Abstract
The phase III ASPEN study demonstrated the comparable efficacy and improved safety of zanubrutinib versus ibrutinib in patients with Waldenström macroglobulinemia (WM). Here, we report long-term follow-up outcomes from ASPEN. The primary end point was the sum of very good partial response (VGPR) + complete response (CR) rates; secondary and exploratory end points were also reported. Cohort 1 comprised 201 patients (myeloid differentiation primary response 88–mutant WM: 102 receiving zanubrutinib; 99 receiving ibrutinib); cohort 2 comprised 28 patients (myeloid differentiation primary response 88 wild-type WM: 28 zanubrutinib; 26 efficacy evaluable). At 44.4-month median follow-up, VGPR + CR rates were 36.3% with zanubrutinib versus 25.3% with ibrutinib in cohort 1 and 30.8% with one CR in cohort 2. In patients with CXC motif chemokine receptor 4 mutation, VGPR + CR rates were 21.2% with zanubrutinib versus 10.0% with ibrutinib (cohort 1). Median progression-free survival and overall survival were not reached. Any-grade adverse events (AEs) of diarrhea (34.7% v 22.8%), muscle spasms (28.6% v 11.9%), hypertension (25.5% v 14.9%), atrial fibrillation/flutter (23.5% v 7.9%), and pneumonia (18.4% v 5.0%) were more common with ibrutinib versus zanubrutinib; neutropenia (20.4% v 34.7%) was less common with ibrutinib versus zanubrutinib (cohort 1). Zanubrutinib was associated with lower risk of AE-related treatment discontinuation. Overall, these findings confirm the long-term response quality and tolerability associated with zanubrutinib.
Zanubrutinib continues to demonstrate meaningful efficacy and favorable safety in patients with WM.
INTRODUCTION
Zanubrutinib is a potent, selective next-generation covalent Bruton tyrosine kinase inhibitor approved in several countries for Waldenström macroglobulinemia (WM) in adults.1-4 Despite not meeting its primary end point at a median follow-up of 19.4 months in ASPEN, zanubrutinib demonstrated comparable efficacy and favorable safety compared with ibrutinib.5 With 2 years of additional follow-up in ASPEN, we present long-term efficacy and safety analyses.
METHODS
