ABSTRACT
The transcription factor OTX2 is required for photoreceptor and bipolar cell formation in the retina. It directly activates the transcription factors Prdm1 and Vsx2 through cell type-specific enhancers. PRDM1 and VSX2 work in opposition, such that PRDM1 promotes photoreceptor fate and VSX2 bipolar cell fate. To determine how OTX2+ cell fates are regulated in mice, we deleted Prdm1 and Vsx2 or their cell type-specific enhancers simultaneously using a CRISPR/Cas9 in vivo retina electroporation strategy. Double gene or enhancer targeting effectively removed PRDM1 and VSX2 protein expression. However, double enhancer targeting favored bipolar fate outcomes, whereas double gene targeting favored photoreceptor fate. Both conditions generated excess amacrine cells. Combined, these fate changes suggest that photoreceptors are a default fate outcome in OTX2+ cells and that VSX2 must be present in a narrow temporal window to drive bipolar cell formation. Prdm1 and Vsx2 also appear to redundantly restrict the competence of OTX2+ cells, preventing amacrine cell formation. By taking a combinatorial deletion approach of both coding sequences and enhancers, our work provides new insights into the complex regulatory mechanisms that control cell fate choice.
Keywords: Prdm1, Vsx2, Photoreceptors, Bipolar, Retina, Enhancers, Mouse
Highlighted Article: Simultaneous mutagenesis of two transcription factors or their cell type-specific enhancers causes divergent fate specification. Fate decisions involve the complex interplay of multiple factors within a temporally narrow window.
INTRODUCTION
One of the fundamental questions in development is how progenitor cells give rise to the incredible variety of cell types found in the central nervous system. The retina is an excellent system for studying the mechanisms of fate specification because the major cell types are readily discernable. The seven principal cell types are derived from a population of retinal progenitor cells in a stereotyped overlapping fashion during embryonic and early postnatal development in the mouse (Sidman, 1961; Carter-Dawson and LaVail, 1979; Young, 1985; Turner and Cepko, 1987; Turner et al., 1990; Rapaport et al., 2004). Some cell types, such as cone photoreceptors, horizontals and ganglion cells, permanently exit the cell cycle (i.e. they are born) embryonically. Rod photoreceptors, bipolar cells and Müller glia are primarily born in the postnatal period. Amacrine cells are mostly formed embryonically, but a small population is born postnatally. It is widely thought that retinal progenitors progressively pass through stages in which they have the competence (potential) to give rise to certain cell types early and others later (Reh and Cagan, 1994; Cepko et al., 1996; Livesey and Cepko, 2001; Brzezinski and Reh, 2010; Bassett and Wallace, 2012; Xiang, 2013; Boije et al., 2014; Cepko, 2014). However, the means by which progenitors change their competence and adopt their final cell fates are only partially understood.
The transcription factor OTX2 sits at the top of a regulatory network that controls photoreceptor and bipolar cell genesis (Brzezinski and Reh, 2015). It is expressed by all mature photoreceptors and bipolar cells (Nishida et al., 2003; Fossat et al., 2007; Beby and Lamonerie, 2013; Shekhar et al., 2016). Mice that lack Otx2 do not generate photoreceptors or bipolar cells, but instead produce excess amacrine cells (Nishida et al., 2003; Sato et al., 2007; Yamamoto et al., 2020). OTX2 is first upregulated in a large fraction of retinal progenitors as they permanently exit the cell cycle (Muranishi et al., 2011), and directly regulates the genes encoding two additional transcription factors, Prdm1 (also known as Blimp1) and Vsx2 (Chx10), through discrete enhancer sequences (Kim et al., 2008; Wang et al., 2014; Mills et al., 2017). In the absence of Prdm1, OTX2+ cells precociously upregulate Vsx2 and form bipolar cells at the expense of photoreceptors (Brzezinski et al., 2010; Katoh et al., 2010; Brzezinski et al., 2013). Many of these Prdm1 mutant OTX2+ cells appear by morphology and immunohistochemistry to start off as photoreceptors and subsequently transition (transdifferentiate) into VSX2+ bipolar cells in the early postnatal period (Brzezinski et al., 2013). Conversely, overexpression of PRDM1 suppresses VSX2 and bipolar cell formation (Brzezinski et al., 2010; Katoh et al., 2010). These data argue that PRDM1 does not instruct photoreceptor fate, but instead inhibits bipolar cell formation. VSX2 overexpression suppresses photoreceptor genes, whereas Vsx2 mutants fail to generate bipolar cells (Burmeister et al., 1996; Green et al., 2003; Dorval et al., 2006; Livne-Bar et al., 2006; Phillips et al., 2014). Taken together, this suggests that OTX2 establishes competence for photoreceptor and bipolar cell fates, whereas PRDM1 and VSX2 form a mutually inhibitory network to influence the fate choice made by these competent OTX2+ cells (Fig. 1A).
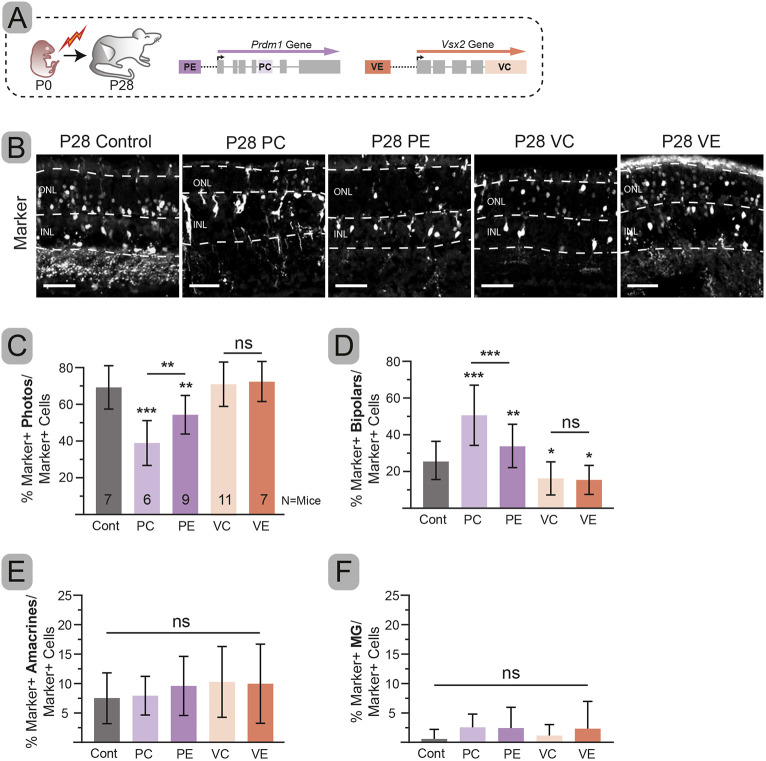
Fig. 1.
Deletion of Prdm1 and Vsx2 enhancers blocks protein expression. (A) Schematic for the Otx2 gene regulatory network. (B) CRISPR targeting guide design and nomenclature. (C,D,G) Schematic of experimental approaches. Note that electroporations carried out at P0 will not target retinal neurons that are formed embryonically (e.g. cones, horizontals and ganglion cells). (E,H) Immunohistochemistry from electroporated cells. (F) Percentage of electroporated cells that also express PRDM1. (I) Quantification of electroporated cells that also express VSX2. Error bars represent s.d. Significance determined by one-tailed unpaired t-test. ***P<0.001. Notched arrowheads, double-labeled cells; arrowheads, single-labeled cells. Insets show magnification of boxed areas. IHC, immunohistochemistry; N, number of mice used for statistics; ns, not significant; L, lens; ON, optic nerve; R, retina; RPE, retinal pigmented epithelium. Scale bars: 50 µm; 25 µm in insets.
Several non-exclusive mechanisms may explain the role of PRDM1 and VSX2 in OTX2+ cell fate determination (Fig. 1A). VSX2 and PRDM1 could fine-tune the numbers of bipolars and photoreceptors formed by repressing instructive factors (Fig. 1A). Another possibility is that these transcription factors stabilize specification after a fate choice has occurred. Finally, it is possible that either photoreceptor or bipolar cell identity is a default outcome during retinal development and that PRDM1 and VSX2 are used to break away from such a default state to ensure both fates are formed. We reasoned that removing the function of PRDM1 and VSX2 simultaneously in OTX2+ cells would discriminate between these potential developmental mechanisms.
To simultaneously remove PRDM1 and VSX2, we developed a loss-of-function CRISPR/Cas9 targeted gene disruption system that could be delivered in vivo to the developing mouse retina. We created two versions of this system. The first targets the coding region of each gene to remove its function via deletion in a non-cell type-specific manner from transfected retinal cells. The second targets the Prdm1 and Vsx2 enhancers that are directly regulated by OTX2. We then delivered these CRISPR/Cas9 plasmids via in vivo electroporation into hundreds of newborn mouse retinas and tracked cell fate under a variety of conditions across multiple time points. Similar to previous loss-of-function experiments (Burmeister et al., 1996; Green et al., 2003; Brzezinski et al., 2010; Katoh et al., 2010; Phillips et al., 2014), we observed that singly targeting Prdm1 and Vsx2, either through their enhancers or coding sequences, affected the number of photoreceptors and bipolar cells that were formed. Removing Prdm1 and Vsx2 simultaneously yielded different fate changes depending on how they were removed. From these data we conclude that photoreceptors are a default fate choice during development, but that there is a critical window when bipolar fate can be driven by VSX2. We found that VSX2 is dispensable for bipolar fate maintenance and we also observed that Prdm1 and Vsx2 act redundantly in OTX2+ cells to restrict their ability to form amacrine cells. By targeting multiple genes and enhancers simultaneously in vivo, we have gained novel insights into the complex regulation of cell fate choice within the retina.
