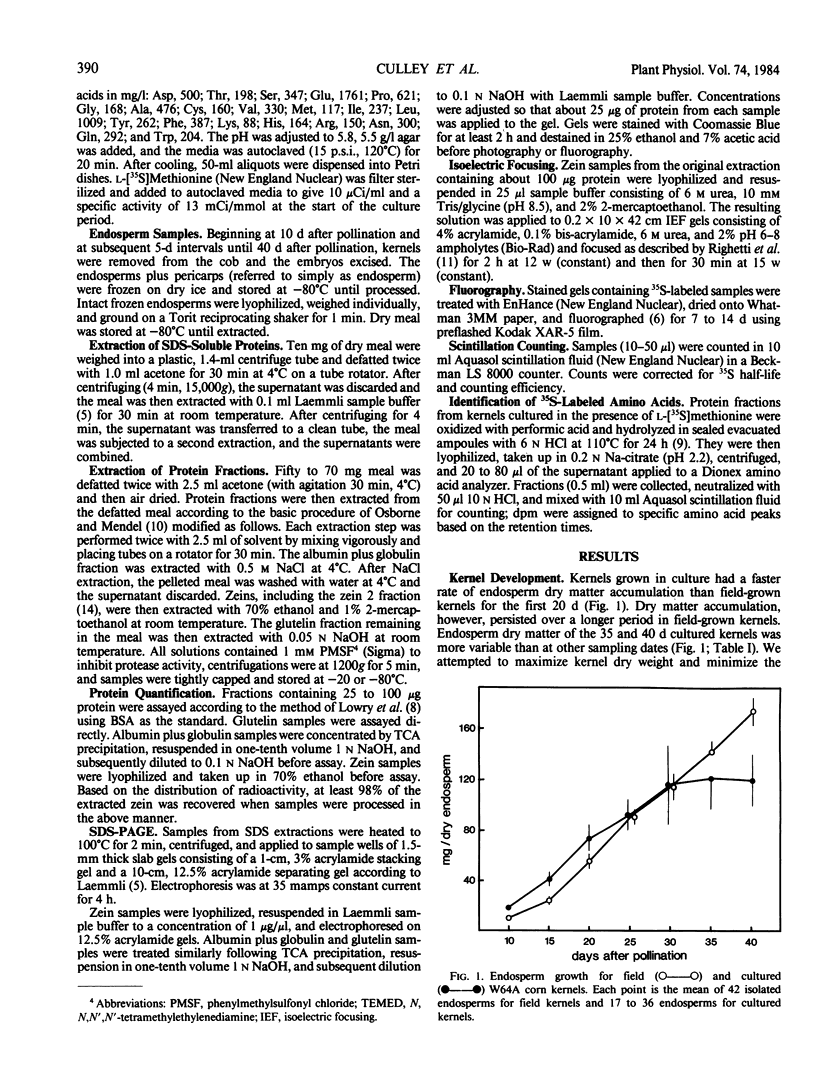
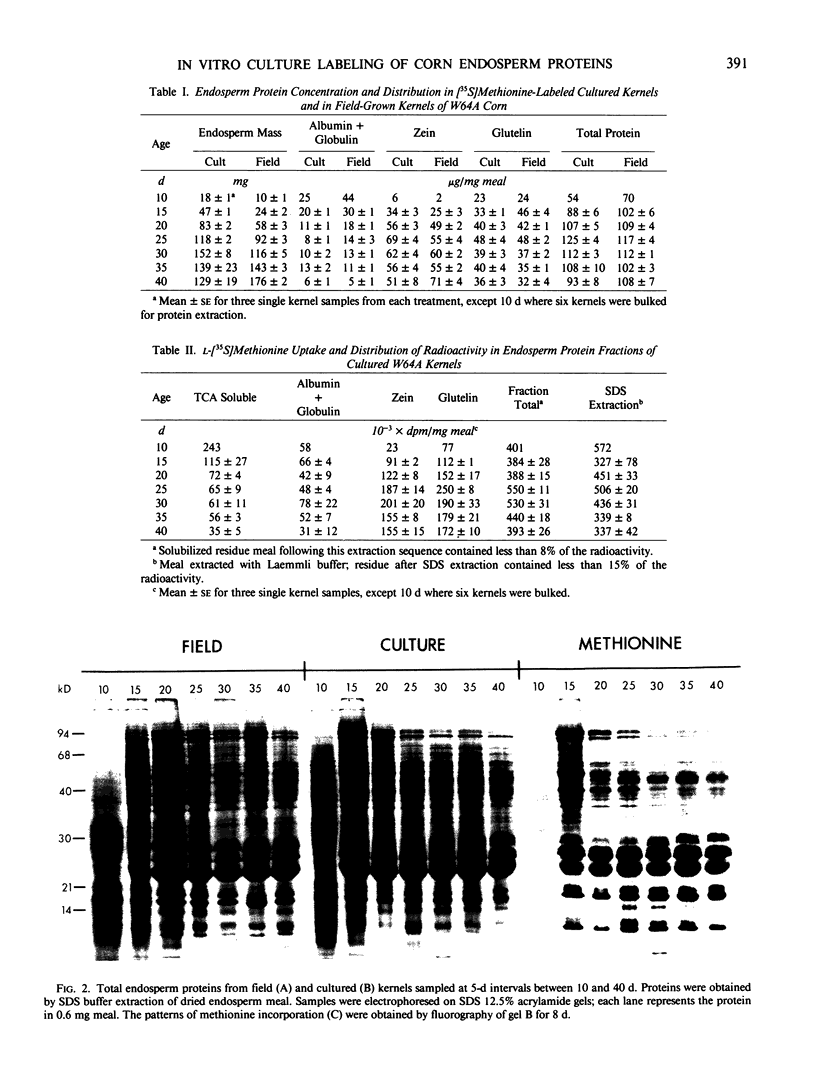
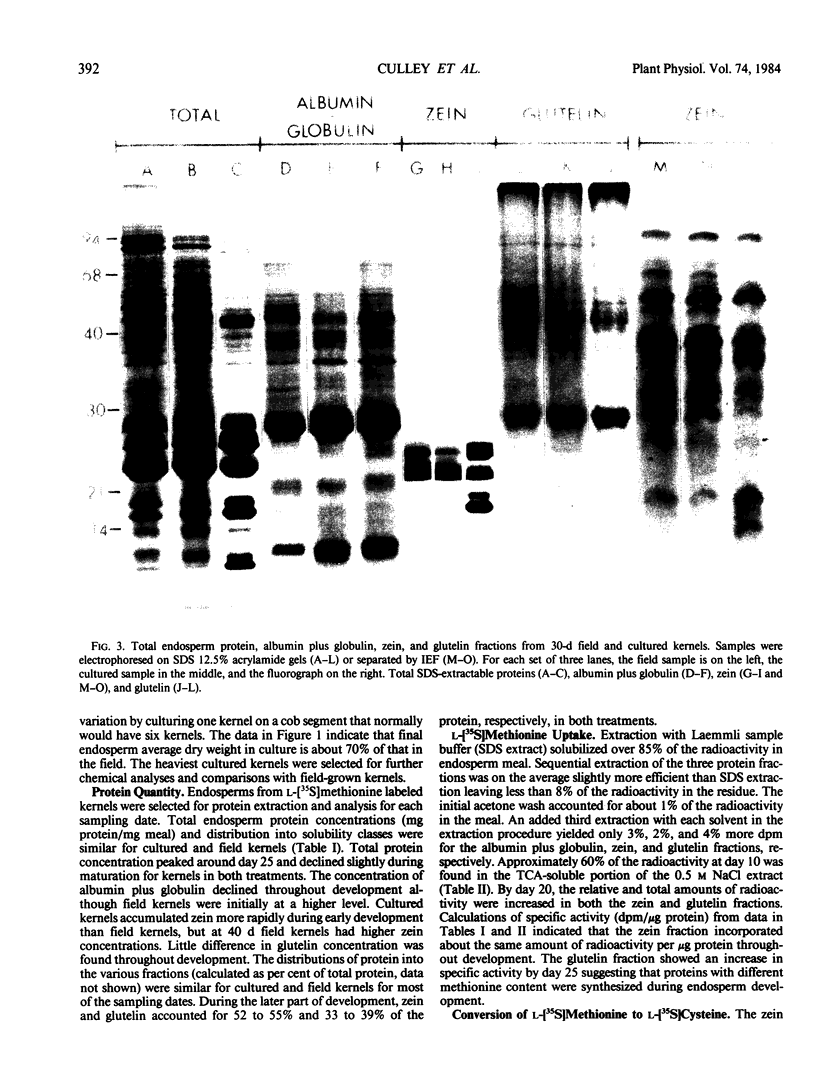
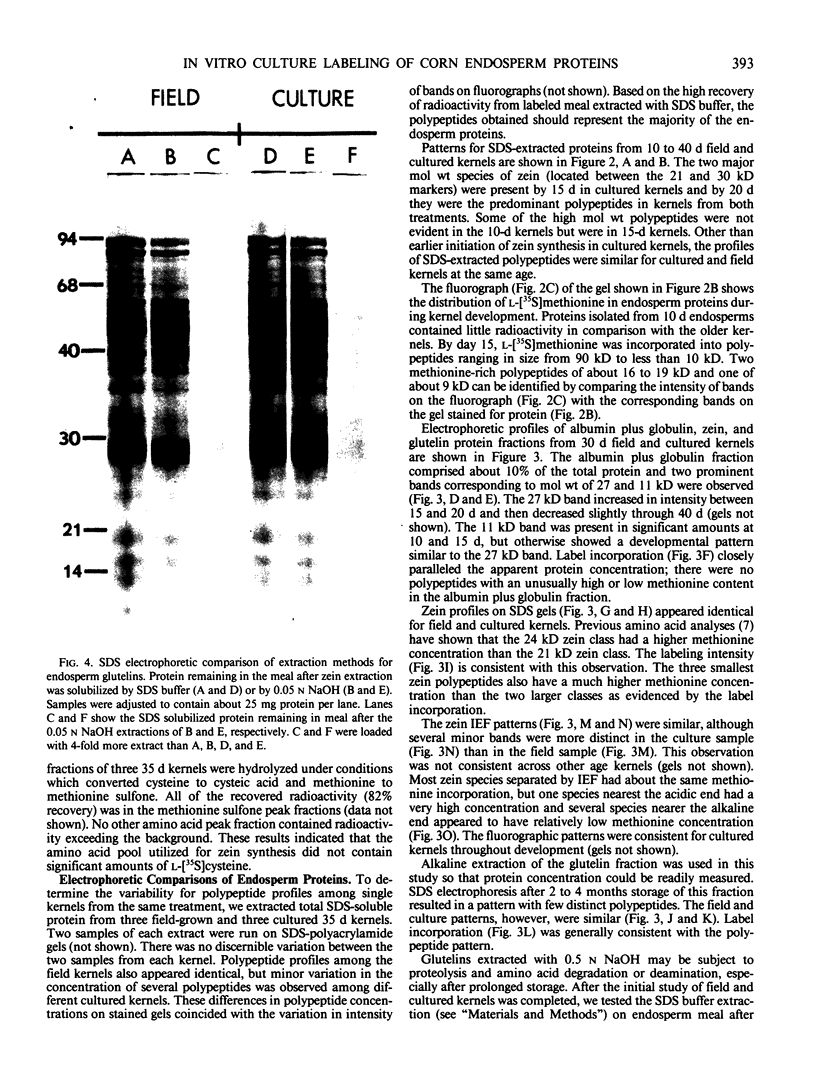
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and isoelectric focusing patterns for endosperm proteins were similar for field and cultured kernels throughout development. By 15 days, over 70% of the l-[35S]methionine taken up was present in endosperm proteins. Label incorporation visualized by fluorography generally followed the protein intensity of the stained gels. The high methionine content, low molecular weight zeins (i.e. 15 and 9 kilodaltons) were highly labeled. All of the radioactivity in hydrolyzed zein samples was recovered in the methionine peak indicating minimal conversion to l-[35S]cysteine. The procedure described here is suitable for long term culture and labeling experiments in which continued kernel development is required.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Jones R. A., Dalby A., Tsai C. Y. Genetic regulation of storaage protein content in maize endosperm. Biochem Genet. 1976 Aug;14(7-8):641–650. doi: 10.1007/BF00485842. [DOI] [PubMed] [Google Scholar]
- Shimamoto K., Nelson O. E. Movement of C-compounds from Maternal Tissue into Maize Seeds Grown in Vitro. Plant Physiol. 1981 Mar;67(3):429–432. doi: 10.1104/pp.67.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek L., da Silva W. J. Glutamate synthase: a possible role in nitrogen metabolism of the developing maize endosperm. Plant Physiol. 1977 Oct;60(4):602–605. doi: 10.1104/pp.60.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. Y., Salamini F., Nelson O. E. Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol. 1970 Aug;46(2):299–306. doi: 10.1104/pp.46.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]