The open-label, phase III ASPEN study (ClinicalTrials.gov identifier: NCT03053440) compared ibrutinib versus zanubrutinib in patients with WM. Cohort 1 included patients with mutant myeloid differentiation primary response 88 (MYD88MUT) randomly assigned 1:1 to zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily; cohort 2 included patients with wild-type MYD88 (MYD88WT) who received zanubrutinib 160 mg twice a day.5 Study design, methods, and primary analysis results have been described.5,6 The ASPEN study was approved by the independent institutional review board or independent ethics committee at each study site and was conducted in accordance with applicable regulatory requirements, the principles of the Declaration of Helsinki, and Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent.
RESULTS
Patient Disposition and Characteristics
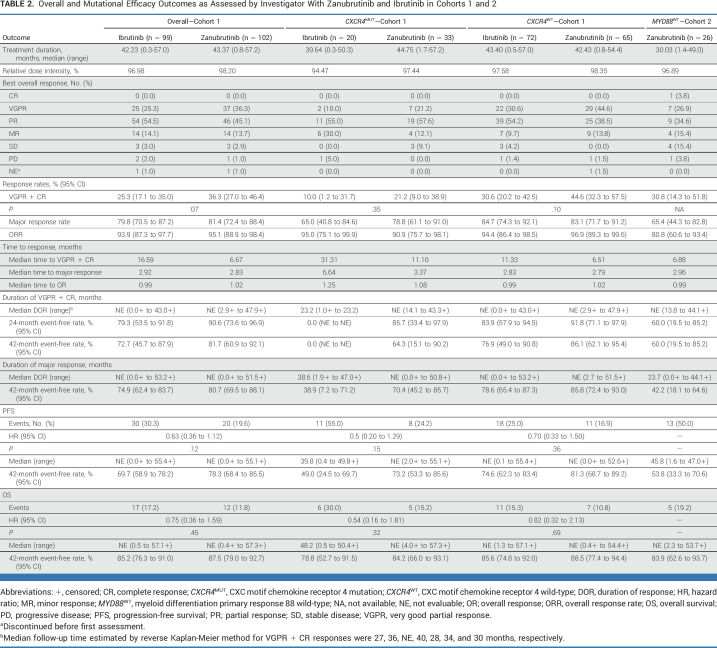
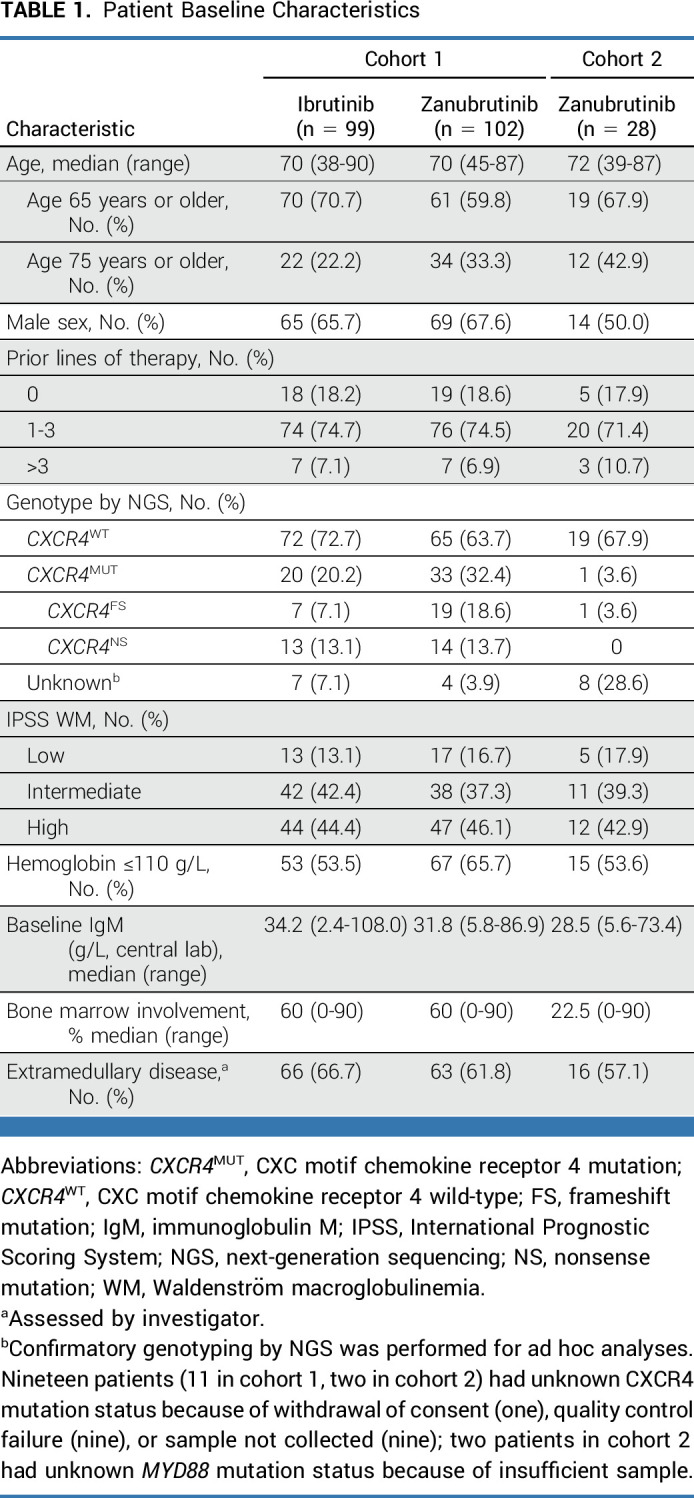
From January 2017 to July 2018, 201 patients with MYD88MUT WM were enrolled in cohort 1 (102 receiving zanubrutinib; 99 receiving ibrutinib); 28 patients were enrolled in cohort 2 (26 MYD88WT; two unknown). More patients randomly assigned to zanubrutinib than ibrutinib were older than 75 years (33.3% v 22.2%, respectively; P = .084) and had CXC motif chemokine receptor 4 mutation (CXCR4MUT) disease (32.4% v 20.2%, respectively; Table 1; P = .073). At a median follow-up of 44.4 months (range, 0.4-57.3), 65.7% of patients on zanubrutinib and 51.5% on ibrutinib remained on treatment (cohort 1). At a median follow-up of 42.9 months (range, 2.3-53.7), 35.7% of patients remained on zanubrutinib (cohort 2; Data Supplement, Fig 1 [online only]).
TABLE 1.
Patient Baseline Characteristics
Efficacy
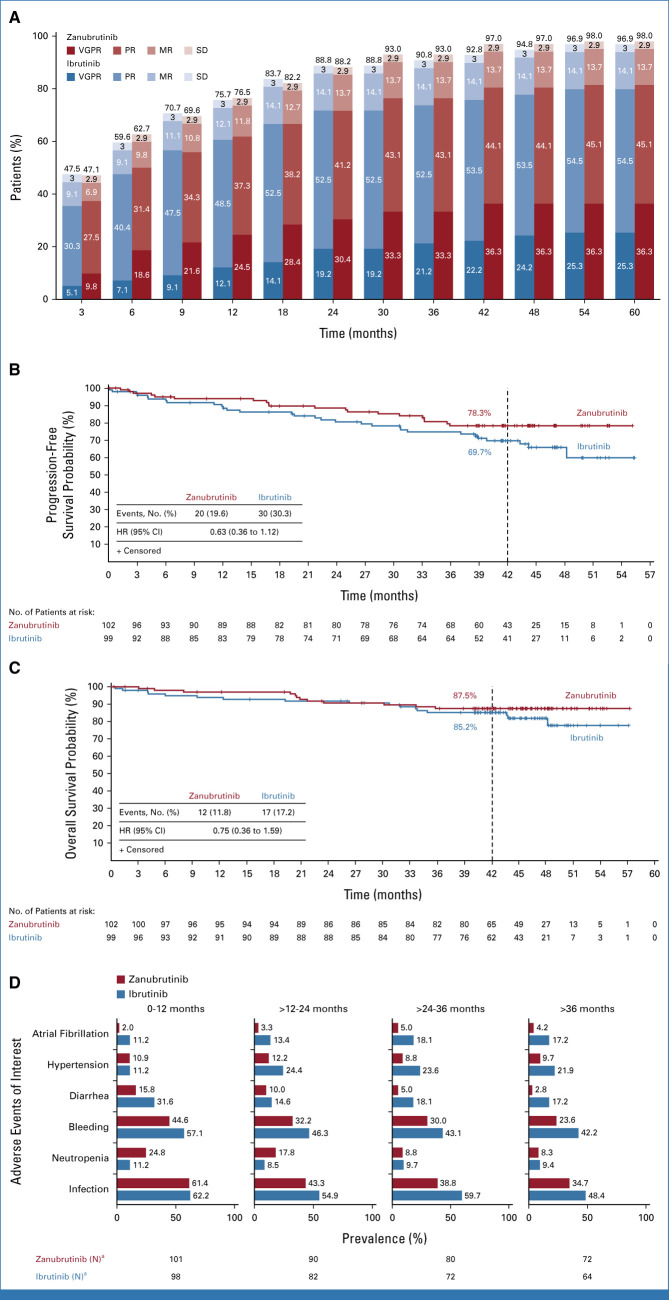
Very good partial response (VGPR) rates increased over time and were numerically higher with zanubrutinib than ibrutinib at all time points (Fig 1A). The median time to VGPR was faster for patients on zanubrutinib (6.7 months) versus ibrutinib (16.6 months); the median time to overall (minor response or better) or major (partial response or better) responses were similar between arms. Median durations of response were not reached (Table 2).