RESULTS
Vsx2 and Prdm1 enhancers are necessary for protein expression at P2 and P7
OTX2 is required for the formation of photoreceptors and bipolar cells (Nishida et al., 2003; Sato et al., 2007). Prdm1 and Vsx2 are direct targets of OTX2 and appear to have a cross-repressive relationship (Kim et al., 2008; Brzezinski et al., 2010; Katoh et al., 2010; Brzezinski et al., 2013; Wang et al., 2014; Mills et al., 2017) (Fig. 1A). We reasoned that deleting Prdm1 and Vsx2 simultaneously would unmask the processes controlling photoreceptor and bipolar fate choices. To test this, we developed a CRISPR/Cas9 system to specifically delete these transcription factors during the postnatal period, when OTX2+ cells primarily choose between rod photoreceptor and bipolar fates (Carter-Dawson and LaVail, 1979; Young, 1985; Rapaport et al., 2004).
We first modified existing plasmid constructs (Ran et al., 2013) so that Streptococcus pyogenes Cas9 was driven by a ubiquitous EF1α promotor and, in some cases, GFP was replaced with RFP (Fig. 1B). Guide RNAs were designed to triply target a key coding exon of Prdm1 (PC) and Vsx2 (VC) (Fig. 1B). By co-electroporating these plasmids into newborn retinas, Vsx2 and Prdm1 can be targeted in any transfected cell (Fig. 1C).
Previous studies identified OTX2-regulated enhancers for Prdm1 and Vsx2. This included a 139 bp retina-specific enhancer approximately 6 kb upstream of Prdm1 and a 164 bp bipolar-specific enhancer about 18 kb upstream of Vsx2 (Kim et al., 2008; Wang et al., 2014; Mills et al., 2017). Although both enhancers require OTX2 for their activity, additional factors are needed to direct their distinct spatial and temporal activity patterns in the retina (Kim et al., 2008; Wang et al., 2014; Mills et al., 2017). Because Vsx2 plays a role in promoting retinal progenitor proliferation in addition to its role in bipolar cell genesis (Burmeister et al., 1996; Green et al., 2003; Livne-Bar et al., 2006; Sigulinsky et al., 2015), we generated another targeting strategy that would prevent Vsx2 and Prdm1 expression specifically within postmitotic OTX2+ cells. To do this we designed a separate trio of guide RNAs to target the Prdm1 (PE) and Vsx2 enhancers (VE) (Fig. 1B). All guides – PC, VC, PE, and VE, along with a non-targeting control – were cloned into plasmids that expressed either RFP or GFP markers.
To test whether this CRISPR/Cas9 strategy effectively lowered PRDM1 and VSX2 protein expression, we electroporated postnatal day (P)0 wild-type CD1 mouse retinas with plasmids (PE, PC, VE, VC or non-targeting control) and raised the pups until the desired age of tissue collection (Fig. 1C). PC, PE and control eyes were collected at P2, when PRDM1 is quantifiable via immunohistochemistry (Fig. 1D). Retinas were stained for PRDM1 and the fluorescent marker (Fig. 1D,E). We observed a significant decrease in PRDM1+ cells in PC and PE (P<0.001, both) compared with control (Fig. 1F), but no difference between PC and PE (P=0.075). This argues that the Prdm1 enhancer is necessary for PRDM1 expression in the retina. Next, we conducted electroporations with control, VC and VE targeting plasmids and collected pups at P7, a time when bipolar-specific VSX2 expression is high (Fig. 1G,H). The percentage of electroporated cells that co-expressed VSX2 was significantly lower in VC and VE (P<0.001, both) compared with control (Fig. 1I). There was no difference between VC and VE (P=0.39).
As VSX2 is made by progenitors, it is possible that both VC and VE affect expression in progenitors. To test this, we electroporated embryonic day (E)13.5 retinal explants with VC, VE or control guides and cultured them for 72 h (Fig. S1A). We then quantified the percentage of electroporated cells (GFP+) that co-expressed VSX2. At this time point, VSX2 only marks progenitors. The VC-targeted cells had significantly reduced co-expression (P<0.001), whereas the VE cells co-expressed VSX2 at the same frequency as control electroporated cells (Fig. S1B,C). Thus, the Vsx2 enhancer is only necessary for bipolar-specific VSX2 expression. Taken together, both coding (PC, VC) and enhancer (PE, VE) CRISPR/Cas9 systems were able to equivalently reduce PRDM1 and VSX2 expression.
Vsx2 and Prdm1 cell type-specific enhancers control fate choice
If VE and PE are necessary for VSX2 and PRDM1 expression in OTX2+ cells, then targeting these enhancers should mimic targeting the coding regions and recapitulate the phenotypes reported in Prdm1 and Vsx2 loss-of-function studies (Burmeister et al., 1996; Green et al., 2003; Brzezinski et al., 2010; Katoh et al., 2010). To test this, we raised PC, PE, VC, VE and control electroporated mice to P28 and quantified the fate of electroporated cells (Fig. 2). Fates were assigned based on cell morphology and their location within the retina (Fig. S1D).
Fig. 2.
Targeting PC, PE, VC or VE shifts cell fates in mature retinas. (A) Experimental schematic. (B) Representative immunohistochemistry of electroporated cells (gray) for each condition. (C-F) Percentage of electroporated cells that are photoreceptors (C), bipolars (D), amacrines (E) and Müller glia (F). Error bars represent s.d. Significance determined by one-way ANOVA followed by Tukey post-hoc tests. *P<0.05, **P<0.01, ***P<0.001. Dashed lines indicate layer boundaries. ns, not significant. Scale bars: 50 µm.
At P28, PE and PC significantly reduced photoreceptors (PE P=0.002, PC P<0.001) and increased bipolar cells (PE P=0.006, PC P<0.001) (Fig. 2C,D). There were no changes in the percentage of electroporated amacrine interneurons or Müller glia (Fig. 2E,F). These data mimic Prdm1 knockout mice and suggest that the Prdm1 enhancer is essential for protein expression. PC produced a more robust shift from photoreceptors (P=0.006) to bipolars (P<0.001) than PE (Fig. 2C,D). We next examined VC- and VE-targeted mice at P28 (Fig. 2). Both conditions had a significant loss of electroporated bipolar cells (VC P=0.039, VE P=0.040) (Fig. 2D). There was no significant change in the percentage of electroporated cells that became photoreceptors, amacrines or Müller glia in VE or VC (Fig. 2C,E,F). There were more photoreceptors in VE and VC compared with control; however, our methods did not have the power to detect a significant photoreceptor increase (Fig. 2B-D). Targeting the Vsx2 coding and enhancer regions affected cell fate equally (P=0.975). These results are similar to Vsx2 mutant mice (Burmeister et al., 1996; Green et al., 2003), suggesting that the Vsx2 enhancer is necessary for bipolar cell genesis.
Simultaneous deletion of Vsx2 and Prdm1 enhancers dysregulates cell fate
Targeting Prdm1 or Vsx2 was robust, but never reached 100% efficiency in our experiments. To ensure that we knocked-out multiple targets consistently, we electroporated retinas with PCVC or PEVE, such that PC and PE were labeled with GFP and VC and VE were marked by RFP (Fig. 3). RFP and GFP overlapped 85% of the time regardless of condition (Fig. 3A-C, data not shown). Based on this high degree of overlap, we proceeded with a single fluorescent label for conditions that received multiple constructs.
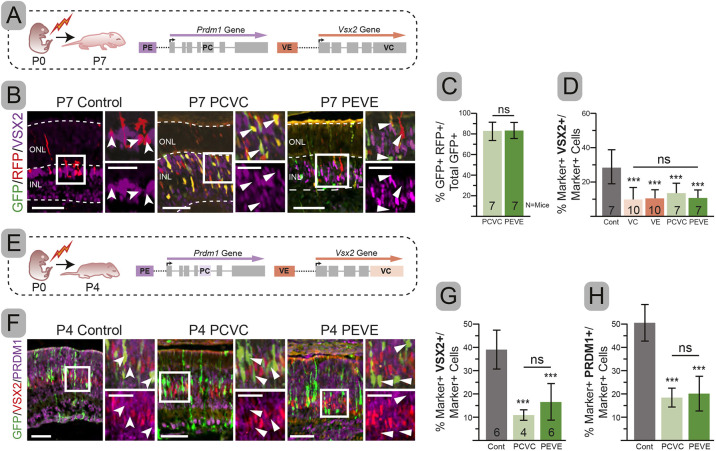
Fig. 3.
Double-targeting is as efficient as single-targeting at blocking expression. (A,E) Experimental schematics. (B) Representative immunohistochemistry of electroporated (RFP or GFP) cells co-stained with VSX2. (C) Percentage overlap between GFP and RFP. (D) Percentage of electroporated cells (marker+) that co-express VSX2. (F) Electroporated cells co-stained with VSX2 and PRDM1. (G,H) Percentage of electroporated cells that co-express VSX2 (G) or PRDM1 (H). Error bars represent s.d. Significance determined by one-tailed unpaired t-test. ***P<0.001. Insets show magnification of boxed areas. Dashed lines indicate layer boundaries. Notched arrowheads, double-labeled cells; arrowheads, electroporated cells without additional co-labeling. ns, not significant. Scale bars: 50 µm; 25 µm in insets.
To determine whether targeting multiple genes was as effective as targeting just one, we stained VC, VE, PCVC, and PEVE for VSX2 expression at P7. We observed an equivalent reduction in the percentage of electroporated cells that co-expressed VSX2 in all four conditions (Fig. 3D). This suggested that the effect was not diluted by including guides targeting other sequences. Next, we asked whether simultaneously targeting Vsx2 and Prdm1 (PEVE, PCVC) diluted its effect (Fig. 3E). We quantified the percentage of electroporated cells that expressed VSX2 and PRDM1 at P4 and observed a significant reduction of VSX2+ and PRDM1+ cells in both conditions (Fig. 3F-H). We concluded that we can simultaneously eliminate PRDM1 and VSX2 using either gene or enhancer targeting. Although effective, we chose to utilize a larger number of biological replicates throughout our studies to overcome the limitations of incomplete targeting that we observed.
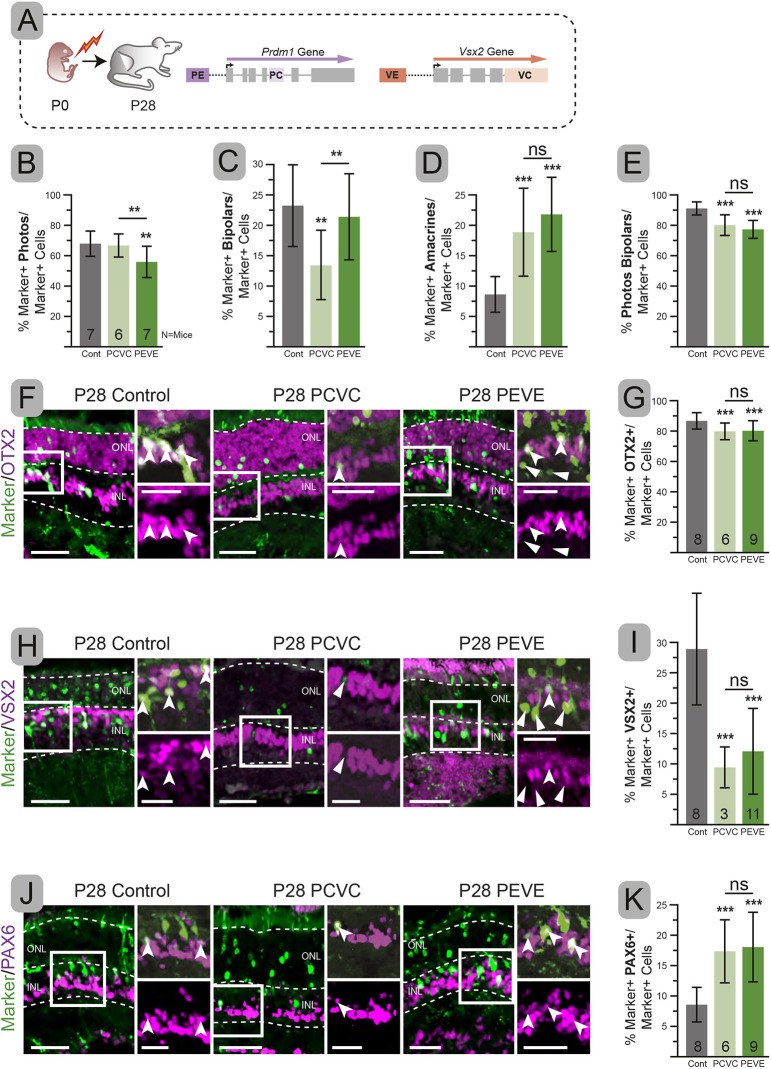
With the ability to target Vsx2 and Prdm1 simultaneously, we next examined the fate of doubly targeted cells. To do this, we performed in vivo electroporations at P0 with control, PCVC and PEVE plasmids, raised pups to P28, and quantified the fate of electroporated cells based on their morphology and location (Fig. 4A). Control electroporated cells were mostly photoreceptors and bipolars, but a modest number of OTX2− amacrines and Müller glia were also seen (Fig. 4). Mice targeted with PEVE had a significantly lower percentage of photoreceptors (P=0.014) compared with controls (Fig. 4B). In contrast, PCVC photoreceptors were indistinguishable from controls (P=0.366) (Fig. 4B). PCVC had a smaller fraction of bipolar cells compared with controls (P=0.002), whereas there was no change in the fraction of bipolars in PEVE electroporations (P=0.261) (Fig. 4B,C). These results were in contrast to single VC and VE electroporations that experienced a loss of bipolar cells and single PC and PE electroporations that had a loss of photoreceptors (Fig. 2). Thus, perturbing both genes or both enhancers simultaneously was not simply the sum of the individual electroporations.
Fig. 4.
Targeting PCVC or PEVE increases amacrines, but results in divergent bipolar and photoreceptor changes. (A) Experimental schematic. (B-D) Percentage of electroporated cells with photoreceptor (B), bipolar (C), or amacrine (D) cell morphology. (E) Combined percentage of electroporated cells that are photoreceptors, bipolars and Müller glia. (F,H,J) Representative immunohistochemistry of electroporated retinas co-stained with OTX2 (F), VSX2 (H) and PAX6 (J). (G,I,K) Percentage of electroporated cells that are also OTX2+ (G), VSX2+ (I) and PAX6+ (K). Error bars represent s.d. Significance determined by one-tailed unpaired t-test. **P<0.01, ***P<0.001. Insets show magnification of boxed areas. Dashed lines indicate layer boundaries. Notched arrowheads, double-labeled cells; arrowheads, electroporated cells without additional co-labeling. ns, not significant. Scale bars: 50 µm; 25 µm in insets.
We expected that targeting Prdm1 and Vsx2 would only alter the fate of OTX2+ cells. However, the sum of bipolar and photoreceptor cells was lower than controls in both PEVE and PCVC conditions (Fig. 4E). This suggested that there should be changes in the number of OTX2− Müller glia and amacrine cells. Upon quantification, we saw no changes in the number of Müller glia (not shown). However, we observed a two- to threefold increase in electroporated cells that become amacrines in both PCVC and PEVE conditions compared with control (P<0.001, both) (Fig. 4D). Both conditions had an equivalent increase in the fraction of amacrines observed (P=0.178) (Fig. 4D), which accounted for the reduction in photoreceptors (PCVC) or bipolar cells (PEVE) (P<0.001, both) in doubly targeted conditions (Fig. 4D,E).
One explanation for this unexpected result is that some bipolar cells migrate to the wrong portion of the inner nuclear layer (INL) and display amacrine-like morphology. To determine whether the morphologic characterization was misleading, we examined these mice by immunohistochemistry with cell type-specific markers. Both PCVC and PEVE had significantly fewer OTX2+ cells compared with controls (P<0.001, both), matching what we observed morphologically (Fig. 4F,G). PCVC and PEVE both showed a significant (P<0.001, both) and equal reduction in VSX2 co-staining compared with control, despite the fact that PEVE did not lose morphologically identified bipolars (Fig. 4C,H,I). The few bipolars seen in PCVC typically co-expressed VSX2 (rare exception highlighted in center panel, Fig. 4H). In contrast, PEVE had numerous clear VSX2− bipolars (Fig. 4H). These data argue that bipolar cells could form or survive without VSX2 or that their morphology remained misleading. To test this, we stained with additional bipolar markers (Fig. S2). We found that co-expression with ISLET1/2 (ISL1/2) (Cone ON and rod bipolars) (Elshatory et al., 2007a,b) matched the morphologically categorized bipolars, showing a significant decrease in PCVC (P=0.002) and no change in PEVE compared with controls (Fig. S2B,C). The co-expression with secretagogin, which marks a subset of ON and OFF cone bipolars (Puthussery et al., 2010), paralleled our observations of VSX2 and showed a significant decrease in PEVE and PCVC compared with control (P<0.001, P=0.003, respectively) (Fig. S2D,E). There was no difference between the percentage of morphological bipolar cells that lacked both ISLET1/2 and secretagogin between the conditions (Fig. S2F). Taken together, these data indicate that most (perhaps all) morphologically identified bipolar cells co-express other bipolar markers even though they lack VSX2. Thus, our morphological assessment is robust and suggests that bipolars were formed in the absence of bipolar-specific VSX2. Nonetheless, loss of VSX2 may alter the subtypes of bipolar cells formed.
To corroborate the change in morphologically identified amacrine cells, we stained for the pan-amacrine marker PAX6 (de Melo et al., 2003). The percentage of electroporated cells that co-expressed PAX6 in controls was indistinguishable from the value determined by morphology (Fig. 4D,J,K). We observed a significant increase in PAX6+ cells to about 20% in both PCVC and PEVE (P<0.001, both) (Fig. 4J,K). This increase was only observed in the PEVE and PCVC conditions and was remarkably consistent across litters (Figs S4F and S5E). We next stained for GLYT1 (SLC6A9) and EBF3, which mark different populations of late-born amacrine cells (Menger et al., 1998; Pow and Hendrickson, 1999; Cherry et al., 2009; Voinescu et al., 2009; Jin et al., 2010; Kay et al., 2011). Like PAX6, the percentage of electroporated cells that co-expressed EBF3 doubled in both PCVC (P=0.054) and PEVE (P=0.051) compared with controls (Fig. S3A-C). Similarly, the percentage of electroporated cells that co-expressed GLYT1 was strongly increased in both PCVC and PEVE (P<0.001, both) conditions (Fig. S3D,E). Thus, morphologically identified amacrines express pan-specific and late-born markers. We also observed that the number of cells co-expressing EBF3 increased in proportion to the total number of amacrines (Fig. S3F). The extra amacrines in PEVE and PCVC conditions appear to proportionally adopt late-born subtype fates, similar to amacrines formed postnatally in controls.