FIG 1.
(A) Best overall response rates over time as assessed by investigator, (B) progression-free survival, and (C) overall survival in the intent-to-treat patients (99 receiving ibrutinib; 102 receiving zanubrutinib at each time point) and (D) prevalence analysis for adverse events of interest from 0 to >36 months (cohort 1). Data cutoff: October 31, 2021. aN is the number of patients who are on treatment in each time interval or who discontinued treatment. The time from first dose date to the earliest date (last dose date + 30 days, initiation of new anticancer therapy, end of study, death or cutoff date) is within the time interval. The prevalence of each interval is the No. of patients with a new or ongoing event during the interval, shown as % of N. HR, hazard ratio; MR, minor response; PR, partial response; SD, stable disease; VGPR, very good partial response.
TABLE 2.
Overall and Mutational Efficacy Outcomes as Assessed by Investigator With Zanubrutinib and Ibrutinib in Cohorts 1 and 2
In patients with CXCR4MUT, higher major response rates and faster median time to response were observed with zanubrutinib versus ibrutinib (Table 2). Regardless of CXCR4 mutational status or mutation type (nonsense v frameshift), VGPR + complete response (CR) rates were numerically higher for zanubrutinib versus ibrutinib.7 In patients with baseline extramedullary disease, the VGPR + CR rate difference was 18.8% (95% CI, 2.4 to 35.1) favoring zanubrutinib, consistent with the greater median reduction observed in lymphadenopathy (65.9% v 52.5%) and splenomegaly (20.0% v 15.0%) for zanubrutinib versus ibrutinib, respectively. VGPR + CR rates were 36.8% versus 22.2% in patients on zanubrutinib versus ibrutinib, respectively, with zero lines of prior therapy; 36.8% versus 25.7% with one to three lines of prior therapy; 28.6% versus 28.6% with greater than three lines of prior therapy. One CR was reported (cohort 2); the VGPR + CR rate was 30.8% and the major response rate was 65.4% in 26 patients with confirmed MYD88WT WM.
Fewer progression-free survival (PFS; hazard ratio [HR], 0.63 [95% CI, 0.36 to 1.12]) and overall survival (OS; HR, 0.75 [95% CI, 0.36 to 1.59]) events were observed on zanubrutinib (cohort 1); median PFS or OS were not reached in the intent-to-treat population (Table 2; Figs 1B and 1C). In patients with CXCR4MUT WM on ibrutinib, the median PFS was 39.8 months (Data Supplement, Figs 2 and 3). In cohort 2, 42-month event-free rates for PFS were lower than cohort 1; OS was comparable between cohorts (87.5% v 83.9%; Table 2; Data Supplement [Fig 4]).
Long-Term Safety
Most common reasons for discontinuing treatment were AEs (cohort 1: nine zanubrutinib, 20 ibrutinib; cohort 2: six) and disease progression (cohort 1: 14 zanubrutinib, 13 ibrutinib; cohort 2: eight; Data Supplement [Fig 1]). Median treatment duration and relative dose intensities were similar between arms (cohort 1).
Any-grade AEs of diarrhea, muscle spasms, hypertension, atrial fibrillation/flutter, and pneumonia were more common with ibrutinib versus zanubrutinib; neutropenia was less common with ibrutinib versus zanubrutinib (cohort 1; Data Supplement, Tables 1 and 2). Incidences of AEs observed with zanubrutinib were similar between cohorts (Data Supplement, Table 3). More patients on ibrutinib experienced cardiovascular AEs, including one incidence of ventricular arrhythmia (Data Supplement, Table 4).