Fate changes do not result from selective survival
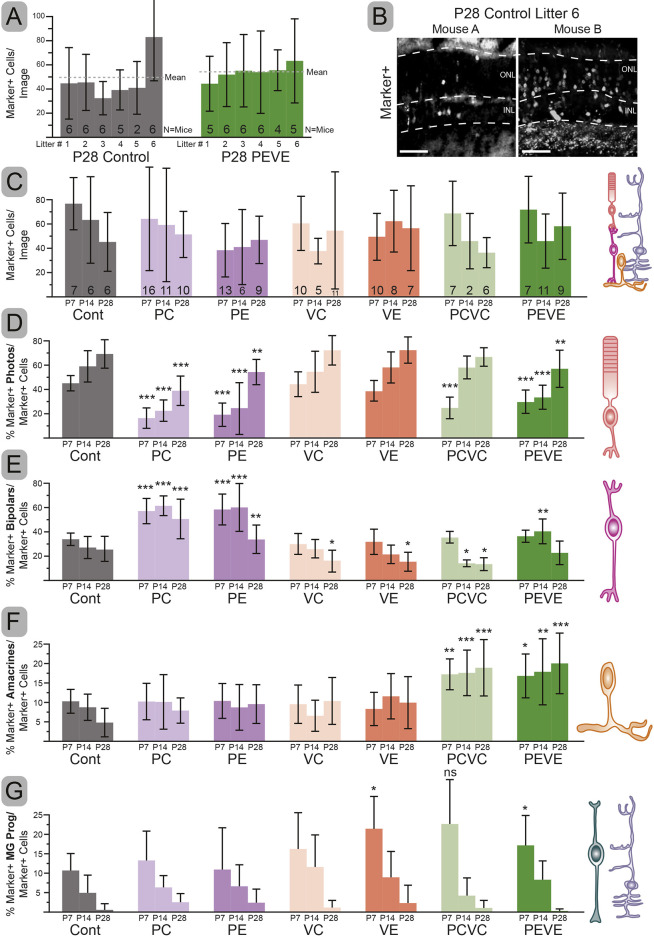
We reasoned that our Prdm1 and Vsx2 perturbations could alter cell survival, biasing the observed fate changes. Because of the temporally precise nature of cell death markers and our sparse-label technique, we were not able to directly test differences in cell death. Instead, we took three complementary indirect approaches to show that cell survival was equivalent between conditions. First, we quantified the number of cells per image across six litters of control and six litters of PEVE (63 total mice and 116 images) and found no differences between conditions (Fig. 5A). This suggests that cell survival is comparable at P28 between the two conditions. There was one exception where a control litter had significantly more cells per image compared with the rest of the controls and PEVE mice (Fig. 5A, Fig. S4). However, the variance within a litter was equal to or greater than between litters (Fig. 5B). Second, we asked whether variance in the total number of electroporated cells measured in any given litter skews the assessment of cell fate. Across six control and six PEVE litters we observed no differences in the percentages within a condition, but significant differences between conditions in the number of photoreceptors and amacrines (Fig. S4). Indeed, we could compare any control litter to any test litter and arrive at the same conclusion. Third, we asked whether there was a progressive loss in electroporated cells by quantifying the number of cells per image at intermediate developmental time points. In no condition was there significantly more cells at P7 or P14 compared with P28 (Fig. 5C), arguing against a progressive loss of electroporated cells. Taken together, our data strongly suggests that cell survival is unaffected by our perturbations and changes are a result of shifting cell fates.
Fig. 5.
Cell fates quantified across all conditions from P7, P14, and P28. (A) Number of electroporated cells per image showing the variability both within and between conditions. These are the average cells per image across 12 unique litters electroporated at P0 and collected at P28. The number of mice with quantifiable electroporations is shown in the bars. (B) Representative immunohistochemistry of two mice from the same litter showing electroporation efficiency differences within a condition. (C) Average electroporated cells per image by condition over time. (D-G) Percentage of electroporated cells with photoreceptor (D), bipolar (E), amacrine (F) or glial/progenitor-like (G) morphologies by condition over time. Error bars represent s.d. Significant differences from the time-matched control determined by one-way ANOVA with Dunnett's Multiple Comparisons test. *P<0.05, **P<0.01, ***P<0.001. Dashed lines indicate layer boundaries. ns, not significant. Scale bars: 50 µm.
Cell fate changes mostly manifest during the early postnatal period
Perturbing Prdm1 and Vsx2 could alter cell fate when these choices are normally being made during the first postnatal week of development. Alternatively, these changes may be delayed and manifest at a later time point. To explore this question, we repeated PC, PE, VC, VE, PCVC, PEVE and control electroporations at P0 and collected animals at P7 and P14. Electroporated cells were screened by morphology to determine whether fate shifts occurred early (by P7) or were delayed (Fig. 5). As it was difficult to assign cell fate at early time points (Fig. S1D), we considered all radially oriented cells that lacked clear neuronal morphology to be progenitors, undifferentiated neurons or Müller glia. For simplicity, we pooled all of these cells in our quantification of electroporated retinas (Fig. 5G, Fig. S1D).
Targeting PC and PE revealed an early fate shift, such that there were more bipolars and fewer photoreceptors at all time points (Fig. 5D,E). In contrast, perturbing VC and VE only showed a significant loss of bipolars at the P28 time point (Fig. 5E). This is likely owing to over-attributing bipolar cell identity at the early time points (Fig. S1D). PCVC and PEVE animals showed an increase in amacrines at all time points (Fig. 5F), which was not observed in the single loss-of-function conditions (Fig. 5D-G). The number of progenitor-like cells progressively decreased in all conditions, suggesting that some electroporated cells had not fully differentiated by P7 or P14 (Fig. 5G). This likely reflects a delay in these cells adopting clear photoreceptor versus bipolar cell morphologies (Fig. 5D). In particular, the PEVE and PCVC conditions had excess undifferentiated cells at the P7 time point compared with control and most of the single mutant conditions. This correlates with the early deficit and protracted accumulation of photoreceptors in PCVC and PEVE conditions. Taken together, our data suggest that cell fate changes mostly occur during the first postnatal week, but that some changes may take longer to manifest.
Birthdating shows an increase in late-born amacrines
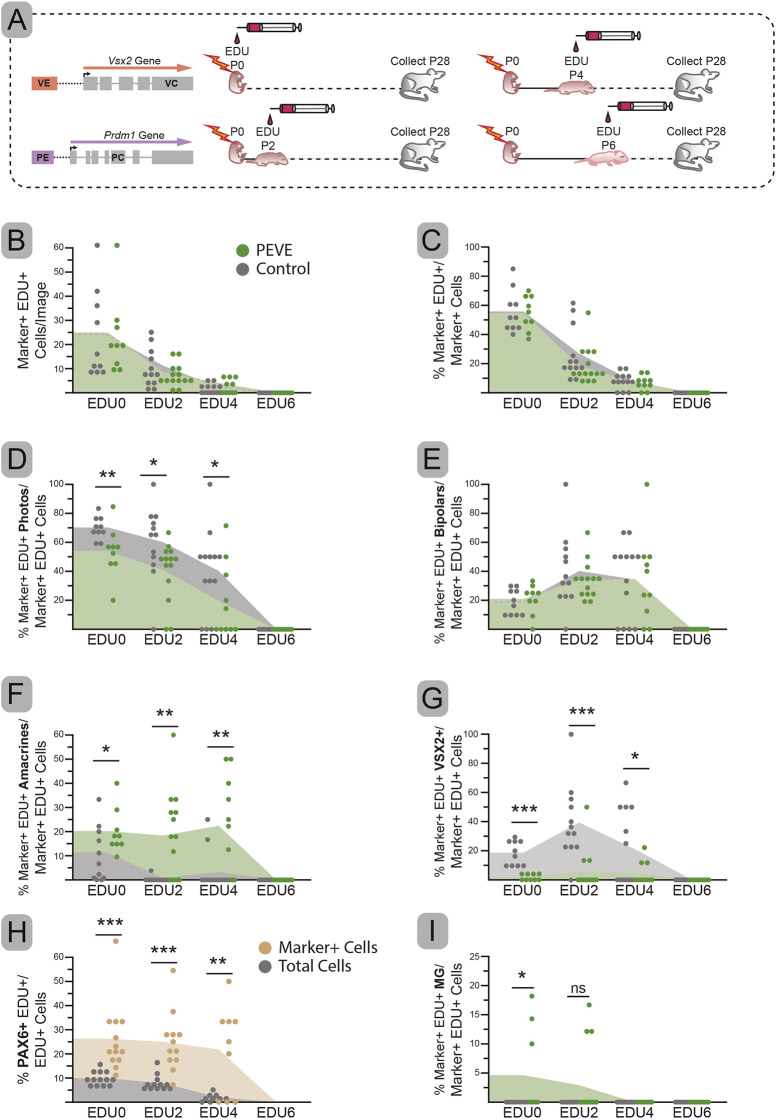
The double mutant conditions may perturb cell cycle exit timing and thus fate choice. To explore this possibility, we conducted an EdU birthdating experiment. Eight litters of P0 pups were electroporated with control or PEVE and subsequently received a single EdU pulse at either P0, P2, P4 or P6 (Fig. 6A). All mice were raised to P28 and examined by morphology or with cell type-specific markers to determine the fate of birthdated (EdU+) cells. The total number and percentage of electroporated cells marked by EdU did not vary between control and PEVE conditions (Fig. 6B,C). We concluded there were no major differences in cell cycle exit timing between PEVE and control. Nonetheless, there were differences in the fate of cells born at P0, P2 and P4 (Fig. 6, Fig. S5). Significantly fewer photoreceptors were born in PEVE compared with controls at P0 (P=0.008), P2 (P=0.025) and P4 (P=0.038) (Fig. 6D). The fraction of birthdated photoreceptors was reduced to a similar degree over time, suggesting that the fate shift was independent of cell cycle exit timing (Fig. 6D). In contrast, there was no difference at any time point in the number of bipolar cells born (Fig. 6E). The birthdating pattern of PEVE closely matched controls and the unchanged number of morphologically identified bipolars that we observed (Figs 4-5, 6E). However, when we stained for VSX2 we saw a significant decrease in birthdated VSX2+ cells in PEVE compared with controls at P0 (P<0.001), P2 (P<0.001) and P4 (P=0.005) (Fig. 6G, Fig. S5C). This paralleled the decrease in VSX2+ bipolars that we observed in PEVE conditions (Fig. 4H). There were slightly more Müller glia born in the PEVE condition at P0 (P=0.035), although this did not result in significantly more total glia and appeared to be caused by three outliers (Fig. 6I, Fig. S4G PEVE litter 1). Taken together, these data indicate that cell cycle exit timing is not contributing to the differences seen in photoreceptor and bipolar cell numbers in PEVE.