Except for neutropenia, prevalence of AEs of interest (Data Supplement, Table 5) were lower with zanubrutinib than ibrutinib at all time points (Fig 1D). Exposure-adjusted incidences of atrial fibrillation/flutter, hypertension, and diarrhea were significantly lower with zanubrutinib versus ibrutinib, respectively (descriptive P < .05; Data Supplement [Fig 5]). With zanubrutinib, the prevalence of neutropenia and infection decreased over time. By >36 months of treatment, the prevalence of infection was lower in patients receiving zanubrutinib than ibrutinib; the prevalence of neutropenia was similar between arms (Fig 1D).
More patients on ibrutinib than zanubrutinib required dose reductions because of AEs (cohort 1; Data Supplement [Table 3]). Treatment-emergent AEs led to discontinuation in 20 (20.4%) patients on ibrutinib versus nine (8.9%) on zanubrutinib. Most common AEs leading to discontinuation with ibrutinib were cardiac disorders and infections and infestations, with zanubrutinib as second malignancy (Data Supplement, Table 3). Higher risk of treatment discontinuation because of AEs (P < .05) and initiation of next treatment (P = .0977) was observed for ibrutinib versus zanubrutinib (Data Supplement, Figs 6 and 7).
Eight AE-related deaths occurred in cohort 1 (five ibrutinib; three zanubrutinib); three AE-related deaths occurred in cohort 2 (Data Supplement, Table 3).
DISCUSSION
In ASPEN, zanubrutinib demonstrated meaningful efficacy by consistently exhibiting high-quality responses and favorable safety across 2 years of additional follow-up. High VGPR + CR rates observed with zanubrutinib across mutational groups also reflect a clinical benefit because achieving immunoglobulin M (IgM) reduction of >90% is associated with less IgM-related morbidity.
In other studies, no patients with MYD88WT WM achieved a major response with ibrutinib or a VGPR/CR with acalabrutinib.8,9 In the ASPEN study, 31% of patients with MYD88WT WM achieved a VGPR/CR with zanubrutinib, including one CR, after 44-month follow-up. Furthermore, PFS and OS in patients with MYD88WT WM in our study were compared favorably with those receiving ibrutinib ± rituximab treatment in other studies, although all were limited by small sample size, and cross trial comparison was not possible.10,11 Our findings support zanubrutinib as the preferred treatment for patients with MYD88WT WM.
Zanubrutinib exhibited fewer side effects associated with off-target binding, especially cardiovascular toxicities. With zanubrutinib, no cases of ventricular arrhythmia were observed; neutropenia occurred early and was neither treatment-limiting nor associated with a higher infection rate. Zanubrutinib was associated with longer treatment duration and lower risk of dose reduction or discontinuation because of AEs.12 Patients previously intolerant to ibrutinib or acalabrutinib did not experience a recurrence of treatment-related AEs with zanubrutinib.12
Study limitations include an open-label design, unknown CXCR4 mutational status, and more patients with CXCR4 mutations randomly assigned to zanubrutinib versus ibrutinib (cohort 1), all of which may have influenced the VGPR + CR rates observed. VGPR + CR rate was chosen as the primary end point for this study because of the prolonged responses and infrequent PFS/OS events expected and because response rates and depth of response are associated with PFS and time to next treatment in patients with WM.13-15 Although potential false negatives may have occurred because of assay sensitivity or lower bone marrow disease involvement in patients with MYD88WT WM, the assay was sufficient for detection congruent with expected mutation rates.16 Potential associations between CXCR4 nonsense versus frameshift mutations and treatment outcomes were evaluated (manuscript in preparation).
Extended follow-up results confirm improved long-term safety and tolerability of zanubrutinib compared with ibrutinib and support deeper, earlier, and more durable responses in patients with WM regardless of previous treatment or CXCR4 and MYD88 mutational statuses.
ACKNOWLEDGMENT
We thank study patients, their supporters, and investigators and clinical research staff at study centers. Medical writing and editorial assistance were provided, under the direction of the authors, by Laura S. Moye, PhD and Elizabeth G. Wheatley, PhD of Bio Connections, LLC, (Chicago, IL) supported by BeiGene.