Fig. 6.
Birthdating of control and PEVE electroporated cells. (A) Schematic of experimental design. (B-I) The total number (B) and the percentage (C) of electroporated cells that are also EdU+ over time. Percentage of electroporated and EdU+ cells with photoreceptor (D), bipolar (E), amacrine (F) or Müller glial (MG) (I) morphologies. Percentage of electroporated and EdU+ cells that co-express VSX2 (G). Percentage of electroporated and EdU+ cells that express PAX6 compared with the percentage of all EdU+ cells that expressed PAX6 in the same image (H). Dots represent specific quantified images; top of the shaded area represents the mean. N for EDU0: control=6, PEVE=6; for EDU2: control=7, PEVE=6; for EDU4: control=7, PEVE=6; and for EDU6: control=5, PEVE=7. Significance determined by one-tailed unpaired t-test for all except panel H, which used a one-tailed paired t-test. *P<0.05, **P<0.01, ***P<0.001. ns, not significant.
Next, we examined amacrine cell birthdates. We observed an increase in birthdated amacrines in PEVE compared with controls at P0 (P=0.048), P2 (P=0.004) and P4 (P=0.004) (Fig. 6F). This increase matched the loss of photoreceptors at each time point (Fig. 6D). Unlike photoreceptors (Fig. 6D), the birthdating profiles of control and PEVE amacrine cells were not parallel, with PEVE amacrine births extending beyond their normal window. We next asked whether the percentage of electroporated and birthdated PAX6+ amacrines exceeded the total percentage of all birthdated amacrines in PEVE. Indeed, there were far more electroporated and birthdated amacrines (Marker+, PAX6+, EDU+) at P0 (P<0.001, pairwise t-test), P2 (P<0.001) and P4 (P=0.005) compared with the percentage of all birthdated (EDU+ PAX6+) cells in those same images (Fig. 6H, Fig. S5D). Together, these data reveal that many of the increased amacrines in PEVE conditions are born later than normal, likely at the direct expense of rods.
Bipolar fate is selected before VE activation and does not require VSX2 for maintenance
Previous loss-of-function work (Burmeister et al., 1996; Green et al., 2003; Phillips et al., 2014) and our own study have shown that VSX2 is necessary for bipolar formation, leading to the conclusion that bipolar-specific VSX2 is a fate-determining regulatory element. Yet, our PEVE data suggest that, in the absence of PRDM1, bipolar-specific VSX2 is not necessary to form or maintain bipolar cell identity. We wondered whether VE activity is in fact downstream of bipolar cell fate choice and that this outcome has been obscured by the effects of PRDM1.
To test this, we designed a system to compare the timing of bipolar fate choice with the expression of the Vsx2 enhancer. We built a plasmid containing the Vsx2 bipolar-specific enhancer driving Cre recombinase (VE-CRE) to fate map newly formed bipolar cells (Fig. 7A). We co-electroporated this construct along with control or VC-Cas9-GFP targeting plasmids into newborn ROSA-RFP reporter mice (Madisen et al., 2010). In this system, control and VC targeted cells will be GFP+, but only cells that activated the Vsx2 enhancer will become permanently RFP+ (Fig. 7). Mice were examined at P7 (VC and control) and P28 (VC) for RFP, GFP and VSX2 expression (Fig. 7B,I). As the control and VC plasmids are expressed under the ubiquitous EF1α enhancer, there were more GFP+ cells in both conditions than RFP+ cells (Fig. 7B). We saw no difference in the percentage of total GFP+ cells that co-expressed RFP between control and VC conditions at P7 (P=0.162) nor between VC conditions at P7 and P28 (P=0.168) (Fig. 7C). RFP+ cells in control animals overwhelmingly adopted bipolar cell morphology, although about one in seven cells showed rod photoreceptor morphology (Fig. 7B). This is consistent with VE becoming activated in cells that are poised between photoreceptor and bipolar fates, with most adopting a bipolar identity. We then examined the VC condition to determine whether there was a fate change in the VE lineage. If VSX2 acts only after the onset of VE activity, we expected to see an increase in the fraction of lineage-traced (RFP+) cells that become photoreceptors at the expense of bipolars. However, we observed the opposite in VC conditions. An even higher fraction of the VC RFP+ cells had bipolar morphology (P=0.017) (Fig. 7D), which came at the direct expense of cells with photoreceptor morphology in the outer nuclear layer (ONL) (P=0.017) (Fig. 7E). The number of cells that co-stained with GFP, RFP and VSX2 were significantly reduced in the VC condition compared with control at both P7 and P28 (P<0.001, both) (Fig. 7F). The reduction in VSX2 co-expression in VC was similar at P7 and P28 (P=0.156) (Fig. 7F). Together, these data suggest that VE lineage-traced cells (RFP+) overwhelmingly adopt bipolar fate despite loss of VSX2 expression, and that their fate choice is stably adopted by P7. This sharply contrasted what happened to the overall VC electroporated (GFP+) population. At P28, we observed that ∼75% of VC (GFP+) cells were photoreceptors located in the ONL compared with only ∼7% of the RFP+ cells (P<0.001) (Fig. 7G,H). There was a smaller fraction of GFP+ bipolar cells and many failed to co-express VSX2 compared with the RFP+ cells within the same image (P<0.001 both) (Fig. 7B,I,J).
Fig. 7.
VSX2 enhancer lineage tracing in control and Vsx2 targeted cells. (A) Schematic of the experimental design. (B) Immunohistochemistry showing RFP+ (lineage traced) and GFP+ (control or VC-Cas9-GFP) electroporated cells with VSX2 at P7 and P28. VE-CRE driven RFP has low efficiency, resulting in small numbers of cells and elevated variance. (C) Percentage of GFP+ cells that are RFP+. (D,E) RFP+ cells within the INL (D) and ONL (E) as a percentage of total RFP+ cells. (F) Percentage of cells that co-express RFP, GFP and VSX2 as a percentage of total RFP+ cells. (G-J) Comparison of the fates of GFP+ and RFP+ cells as a percentage of their respective total cells in P28 VC+VE-CRE animals. The RFP+ population at P28 is distinct from the GFP population in the percentage of cells (photoreceptors) in the ONL (G), in the INL (H), that co-express VSX2 (I), and that have bipolar morphology (J). (K) Schematic showing that VE enhancer activation can occur after the decision to become a bipolar in VC-targeted cells. Error bars represent s.d. Significance for C-F determined using unpaired t-tests and for G-J using a paired t-test. *P<0.05, **P<0.01, ***P<0.001. Insets show magnification of boxed areas. Dashed lines indicate layer boundaries. Notched arrowheads, VSX2+ cells; arrowheads, VSX2− cells. ns, not significant. Scale bars: 50 µm; 25 µm in insets.
The loss of VSX2 via VC targeting caused a shift in cell fate from bipolars to photoreceptors. Despite this shift, cells that expressed VE-CRE-driven RFP were more likely to remain bipolar cells than their control counterparts. Targeting VC can alter the fate of OTX2+ cells before VE activation, suggesting that VE expression is downstream of the fate-choice to become a bipolar cell (Fig. 7K). in addition, nearly all of the GFP+ cells and most of the RFP+ cells lacked VSX2 at both P7 and P28 (Fig. 7F,I). This, along with our PEVE findings (Fig. 4, Fig. S2), suggest that VSX2 is not required for bipolar cell maintenance.
DISCUSSION
OTX2 activates the expression of two downstream transcription factors (Prdm1 and Vsx2) to influence whether cells adopt bipolar or photoreceptor fates (Fig. 8A). Lineage tracing experiments of the Prdm1 (PE) and Vsx2 bipolar (VE) enhancers show that each can give rise to bipolars and photoreceptors (Kim et al., 2008; Wang et al., 2014; Mills et al., 2017). This indicates that OTX2+ cells are poised between fates and that Prdm1 and Vsx2 compete to regulate the choice. To investigate how these genes interact, we used a CRISPR/Cas9 approach to simultaneously remove Prdm1 and Vsx2 activity from the retina. Our results suggest that: (1) the PE and VE elements are necessary in the retina; (2) photoreceptors are a default outcome in OTX2+ cells; (3) there is a critical period during which VSX2 is needed to drive bipolar fate choice; (4) bipolar fate maintenance does not require VSX2; and (5) PRDM1 and VSX2 act redundantly to suppress amacrine formation. Our findings also provide a framework for understanding how the deletion of necessary enhancers and their target genes can cause divergent results.
Fig. 8.
Summary of results from single and double targeting. (A) Under control conditions, progenitors express VSX2 and activate OTX2 as they exit the cell cycle. These OTX2+ cells generate both photoreceptors and bipolars. The presence of PRDM1 and bipolar-specific VSX2 control the numbers of each cell that forms. VSX2 made by progenitors is controlled by unknown regulatory elements. (B) OTX2 drives PRDM1 and VSX2 expression, leading to photoreceptor and bipolar fates, respectively. (C) PC leads to a major loss of photoreceptors and VSX2 is able to drive excess bipolars. (D) PE leads to a more modest loss of photoreceptors and an increase in bipolars. (E) OTX2+ cells without PRDM1 shift to adopt bipolar fates. (F,G) VC (F) and VE (G) cause a loss of bipolars, which may instead become rods. (H) OTX2+ cells are unable to adopt bipolar fate without VSX2 and may adopt rod fate because PRDM1 is intact. (I) PCVC blocks VSX2 and PRDM1 expression. Without VSX2 carry-over from progenitors, OTX2+ cells can adopt photoreceptor identity. Some cells become amacrines and lose OTX2 expression. (J) PEVE-targeted cells retain progenitor-derived VSX2, resulting in a preference for bipolars in the absence of PRDM1. (K) In the absence of both VSX2 and PRDM1, some cells lose OTX2 expression and adopt amacrine fates. Thus, Vsx2 and Prdm1 may redundantly suppress amacrine competence in OTX2+ cells.