Meletios A. Dimopoulos
Honoraria: Amgen, Takeda, Janssen-Cilag, Bristol Myers Squibb, BeiGene, Sanofi
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb, BeiGene, Sanofi
Stephen Opat
Honoraria: AbbVie, AstraZeneca, Janssen, Roche, Gilead Sciences, Mundipharma, Takeda, Merck, BeiGene
Consulting or Advisory Role: AbbVie, AstraZeneca, Janssen, Celgene, Novartis, Gilead Sciences, Takeda, Merck, Mundipharma, CSL Behring
Research Funding: AstraZeneca (Inst), BeiGene (Inst), Roche (Inst), AbbVie (Inst), Gilead Sciences (Inst), Takeda (Inst), Pharmacyclics (Inst), Janssen (Inst), Celgene (Inst), Merck (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: EUSA Pharma
Shirley D'Sa
Honoraria: BeiGene (Inst)
Consulting or Advisory Role: BeiGene, Sanofi (Inst), Kite, a Gilead company (Inst)
Speakers' Bureau: BeiGene (Inst)
Research Funding: BeiGene (Inst)
Travel, Accommodations, Expenses: BeiGene
Wojciech Jurczak
Consulting or Advisory Role: BeiGene, AstraZeneca, Lilly
Research Funding: Roche, Takeda, Janssen-Cilag, BeiGene, Morphosys, AstraZeneca, Lilly
Hui-Peng Lee
Stock and Other Ownership Interests: CSL Behring
Honoraria: Takeda
Consulting or Advisory Role: BeiGene
Uncompensated Relationships: HSANZ
Gavin Cull
Research Funding: BeiGene (Inst), AstraZeneca (Inst), Glyomimetics (Inst)
Roger G. Owen
Honoraria: Janssen-Cilag, BeiGene, AstraZeneca
Consulting or Advisory Role: BeiGene, Janssen-Cilag
Travel, Accommodations, Expenses: BeiGene
Paula Marlton
Consulting or Advisory Role: Novartis, AbbVie, Astellas Pharma, Pfizer, BeiGene, Jazz Pharmaceuticals, Gilead Sciences, AstraZeneca, Janssen, Menarini, MSD, Otsuka, Servier
Speakers' Bureau: AbbVie
Björn E. Wahlin
Stock and Other Ownership Interests: Genmab
Research Funding: Roche
Ramon Garcia-Sanz
Honoraria: Janssen, Takeda, Amgen, BeiGene, Novartis, AstraZeneca Spain
Consulting or Advisory Role: Janssen
Research Funding: Incyte (Inst), Astellas Pharma, Takeda (Inst), Takeda (Inst)
Patents, Royalties, Other Intellectual Property: BIOMED 2 primers (Inst)
Travel, Accommodations, Expenses: Janssen, Takeda
Other Relationship: Spanish Society of Hematology
Helen McCarthy
Honoraria: Janssen/Pharmacyclics
Consulting or Advisory Role: BeiGene
Travel, Accommodations, Expenses: BeiGene
Stephen Mulligan
Honoraria: Janssen, BeiGene
Consulting or Advisory Role: Janssen, BeiGene
Speakers' Bureau: Janssen
Alessandra Tedeschi
Consulting or Advisory Role: Janssen, BeiGene, AstraZeneca, AbbVie
Speakers' Bureau: AbbVie, AstraZeneca, Janssen, BeiGene
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, BeiGene, AbbVie/Pharmacyclics, Cellectar, Loxo/Lilly, Kite/Gilead
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), BeiGene (Inst), TG Therapeutics (Inst), AstraZeneca (Inst), Cellectar (Inst), Loxo/Lilly (Inst)
Carlos Fernández de Larrea
Honoraria: Janssen, BeiGene, Bristol Myers Squibb/Celgene, Pfizer, Amgen
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Amgen, Pfizer, Sanofi, BeiGene
Research Funding: Janssen (Inst), Bristol Myers Squibb/Celgene (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Janssen, Amgen, GlaxoSmithKline, Bristol Myers Squibb/Celgene
David Belada
Consulting or Advisory Role: Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys, BeiGene, Genmab, AstraZeneca, Pharmacyclics
Research Funding: Roche (Inst), Gilead Sciences (Inst), Janssen-Cilag (Inst), Takeda (Inst), MorphoSys (Inst), Pharmacyclics (Inst), Dr Reddy's (Inst), Genmab (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Roche
Edward Libby
Honoraria: Curio Science, Pharmacyclics
Consulting or Advisory Role: Pharmacylics/Janssen
Research Funding: GlaxoSmithKline (Inst), Janssen (Inst), Genentech (Inst), BeiGene (Inst)
Jeffrey Matous
Consulting or Advisory Role: Pharmacyclics, BeiGene
Tanya Siddiqi
Consulting or Advisory Role: AstraZeneca, BeiGene, Celgene, Bristol Myers Squibb/Celgene, AbbVie, Kite, a Gilead company
Speakers' Bureau: AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene
Research Funding: Juno Therapeutics (Inst), Kite, a Gilead company (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), BeiGene (Inst), Pharmacyclics (Inst), Celgene (Inst), Oncternal Therapeutics (Inst), Bristol Myers Squibb/Sanofi (Inst), Ascentage Pharma (Inst), AstraZeneca (Inst)
Monica Tani
Consulting or Advisory Role: Incyte, AbbVie, Kirin Pharmaceuticals
Marek Trněný
Honoraria: Janssen, Gilead Sciences, Takeda, Bristol Myers Squibb, Amgen, AbbVie, Roche, MorphoSys, Novartis
Consulting or Advisory Role: Takeda, Bristol Myers Squibb, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, MorphoSys, Novartis, Genmab, Sobi
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Bristol Myers Squibb, Roche, Janssen, AbbVie
Monique C. Minnema
Consulting or Advisory Role: Janssen-Cilag (Inst), CDR-life (Inst), GlaxoSmithKline (Inst)
Speakers' Bureau: Celgene/Bristol Myers Squibb (Inst), Medscape (Inst), Janssen Medical Affairs (Inst)
Research Funding: BeiGene (Inst)
Christian Buske
Honoraria: Roche/Genentech, Janssen, BeiGene, Novartis, Pfizer, AbbVie, Gilead Sciences, Celltrion, MorphoSys, Regeneron, Hexal, Bayer, Sobi
Consulting or Advisory Role: Gilead Sciences, Janssen, Roche, Pfizer, BeiGene, Celltrion, AbbVie, Regeneron, MorphoSys, Novartis, Bayer
Speakers' Bureau: Roche, Janssen, BeiGene, Celltrion, AbbVie, Pfizer, Gilead Sciences, Bayer, Hexal, MorphoSys, Regeneron, Novartis, Sobi
Research Funding: Roche/Genentech, Janssen, Celltrion, MSD, Amgen, Bayer (Inst)
Veronique Leblond
Honoraria: AstraZeneca, BeiGene, Amgen, Janssen Oncology, AbbVie, MSD Oncology, Lilly
Consulting or Advisory Role: BeiGene, Janssen, AstraZeneca, Lilly, AbbVie
Speakers' Bureau: BeiGene, AstraZeneca, AbbVie
Travel, Accommodations, Expenses: AbbVie
Steven P. Treon
Honoraria: AbbVie/Pharmacyclics, Janssen Oncology, BeiGene
Consulting or Advisory Role: Janssen, Pharmacyclics, BeiGene
Research Funding: Pharmacyclics, X4 Pharma (Inst), Lilly (Inst), BeiGene (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: My institution holds patents related to use of MYD88 and CXCR4 testing for which a predetermined financial distribution to the laboratory and individuals is provided. I have not received any income to this date related to these patents (Inst)
Judith Trotman
Research Funding: BeiGene (Inst), Roche/Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda (Inst), BMS (Inst), Cellectar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Wai Y. Chan
Employment: BeiGene USA
Stock and Other Ownership Interests: BeiGene, Bristol Myers Squibb
Jingjing Schneider
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: BeiGene
Heather Allewelt
Employment: BeiGene, Nkarta
Leadership: Nkarta
Stock and Other Ownership Interests: BeiGene, Nkarta
Research Funding: BeiGene, Nkarta
Patents, Royalties, Other Intellectual Property: My husband is a named inventor on a patent held by St Jude Children's Research Hospital regarding the use of expanded NK cells in the treatment of cancer
Travel, Accommodations, Expenses: BeiGene, Nkarta
Sheel Patel
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: BeiGene
Aileen Cohen
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, BeiGene, Pharmacyclics, Roche/Genentech, Loxo/Lilly
Consulting or Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag (Inst), AbbVie (Inst), BeiGene (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2022; the European Hematology Association Congress, Vienna, Austria, June 9-12, 2022; the Pan Pacific Lymphoma Conference, Koloa, HI, July 18-22, 2022; and the International Workshop on Waldenström Macroglobulinemia, Madrid, Spain, October 27-30, 2022.
SUPPORT
BeiGene sponsored the study. Investigators and BeiGene collaborated on protocol development, data collection, and interpretation. BeiGene performed statistical analyses.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The redacted study Protocol is provided online only with this article. All authors had access to the original data for the analyses described here. On request and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to DataDisclosure@beigene.com.
AUTHOR CONTRIBUTIONS
Conception and design: Stephen Opat, Roger G. Owen, Alessandra Tedeschi, Christian Buske, Veronique Leblond, Aileen Cohen, Constantine S. Tam
Provision of study materials or patients: Meletios A. Dimopoulos, Stephen Opat, Shirley D'Sa, Wojciech Jurczak, Hui-Peng Lee, Gavin Cull, Roger G. Owen, Paula Marlton, Björn E. Wahlin, Ramon Garcia-Sanz, Helen McCarthy, Stephen Mulligan, Alessandra Tedeschi, Jorge J. Castillo, Jaroslaw Czyz, Carlos Fernández de Larrea, David Belada, Edward Libby, Jeffrey Matous, Marina Motta, Tanya Siddiqi, Monica Tani, Marek Trěný, Monique C. Minnema, Christian Buske, Veronique Leblond, Steven P. Treon, Judith Trotman, Constantine S. Tam
Collection and assembly of data: Wai Y. Chan, Jingjing Schneider, Heather Allewelt, Sheel Patel, Aileen Cohen
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Zanubrutinib Versus Ibrutinib in Symptomatic Waldenström Macroglobulinemia: Final Analysis From the Randomized Phase III ASPEN Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Meletios A. Dimopoulos
Honoraria: Amgen, Takeda, Janssen-Cilag, Bristol Myers Squibb, BeiGene, Sanofi
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda, Bristol Myers Squibb, BeiGene, Sanofi
Stephen Opat
Honoraria: AbbVie, AstraZeneca, Janssen, Roche, Gilead Sciences, Mundipharma, Takeda, Merck, BeiGene
Consulting or Advisory Role: AbbVie, AstraZeneca, Janssen, Celgene, Novartis, Gilead Sciences, Takeda, Merck, Mundipharma, CSL Behring
Research Funding: AstraZeneca (Inst), BeiGene (Inst), Roche (Inst), AbbVie (Inst), Gilead Sciences (Inst), Takeda (Inst), Pharmacyclics (Inst), Janssen (Inst), Celgene (Inst), Merck (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: EUSA Pharma
Shirley D'Sa
Honoraria: BeiGene (Inst)