Prdm1 and Vsx2 enhancers are necessary for gene expression
OTX2 is necessary, but not sufficient for the activity of both the Prdm1 and Vsx2 enhancers (Kim et al., 2008; Brzezinski et al., 2013; Wang et al., 2014; Mills et al., 2017). Targeting the Prdm1 enhancer was equally effective at reducing protein expression compared with targeting its coding sequence (Fig. 1). Targeting PE and PC each caused the same type of cell fate changes, indicating that the Prdm1 enhancer is necessary for PRDM1 expression (Figs 2 and 8A-D). Our results parallel other work that explored the necessity of the PE sequence indirectly (Wang et al., 2014). Despite affecting protein expression equally during development, targeting PE and PC did not result in the same degree of fate changes between photoreceptors and bipolars at P28 (Figs 2 and 8C-E). It is unclear why this was the case. One possibility that accounts for the milder phenotype in the PE condition is that targeting PE may result in slightly later timing of PRDM1 loss, allowing more cells to develop normally.
Targeting the VE sequence significantly reduced VSX2 protein and matched targeting the coding region. Correspondingly, there was no difference in the effect on cell fate between VE and VC conditions (Figs 1,2). In contrast to VC, targeting VE did not affect VSX2 in embryonic progenitors (Fig. S1). Taken together, this suggests that the VE region is necessary for VSX2 expression in bipolars (Fig. 8F-H). This is in line with a recent report in which a large (∼32 kb) upstream region of Vsx2 (including VE) was deleted, resulting in mice that lacked bipolar cells (Norrie et al., 2019). Here, we show that targeting just the 164 bp VE sequence was sufficient to prevent bipolar formation (Fig. 2D).
Photoreceptors are the default outcome in OTX2+ cells
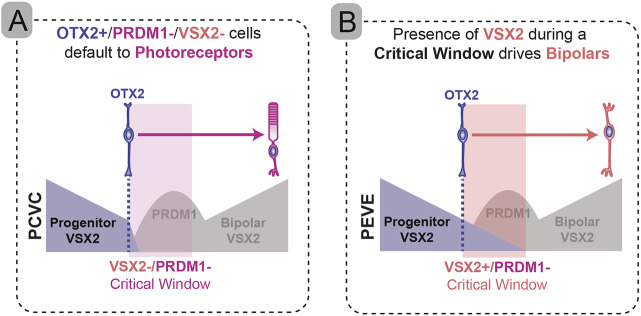
Targeting Prdm1 or Vsx2 resulted in changes in the numbers of photoreceptors and bipolar cells (Fig. 8). Targeting the coding regions of Prdm1 and Vsx2 simultaneously resulted in retinas that had reduced numbers of bipolar cells (Figs 4 and 8I). One possibility is that Vsx2 acts to repress Prdm1 and photoreceptor instructive signals. In this situation, double mutant cells might be expected to form some bipolar cells. Another possibility is that Vsx2 acts instructively in OTX2+ cells to promote bipolar formation. In this situation, double mutant cells would not be expected to form bipolar cells. We observed that the percentage of bipolars formed in PCVC electroporations matched the percentage of cells that failed to lose VSX2 expression (Fig. 4C,I). We almost never observed VSX2− bipolars in PCVC, unlike what was seen in the PEVE condition. This strongly suggests that the morphological bipolars seen in the PCVC condition were predominantly cells that escaped Vsx2 targeting. This indicates that OTX2+ cells that lacked both PRDM1 and VSX2 overwhelmingly adopted photoreceptor fates. Together, these data indicate that Vsx2 is acting instructively and that photoreceptors are a default outcome in postnatal OTX2+ cells (Fig. 9A). This aligns with our findings and other work showing that bipolar cells only form if VSX2 is present (Burmeister et al., 1996; Green et al., 2003; Norrie et al., 2019). Factors beyond Vsx2 and Prdm1 must contribute to the specification of OTX2+ cells (Fig. 1A). The identity of these factors and how they work remains to be uncovered.
Fig. 9.
Summary of changes in OTX2+ cell fates. (A) Loss of PRDM1 and VSX2 leads to a photoreceptor default state in OTX2+ cells. (B) The presence of VSX2 in a critical period when PRDM1 is absent can instruct bipolar fate.
VSX2 is not required for bipolar cell maintenance
Loss-of-function experiments argue that VSX2 is necessary to form bipolar cells. Throughout our study, the bipolars that formed in PCVC, VC and VE conditions closely aligned with the number of VSX2+ cells. We concluded that the bipolars observed in these conditions were a function of the escape rate within our CRISPR/Cas9 technique and not representative of what happens in the absence of VSX2. In contrast, many bipolars in the PEVE conditions lacked VSX2, yet robustly displayed bipolar morphology (Fig. 4), bipolar subtype markers (Fig. S2) and were born in a similar pattern (Fig. 6). These data argue that at some point soon after an OTX2+ cell has decided to become a bipolar, it does not need VSX2 to maintain its identity.
Under adult conditions, most or all bipolars express VSX2 (Shekhar et al., 2016). If VSX2 is not needed for maintenance, why do adult bipolar cells express VSX2? When we quantified the subtype fates of the PEVE bipolars, we observed that ON type bipolar cell markers were comparable with controls, whereas secretagogin-labeled cone bipolars (types 2-6, 8) (Puthussery et al., 2010) were strongly reduced. This suggests that the formation or maintenance of some cone bipolar subtypes requires VE-mediated VSX2 expression. This may be because of complex negative interactions between VSX2 and its paralog VSX1, which is needed for the proper formation of cone bipolars (Chow et al., 2004; Ohtoshi et al., 2004; Clark et al., 2008; Shi et al., 2011; Shi et al., 2012). Another possibility is that VSX2 is used to maintain the physiological functions of mature bipolar cells, which we did not investigate here.
VSX2 affects bipolar fate choice over a critical window of time
We were surprised to discover that simultaneously targeting Prdm1 and Vsx2 coding regions did not yield the same result as targeting their enhancers. In contrast to PCVC electroporations, PEVE cells were more likely to be bipolars and less likely to be rod photoreceptors (Fig. 8J). We reasoned that the most probable explanation for this difference is that there was some VSX2 present in the electroporated cells. As targeting VE has no effect on VSX2 made in progenitors (Fig. S1), it is possible that some VSX2 is carried over as cells become postmitotic (Fig. 8J). This residual VSX2 expression could drive bipolar fate, but only in the absence of PRDM1 because of its ability to suppress bipolar formation in OTX2+ cells (Figs 8 and 9B). This would not be the case in PCVC conditions, in which VSX2 is eliminated in progenitors before it has a chance to carry-over into OTX2+ cells and affect fate (Fig. 8I). The decrease in photoreceptors in the PEVE condition is likely explained by the ability of progenitor-derived VSX2 to promote bipolar fate at the expense of rods. Further support of this carry-over model (Figs 8 and 9B) was found when we traced the Vsx2 enhancer lineage in control and Vsx2-coding mutant cells. The VE lineage did not convert from bipolars into photoreceptors when VSX2 was targeted. Nonetheless, the fate of VC-targeted cells was strongly biased against forming bipolar cells. This suggests that the choice between photoreceptor and bipolar fates occurs before VE is activated (Fig. 7K). The small number of cells that got far enough to activate the VE lineage reporter were more likely to co-express VSX2 protein. This raises the possibility that partial or delayed loss of VSX2 allowed a subset of OTX2+ cells to adopt bipolar fate and activate VE. Most of these VE lineage bipolars lacked VSX2 in the adult retina, suggesting that VSX2 is not necessary for bipolar cell maintenance. These lineage findings suggest that transient VSX2 expression is sufficient to rescue bipolar cell formation (Figs 8 and 9B). If carry-over of VSX2 is sufficient to drive bipolar formation, why are there photoreceptors in the PEVE condition? This could occur if only a subset of OTX2+ cells carry over sufficient VSX2 to rescue bipolar fate. Alternatively, targeting PE may not remove PRDM1 quickly or completely enough to prevent photoreceptor formation.
In the absence of PRDM1, progenitor VSX2 carry-over appears sufficient to rescue bipolar cell formation (Fig. 9). However, VC and VE single targeting equivalently reduced bipolar cell formation (Fig. 2), and mice lacking a 32 kb region of DNA upstream of Vsx2 completely lack bipolars (Norrie et al., 2019). This shows that VSX2 expression driven by VE is essential for bipolar fate choice when PRDM1 is present. VSX2 is not required for the maintenance of bipolar cells as a class. Taken together, these data argue that there is a transient period in which VSX2 is required for bipolar cell formation. This critical period spans a relatively narrow window before and after the onset of VE activity. The mechanisms that VSX2 uses during this window and its interactions with PRDM1 and other factors remain to be determined.
Prdm1 and Vsx2 combine to suppress amacrine formation
As Prdm1 and Vsx2 are downstream of Otx2, we expected that mutating these genes would simply alter the proportion of photoreceptors and bipolar cells that were formed. Indeed, mutating Vsx2 or Prdm1 on their own changed the numbers of OTX2+ cell types that formed. We were surprised to see a proportional increase in amacrine cells when we perturbed Prdm1 and Vsx2 (PEVE and PCVC) simultaneously. This mimics what is seen in Otx2 null retinas, which form excess amacrine cells at the expense of photoreceptors and bipolar cells (Nishida et al., 2003; Sato et al., 2007).
OTX2+ cells have the competence to become photoreceptors and bipolar cells, but some of these cells likely give rise to OTX2− amacrines and horizontals (Brzezinski et al., 2013; Brzezinski and Reh, 2015; Mills et al., 2017; Goodson et al., 2018) (Fig. 1A). We observed that single mutants of Prdm1 or Vsx2 did not alter the number of amacrine cells formed. Thus, it seems unlikely that Prdm1 or Vsx2 are necessary for restricting amacrine competence in OTX2+ cells. As double mutants have excess amacrine cells that are born outside of their normal temporal window, it is possible that Prdm1 and Vsx2 act redundantly to restrict amacrine competence in OTX2+ cells (Fig. 8K). It is unclear why only a subset of these double mutant cells shifted to amacrine fate. One possibility is that there are other factors beyond Prdm1 and Vsx2 that contribute to inhibiting amacrine formation. Another possibility is that signals promoting amacrine identity are limiting in the postnatal retinal environment. Future experiments with Prdm1 and Vsx2 double enhancer mutant mice will help decipher how competence is regulated in the OTX2+ cell population.
MATERIALS AND METHODS
Mice
All animals were used in accordance with procedures approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee. P0 pups were from timed-pregnant wild-type (CD-1) mice that were obtained from Charles River Laboratories. ROSA-RFP mice (Madisen et al., 2010) were obtained from The Jackson Laboratory (#007914). All electroporated pups were housed with their mother and siblings until being collected for histology.
CRISPR
CRISPR constructs were designed based on a plasmid from Dr Feng Zhang's Lab; pSpCas9(BB)-2A-GFP (PX458) (Addgene plasmid #48138) (Ran et al., 2013). The original PX458 plasmid was modified by exchanging the CMV promoter for an EF1α promoter to drive Cas9. In some conditions, the GFP was replaced with the Cherry red fluorescent protein. Guide RNAs were designed using the CRISPR Guide Tool in Benchling (Benchling.com). Potential guides were sorted for their on-target (Doench et al., 2016) and off-target scores (Hsu et al., 2013). Three non-overlapping guides with the highest aggregate score were selected per condition (Table S1). Guides were inserted into the modified PX458 plasmid utilizing Golden Gate Assembly (Ran et al., 2013).
Electroporation
In vivo electroporations were performed on P0 pups following the methods described previously (de Melo and Blackshaw, 2011). Briefly, 0.5 µl of 2 µg/µl DNA in sterile H2O was injected subretinally using a Hamilton syringe. The mouse head was placed between a tweezer electrode (BTX) and electroporated with five 80 V square wave pulses for 50 ms with a 950 ms delay between each pulse using a Bio-Rad Gene Pulser Xcell.
Retina collection and immunohistochemistry
Both eyes were collected immediately after animal death and tissue collection followed our previous methods (Goodson et al., 2018). After fixing in 2% paraformaldehyde for 1-2 h at room temperature followed by a 10%, 20%, 30% sucrose gradient overnight, eyes were flash frozen in O.C.T. compound (Sakura Finetech) and cryosectioned at 12 µm (Microm HM 550, Thermo). Immunohistochemistry procedures were conducted as described previously (Brzezinski et al., 2010; Park et al., 2017; Goodson et al., 2018) using the following primary antibodies: sheep anti-CHX10 (VSX2) (1:400; X1179P, Exalpha Biologicals); mouse anti-EBF3 (1:400; H00253738-M05, Abnova); rabbit anti-GAD65/67 (1:400; AB1511, Millipore); mouse anti-GFP (1:1000; ab13970, Abcam); goat anti-GLYT1 (1:500; AB1770, Millipore); mouse anti-ISL1/2 (1:200, 39.4D5, Developmental Studies Hybridoma Bank); goat anti-OTX2 (1:250; BAF1979, Bio-Techne); rabbit anti-PAX6 (1:500; PRB-278P, Covance); chicken anti-RFP (1:100; 600-901-379, Rockland); mouse anti-RFP (1:1000; ab65856, Abcam); rabbit anti-secretagogin (1:2500; RD181120100, Biovendor); and goat anti-SOX2 (1:500; sc-17320, Santa Cruz Biotechnology). EdU staining was carried out according the manufacturer's instructions (Thermo Fisher Scientific) following immunostaining (Brzezinski et al., 2013).
Imaging and cell counts
Slides were imaged using a 20× objective on a Nikon C2 laser scanning confocal scope. Then 4-6 images 1.5 µm apart were collected as a z-stack, compressed using a maximum intensity stack compression in ImageJ (Schneider et al., 2012), and minimally processed in Adobe Photoshop. Cell counts were conducted manually. Dotplots were generated using ggplot in R (Wickham, 2016).
Images were taken from both male and female mice. Most images covered the central or mid-peripheral retina. Counts were conducted on sections of relatively intact retinas, and disrupted sections were excluded from quantification. We also excluded images with less than 10 electroporated cells. Retinas were imaged in 2-6 locations and counts pooled to calculate the mean and standard deviation. For all plots, error bars represent standard deviation. One-tailed unpaired t-tests with the assumption of heteroscedasticity were used for statistics in Figs 1, 3, 4, 6, 7, Figs S1-S3, S4. Paired t-tests were used in some instances and are specifically noted. The data in Fig. 2 and Fig. S4 were analyzed using a one-way ANOVA followed by Tukey post-hoc test. The data in Fig. 5 were analyzed using a one-way ANOVA followed by Dunnett's Multiple Comparison's test, comparing each group with their time-matched controls. The degrees of freedom were based on the number of animals or retinal explants examined. A P<0.05 was considered significant and was calculated using GraphPad Prism 8.
Supplementary Material
Acknowledgements
The authors thank Santos Franco and Natalia Vergara for advice and critical feedback on the manuscript.
Footnotes
Author contributions
Conceptualization: N.B.G., J.A.B.; Methodology: N.B.G., M.A.K., K.U.P.; Investigation: N.B.G., M.A.K., K.U.P., J.A.B.; Writing - original draft: N.B.G., J.A.B.; Writing - review & editing: N.B.G., M.A.K., J.A.B.; Supervision: J.A.B.; Funding acquisition: J.A.B.
Funding
Work was supported in part by grants from the National Institutes of Health R01-EY024272 (J.A.B.), T32-NS099042 (N.B.G.), and by National Institutes of Health/The National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award Grant Number TL1 TR002533 (N.B.G.). Work was partially supported by a Challenge Grant to the CU Department of Ophthalmology from Research to Prevent Blindness.
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.190272.reviewer-comments.pdf
References
- Bassett, E. A. and Wallace, V. A. (2012). Cell fate determination in the vertebrate retina. Trends Neurosci. 35, 565-573. 10.1016/j.tins.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Beby, F. and Lamonerie, T. (2013). The homeobox gene Otx2 in development and disease. Exp. Eye Res. 111, 9-16. 10.1016/j.exer.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Boije, H., Macdonald, R. B. and Harris, W. A. (2014). Reconciling competence and transcriptional hierarchies with stochasticity in retinal lineages. Curr. Opin. Neurobiol. 27, 68-74. 10.1016/j.conb.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski, J. A. and Reh, T. A. (2010). Retinal histogenesis. In Encyclopedia of the Eye, 1st edn. (ed. D. A. Dartt, J. C. Besharse and R. Dana), pp. 73-80. Boston, MA: Elsevier. [Google Scholar]
- Brzezinski, J. A. and Reh, T. A. (2015). Photoreceptor cell fate specification in vertebrates. Development 142, 3263-3273. 10.1242/dev.127043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski, J. A., Lamba, D. A. and Reh, T. A. (2010). Blimp1 controls photoreceptor versus bipolar cell fate choice during retinal development. Development 137, 619-629. 10.1242/dev.043968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski, J. A., UOON Park, K. and Reh, T. A. (2013). Blimp1 (Prdm1) prevents re-specification of photoreceptors into retinal bipolar cells by restricting competence. Dev. Biol. 384, 194-204. 10.1016/j.ydbio.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister, M., Novak, J., Liang, M.-Y., Basu, S., Ploder, L., Hawes, N. L., Vidgen, D., Hoover, F., Goldman, D., Kalnins, V. I., et al. (1996). Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12, 376-384. 10.1038/ng0496-376 [DOI] [PubMed] [Google Scholar]
- Carter-Dawson, L. D. and Lavail, M. M. (1979). Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J. Comp. Neurol. 188, 263-272. 10.1002/cne.901880205 [DOI] [PubMed] [Google Scholar]
- Cepko, C. (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. Nat. Rev. Neurosci. 15, 615-627. 10.1038/nrn3767 [DOI] [PubMed] [Google Scholar]
- Cepko, C. L., Austin, C. P., Yang, X., Alexiades, M. and Ezzeddine, D. (1996). Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 93, 589-595. 10.1073/pnas.93.2.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry, T. J., Trimarchi, J. M., Stadler, M. B. and Cepko, C. L. (2009). Development and diversification of retinal amacrine interneurons at single cell resolution. Proc. Natl. Acad. Sci. USA 106, 9495-9500. 10.1073/pnas.0903264106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, R. L., Volgyi, B., Szilard, R. K., Ng, D., Mckerlie, C., Bloomfield, S. A., Birch, D. G. and Mcinnes, R. R. (2004). Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc. Natl. Acad. Sci. USA 101, 1754-1759. 10.1073/pnas.0306520101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. M., Yun, S., Veien, E. S., Wu, Y. Y., Chow, R. L., Dorsky, R. I. and Levine, E. M. (2008). Negative regulation of Vsx1 by its paralog Chx10/Vsx2 is conserved in the vertebrate retina. Brain Res. 1192, 99-113. 10.1016/j.brainres.2007.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo, J. and Blackshaw, S. (2011). In vivo electroporation of developing mouse retina. J. Vis. Exp., e2847. 10.3791/2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo, J., Qiu, X., Du, G., Cristante, L. and Eisenstat, D. D. (2003). Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J. Comp. Neurol. 461, 187-204. 10.1002/cne.10674 [DOI] [PubMed] [Google Scholar]