Consulting or Advisory Role: BeiGene, Sanofi (Inst), Kite, a Gilead company (Inst)
Speakers' Bureau: BeiGene (Inst)
Research Funding: BeiGene (Inst)
Travel, Accommodations, Expenses: BeiGene
Wojciech Jurczak
Consulting or Advisory Role: BeiGene, AstraZeneca, Lilly
Research Funding: Roche, Takeda, Janssen-Cilag, BeiGene, Morphosys, AstraZeneca, Lilly
Hui-Peng Lee
Stock and Other Ownership Interests: CSL Behring
Honoraria: Takeda
Consulting or Advisory Role: BeiGene
Uncompensated Relationships: HSANZ
Gavin Cull
Research Funding: BeiGene (Inst), AstraZeneca (Inst), Glyomimetics (Inst)
Roger G. Owen
Honoraria: Janssen-Cilag, BeiGene, AstraZeneca
Consulting or Advisory Role: BeiGene, Janssen-Cilag
Travel, Accommodations, Expenses: BeiGene
Paula Marlton
Consulting or Advisory Role: Novartis, AbbVie, Astellas Pharma, Pfizer, BeiGene, Jazz Pharmaceuticals, Gilead Sciences, AstraZeneca, Janssen, Menarini, MSD, Otsuka, Servier
Speakers' Bureau: AbbVie
Björn E. Wahlin
Stock and Other Ownership Interests: Genmab
Research Funding: Roche
Ramon Garcia-Sanz
Honoraria: Janssen, Takeda, Amgen, BeiGene, Novartis, AstraZeneca Spain
Consulting or Advisory Role: Janssen
Research Funding: Incyte (Inst), Astellas Pharma, Takeda (Inst), Takeda (Inst)
Patents, Royalties, Other Intellectual Property: BIOMED 2 primers (Inst)
Travel, Accommodations, Expenses: Janssen, Takeda
Other Relationship: Spanish Society of Hematology
Helen McCarthy
Honoraria: Janssen/Pharmacyclics
Consulting or Advisory Role: BeiGene
Travel, Accommodations, Expenses: BeiGene
Stephen Mulligan
Honoraria: Janssen, BeiGene
Consulting or Advisory Role: Janssen, BeiGene
Speakers' Bureau: Janssen
Alessandra Tedeschi
Consulting or Advisory Role: Janssen, BeiGene, AstraZeneca, AbbVie
Speakers' Bureau: AbbVie, AstraZeneca, Janssen, BeiGene
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, BeiGene, AbbVie/Pharmacyclics, Cellectar, Loxo/Lilly, Kite/Gilead
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), BeiGene (Inst), TG Therapeutics (Inst), AstraZeneca (Inst), Cellectar (Inst), Loxo/Lilly (Inst)
Carlos Fernández de Larrea
Honoraria: Janssen, BeiGene, Bristol Myers Squibb/Celgene, Pfizer, Amgen
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Amgen, Pfizer, Sanofi, BeiGene
Research Funding: Janssen (Inst), Bristol Myers Squibb/Celgene (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Janssen, Amgen, GlaxoSmithKline, Bristol Myers Squibb/Celgene
David Belada
Consulting or Advisory Role: Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys, BeiGene, Genmab, AstraZeneca, Pharmacyclics
Research Funding: Roche (Inst), Gilead Sciences (Inst), Janssen-Cilag (Inst), Takeda (Inst), MorphoSys (Inst), Pharmacyclics (Inst), Dr Reddy's (Inst), Genmab (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Roche
Edward Libby
Honoraria: Curio Science, Pharmacyclics
Consulting or Advisory Role: Pharmacylics/Janssen
Research Funding: GlaxoSmithKline (Inst), Janssen (Inst), Genentech (Inst), BeiGene (Inst)
Jeffrey Matous
Consulting or Advisory Role: Pharmacyclics, BeiGene
Tanya Siddiqi
Consulting or Advisory Role: AstraZeneca, BeiGene, Celgene, Bristol Myers Squibb/Celgene, AbbVie, Kite, a Gilead company
Speakers' Bureau: AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene
Research Funding: Juno Therapeutics (Inst), Kite, a Gilead company (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), BeiGene (Inst), Pharmacyclics (Inst), Celgene (Inst), Oncternal Therapeutics (Inst), Bristol Myers Squibb/Sanofi (Inst), Ascentage Pharma (Inst), AstraZeneca (Inst)
Monica Tani
Consulting or Advisory Role: Incyte, AbbVie, Kirin Pharmaceuticals
Marek Trněný
Honoraria: Janssen, Gilead Sciences, Takeda, Bristol Myers Squibb, Amgen, AbbVie, Roche, MorphoSys, Novartis
Consulting or Advisory Role: Takeda, Bristol Myers Squibb, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, MorphoSys, Novartis, Genmab, Sobi
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Bristol Myers Squibb, Roche, Janssen, AbbVie
Monique C. Minnema
Consulting or Advisory Role: Janssen-Cilag (Inst), CDR-life (Inst), GlaxoSmithKline (Inst)
Speakers' Bureau: Celgene/Bristol Myers Squibb (Inst), Medscape (Inst), Janssen Medical Affairs (Inst)
Research Funding: BeiGene (Inst)
Christian Buske
Honoraria: Roche/Genentech, Janssen, BeiGene, Novartis, Pfizer, AbbVie, Gilead Sciences, Celltrion, MorphoSys, Regeneron, Hexal, Bayer, Sobi
Consulting or Advisory Role: Gilead Sciences, Janssen, Roche, Pfizer, BeiGene, Celltrion, AbbVie, Regeneron, MorphoSys, Novartis, Bayer
Speakers' Bureau: Roche, Janssen, BeiGene, Celltrion, AbbVie, Pfizer, Gilead Sciences, Bayer, Hexal, MorphoSys, Regeneron, Novartis, Sobi
Research Funding: Roche/Genentech, Janssen, Celltrion, MSD, Amgen, Bayer (Inst)
Veronique Leblond
Honoraria: AstraZeneca, BeiGene, Amgen, Janssen Oncology, AbbVie, MSD Oncology, Lilly
Consulting or Advisory Role: BeiGene, Janssen, AstraZeneca, Lilly, AbbVie