- Doench, J. G., Fusi, N., Sullender, M., Hegde, M., Vaimberg, E. W., Donovan, K. F., Smith, I., Tothova, Z., Wilen, C., Orchard, R., et al. (2016). Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184-191. 10.1038/nbt.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval, K. M., Bobechko, B. P., Fujieda, H., Chen, S., Zack, D. J. and Bremner, R. (2006). CHX10 targets a subset of photoreceptor genes. J. Biol. Chem. 281, 744-751. 10.1074/jbc.M509470200 [DOI] [PubMed] [Google Scholar]
- Elshatory, Y., Deng, M., Xie, X. and Gan, L. (2007a). Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J. Comp. Neurol. 503, 182-197. 10.1002/cne.21390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory, Y., Everhart, D., Deng, M., Xie, X., Barlow, R. B. and Gan, L. (2007b). Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 27, 12707-12720. 10.1523/JNEUROSCI.3951-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat, N., Le Greneur, C., Béby, F., Vincent, S., Godement, P., Chatelain, G. and Lamonerie, T. (2007). A new GFP-tagged line reveals unexpected Otx2 protein localization in retinal photoreceptors. BMC Dev. Biol. 7, 122. 10.1186/1471-213X-7-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson, N. B., Nahreini, J., Randazzo, G., Uruena, A., Johnson, J. E. and Brzezinski, J. A. T. (2018). Prdm13 is required for Ebf3+ amacrine cell formation in the retina. Dev. Biol. 434, 149-163. 10.1016/j.ydbio.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, E. S., Stubbs, J. L. and Levine, E. M. (2003). Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development 130, 539-552. 10.1242/dev.00275 [DOI] [PubMed] [Google Scholar]
- Hsu, P. D., Scott, D. A., Weinstein, J. A., Ran, F. A., Konermann, S., Agarwala, V., Li, Y., Fine, E. J., Wu, X., Shalem, O., et al. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827-832. 10.1038/nbt.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, K., Jiang, H., Mo, Z. and Xiang, M. (2010). Early B-cell factors are required for specifying multiple retinal cell types and subtypes from postmitotic precursors. J. Neurosci. 30, 11902-11916. 10.1523/JNEUROSCI.2187-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K., Omori, Y., Onishi, A., Sato, S., Kondo, M. and Furukawa, T. (2010). Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. J. Neurosci. 30, 6515-6526. 10.1523/JNEUROSCI.0771-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, J. N., Voinescu, P. E., Chu, M. W. and Sanes, J. R. (2011). Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat. Neurosci. 14, 965-972. 10.1038/nn.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. S., Matsuda, T. and Cepko, C. L. (2008). A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J. Neurosci. 28, 7748-7764. 10.1523/JNEUROSCI.0397-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey, F. J. and Cepko, C. L. (2001). Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2, 109-118. 10.1038/35053522 [DOI] [PubMed] [Google Scholar]
- Livne-Bar, I., Pacal, M., Cheung, M. C., Hankin, M., Trogadis, J., Chen, D., Dorval, K. M. and Bremner, R. (2006). Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc. Natl. Acad. Sci. USA 103, 4988-4993. 10.1073/pnas.0600083103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen, L., Zwingman, T. A., Sunkin, S. M., Oh, S. W., Zariwala, H. A., Gu, H., Ng, L. L., Palmiter, R. D., Hawrylycz, M. J., Jones, A. R., et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger, N., Pow, D. V. and Wässle, H. (1998). Glycinergic amacrine cells of the rat retina. J. Comp. Neurol. 401, 34-46. [DOI] [PubMed] [Google Scholar]
- Mills, T. S., Eliseeva, T., Bersie, S. M., Randazzo, G., Nahreini, J., Park, K. U. and Brzezinski, J. A.IV. (2017). Combinatorial regulation of a Blimp1 (Prdm1) enhancer in the mouse retina. PLoS ONE 12, e0176905. 10.1371/journal.pone.0176905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranishi, Y., Terada, K., Inoue, T., Katoh, K., Tsujii, T., Sanuki, R., Kurokawa, D., Aizawa, S., Tamaki, Y. and Furukawa, T. (2011). An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J. Neurosci. 31, 16792-16807. 10.1523/JNEUROSCI.3109-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, A., Furukawa, A., Koike, C., Tano, Y., Aizawa, S., Matsuo, I. and Furukawa, T. (2003). Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6, 1255-1263. 10.1038/nn1155 [DOI] [PubMed] [Google Scholar]
- Norrie, J. L., Lupo, M. S., Xu, B., AL Diri, I., Valentine, M., Putnam, D., Griffiths, L., Zhang, J., Johnson, D., Easton, J., et al. (2019). Nucleome dynamics during retinal development. Neuron 104, 512-528.e11. 10.1016/j.neuron.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtoshi, A., Wang, S. W., Maeda, H., Saszik, S. M., Frishman, L. J., Klein, W. H. and Behringer, R. R. (2004). Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr. Biol. 14, 530-536. 10.1016/j.cub.2004.02.027 [DOI] [PubMed] [Google Scholar]
- Park, K. U., Randazzo, G., Jones, K. L. and Brzezinski, J. A. T. (2017). Gsg1, Trnp1, and Tmem215 mark subpopulations of bipolar interneurons in the mouse retina. Invest. Ophthalmol. Vis. Sci. 58, 1137-1150. 10.1167/iovs.16-19767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. J., Perez, E. T., Martin, J. M., Reshel, S. T., Wallace, K. A., Capowski, E. E., Singh, R., Wright, L. S., Clark, E. M., Barney, P. M., et al. (2014). Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells 32, 1480-1492. 10.1002/stem.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow, D. V. and Hendrickson, A. E. (1999). Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis. Neurosci. 16, 231-239. 10.1017/S0952523899162047 [DOI] [PubMed] [Google Scholar]
- Puthussery, T., Gayet-Primo, J. and Taylor, W. R. (2010). Localization of the calcium-binding protein secretagogin in cone bipolar cells of the mammalian retina. J. Comp. Neurol. 518, 513-525. 10.1002/cne.22234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, F. A., Hsu, P. D., Wright, J., Agarwala, V., Scott, D. A. and Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281-2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport, D. H., Wong, L. L., Wood, E. D., Yasumura, D. and Lavail, M. M. (2004). Timing and topography of cell genesis in the rat retina. J. Comp. Neurol. 474, 304-324. 10.1002/cne.20134 [DOI] [PubMed] [Google Scholar]
- Reh, T. A. and Cagan, R. L. (1994). Intrinsic and extrinsic signals in the developing vertebrate and fly eyes: viewing vertebrate and invertebrate eyes in the same light. Perspect. Dev. Neurobiol. 2, 183-190. [PubMed] [Google Scholar]
- Sato, S., Inoue, T., Terada, K., Matsuo, I., Aizawa, S., Tano, Y., Fujikado, T. and Furukawa, T. (2007). Dkk3-Cre BAC transgenic mouse line: a tool for highly efficient gene deletion in retinal progenitor cells. Genesis 45, 502-507. 10.1002/dvg.20318 [DOI] [PubMed] [Google Scholar]
- Schneider, C. A., Rasband, W. S. and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar, K., Lapan, S. W., Whitney, I. E., Tran, N. M., Macosko, E. Z., Kowalczyk, M., Adiconis, X., Levin, J. Z., Nemesh, J., Goldman, M., et al. (2016). Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 166, 1308-1323.e30. 10.1016/j.cell.2016.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z., Trenholm, S., Zhu, M., Buddingh, S., Star, E. N., Awatramani, G. B. and Chow, R. L. (2011). Vsx1 regulates terminal differentiation of type 7 ON bipolar cells. J. Neurosci. 31, 13118-13127. 10.1523/JNEUROSCI.2331-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z., Jervis, D., Nickerson, P. E. B. and Chow, R. L. (2012). Requirement for the paired-like homeodomain transcription factor VSX1 in type 3a mouse retinal bipolar cell terminal differentiation. J. Comp. Neurol. 520, 117-129. 10.1002/cne.22697 [DOI] [PubMed] [Google Scholar]
- Sidman, R. L. (1961). Histogenesis of mouse retina studied with thymidine H3. In Structure of the Eye (ed. G. K. Smelser), pp. 487-506. New York: Academic Press. [Google Scholar]
- Sigulinsky, C. L., German, M. L., Leung, A. M., Clark, A. M., Yun, S. and Levine, E. M. (2015). Genetic chimeras reveal the autonomy requirements for Vsx2 in embryonic retinal progenitor cells. Neural Dev. 10, 12. 10.1186/s13064-015-0039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, D. L. and Cepko, C. L. (1987). A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131-136. 10.1038/328131a0 [DOI] [PubMed] [Google Scholar]
- Turner, D. L., Snyder, E. Y. and Cepko, C. L. (1990). Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4, 833-845. 10.1016/0896-6273(90)90136-4 [DOI] [PubMed] [Google Scholar]
- Voinescu, P. E., Kay, J. N. and Sanes, J. R. (2009). Birthdays of retinal amacrine cell subtypes are systematically related to their molecular identity and soma position. J. Comp. Neurol. 517, 737-750. 10.1002/cne.22200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Sengel, C., Emerson, M. M. and Cepko, C. L. (2014). A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell 30, 513-527. 10.1016/j.devcel.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Xiang, M. (2013). Intrinsic control of mammalian retinogenesis. Cell. Mol. Life Sci. 70, 2519-2532. 10.1007/s00018-012-1183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, H., Kon, T., Omori, Y. and Furukawa, T. (2020). Functional and evolutionary diversification of Otx2 and Crx in vertebrate retinal photoreceptor and bipolar cell development. Cell Rep. 30, 658-671.e5. 10.1016/j.celrep.2019.12.072 [DOI] [PubMed] [Google Scholar]
- Young, R. W. (1985). Cell differentiation in the retina of the mouse. Anat. Rec. 212, 199-205. 10.1002/ar.1092120215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.