Speakers' Bureau: BeiGene, AstraZeneca, AbbVie
Travel, Accommodations, Expenses: AbbVie
Steven P. Treon
Honoraria: AbbVie/Pharmacyclics, Janssen Oncology, BeiGene
Consulting or Advisory Role: Janssen, Pharmacyclics, BeiGene
Research Funding: Pharmacyclics, X4 Pharma (Inst), Lilly (Inst), BeiGene (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: My institution holds patents related to use of MYD88 and CXCR4 testing for which a predetermined financial distribution to the laboratory and individuals is provided. I have not received any income to this date related to these patents (Inst)
Judith Trotman
Research Funding: BeiGene (Inst), Roche/Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda (Inst), BMS (Inst), Cellectar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Wai Y. Chan
Employment: BeiGene USA
Stock and Other Ownership Interests: BeiGene, Bristol Myers Squibb
Jingjing Schneider
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: BeiGene
Heather Allewelt
Employment: BeiGene, Nkarta
Leadership: Nkarta
Stock and Other Ownership Interests: BeiGene, Nkarta
Research Funding: BeiGene, Nkarta
Patents, Royalties, Other Intellectual Property: My husband is a named inventor on a patent held by St Jude Children's Research Hospital regarding the use of expanded NK cells in the treatment of cancer
Travel, Accommodations, Expenses: BeiGene, Nkarta
Sheel Patel
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: BeiGene
Aileen Cohen
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, BeiGene, Pharmacyclics, Roche/Genentech, Loxo/Lilly
Consulting or Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag (Inst), AbbVie (Inst), BeiGene (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Guo Y, Liu Y, Hu N, et al. : Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J Med Chem 62:7923-7940, 2019 [DOI] [PubMed] [Google Scholar]
- 2.BRUKINSA [package insert]. San Mateo, CA. BeiGene USA, Inc, 2021 [Google Scholar]
- 3.BRUKINSA [product monograph]. BeiGene Switzerland GmbH. 2021 [Google Scholar]
- 4. National Medical Products Administration: Approved drug data search. https://www.nmpa.gov.cn/datasearch/
- 5.Tam CS, Opat S, D'Sa S, et al. : A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: The ASPEN study. Blood 136:2038-2050, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos M, Sanz RG, Lee H-P, et al. : Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: A substudy of the phase 3 ASPEN trial. Blood Adv 4:6009-6018, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimopoulos M, Opat S, D'Sa S, et al. : ASPEN biomarker analysis: Response to BTK inhibitor treatment in patients with Waldenström macroglobulinemia harboring CXCR4, TP53, and TERT mutations. 11th International Workshop on Waldenström’s Macroglobulinemia. Madrid, Spain, 2022, pp WM041
- 8.Treon SP, Tripsas CK, Meid K, et al. : Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med 372:1430-1440, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Owen RG, McCarthy H, Rule S, et al. : Acalabrutinib monotherapy in patients with Waldenstrom macroglobulinemia: A single-arm, multicentre, phase 2 study. Lancet Haematol 7:e112-e121, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Treon SP, Meid K, Gustine J, et al. : Long-term follow-up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenstrom macroglobulinemia. J Clin Oncol 39:565-575, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buske C, Tedeschi A, Trotman J, et al. : Ibrutinib plus rituximab versus placebo plus rituximab for Waldenstrom's macroglobulinemia: Final analysis from the randomized phase III iNNOVATE study. J Clin Oncol 40:52-62, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shadman M, Flinn IW, Levy MY, et al. : A phase 2 study of zanubrutinib in patients with previously treated B-cell malignancies intolerant of prior Bruton Tyrosine Kinase inhibitors. Lancet Haematol 10:e35-e45, 2023 [DOI] [PubMed] [Google Scholar]
- 13.Kapoor P, Treon SP: The race to stymie BTK: Zanu zings. Blood 136:1997-1999, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo JJ, Abeykoon JP, Gustine JN, et al. : Partial response or better at six months is prognostic of superior progression-free survival in Waldenstrom macroglobulinaemia patients treated with ibrutinib. Br J Haematol 192:542-550, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paludo J, Abeykoon JP, Gertz MA, et al. : Depth of response in Waldenstrom macroglobulinemia. Blood 132, 2018. (suppl 1; abstr 4141) [Google Scholar]
- 16.Treon SP, Cao Y, Xu L, et al. : Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood 123:2791-2796, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The redacted study Protocol is provided online only with this article. All authors had access to the original data for the analyses described here. On request and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to DataDisclosure@beigene.com.