Abstract
Background and Aims
Substrate preferences are often treated as species traits and are used to distinguish different habits, i.e. an epiphytic, lithophytic or terrestrial habit. Such a categorization, however, ignores substantial intraspecific variation. An approach that takes biological variability within a species into account is needed.
Methods
We focused on four large genera of ferns and lycophytes and found relevant information in >500 sources, such as online databases, checklists, floras and species descriptions. Translating textual information into a quantitative index, we quantified the propensity to grow on either substrate as a continuous trait for 1475 species.
Key Results
Only a minority of species exhibited strict substrate fidelity, but a majority of them showed clear habitat preferences. The relative frequencies of intermediates between strict lithophytes, epiphytes and terrestrials does not support the frequent notion of ecological similarity of the lithophytic and epiphytic habitat.
Conclusions
The compiled data are useful immediately for ecological and evolutionary studies with the focal taxa. More importantly, we propose the replacement of the concept of distinct habits with one of gradual differences. This should have a profound impact on any such study with plants in general.
Keywords: Asplenium, Elaphoglossum, ferns, habit, habitat generalist, habitat specialist, hemiepiphyte, Hymenophyllum, interspecific variation, intraspecific variation, lycophytes, Phlegmariurus
INTRODUCTION
There are numerous schemes to categorize plant diversity, using differences in phenology (evergreen vs. deciduous), life history (annuals vs. perennials), stature (free-standing vs. climbing), photosynthetic pathway (C3 vs. C4 or CAM plants), tolerance against particular stressors (e.g. halophytes vs. glycophytes), habit (epiphytic vs. lithophytic vs. terrestrial) and many more to define different groups of plants. However, in many of these cases there are fundamental doubts concerning whether such categorizations capture natural variation appropriately and do not obscure more gradual differences. For example, Ogburn and Edwards (2010) crisply state that ‘succulence is not a binary trait’, although it is often used in that sense by scientists, as indicated by titles such as ‘succulent plants’ (Griffiths and Males, 2017). A similar case is the common practice of labelling species as ‘epiphytic’, ‘lithophytic’ or ‘terrestrial’. Are these genuinely distinct groups of vascular land plants?
Most land plants root in soil, i.e. they are terrestrials. An epiphyte, in turn, is defined as a non-parasitic plant that grows on another plant throughout its life, without contact with the ground (Zotz, 2016). Lithophytes (also called saxicolous or epipetric plants) are defined as plants ‘that grow on rock and derive their nourishment chiefly from the atmosphere’ (Barnhart, 2005). Lithophytes and epiphytes share growth on a largely impenetrable substrate, with resulting problems of anchorage and procurement of water and nutrients. However, in both cases the growing conditions can be increasingly similar to those of terrestrial plants, e.g. in montane tropical forests with substantial accumulations of organic material on the branches of trees or when rocks are densely covered with moss. Although application of these definitions to an individual plant can work rather well in most cases, it is impossible to show that all individuals of a given species use the same substrate in all but the exceptional case. Hence, there is some inevitable ambiguity in the terms ‘epiphytic species’, ‘lithophytic species’ or ‘terrestrial species’.
Previous work has emphasized interspecific differences, whereas intraspecific variation has been largely ignored. For example, we have recently compiled a global list of epiphyte species, EpiList 1.0 (Zotz et al., 2021b). For each species, at least one reference was given to justify its inclusion. However, such a list undoubtedly lumps species with very different degrees of fidelity to the epiphytic habitat, and additional information will lead to the exclusion of some species and the addition of others. To acknowledge this problem, these authors called their list a compilation of ‘31,000 hypotheses’.
More recently, we presented a literature-based approach to tackle the question of the degree of fidelity to epiphytic, lithophytic or terrestrial growth for the family Hymenophyllaceae (Zotz and Einzmann, 2023). We showed that the literature is a rich source for information on intraspecific variation in growing sites, which was used to arrive at a much more realistic picture of habitat preferences in that family than any previous work.
The present study applies this approach in a larger taxonomic context. Specifically, we picked the four globally distributed genera with the largest absolute numbers of epiphytic species in Epilist 1.0 (Zotz et al., 2021b), namely Asplenium, Elaphoglossum and Hymenophyllum among ferns with ~250–450 epiphytic taxa each, and Phlegmariurus among lycophytes with >100 epiphytic taxa. We quantified the propensity of epiphytic, lithophytic or terrestrial growth in these four genera as a continuous variable with a numerical approach.
Fawcett et al. (2022) have recently emphasized the importance of making information that is hidden in taxonomic treatments, printed floras and other scientific literature available in digital, accessible form. We did this, with the presentation of data of 1475 species from >500 publications. We believe that our data will be a rich resource for future ecological and phylogenetic studies with these four genera and will also help to arrive at a more realistic view of epiphytic, lithophytic and terrestrial growth in the plant kingdom at large.
MATERIALS AND METHODS
The present study follows the approach presented by Zotz and Einzmann (2023). We augmented the data set on Hymenophyllum used in that study with 120 additional entries and seven additional species. For the other three genera (Asplenium, Elaphoglossum and Phlegmariurus), we obtained information on growing sites of almost 1300 species from the taxononomic literature (species descriptions, checklists and floras), the ecological literature (community studies and vegetation descriptions) and numerous online data bases and other web sites, e.g. Brazilian Flora 2020 in construction (continuously updated) or eFloras (2020). An important criterion to decide whether to accept a source for our purpose was that the source acknowledged that a species might occur on different substrates. This was mostly not stated explicitly in a publication, but we considered it to be given implicitly when species lists included at least one species, e.g. as growing both as an epiphyte or lithophyte. Thus, all studies with an exclusive focus on vascular epiphytes (e.g. Hietz and Hietz-Seifert, 1995; Benavides et al., 2005; Einzmann et al., 2015) were excluded, unless the authors included accidental or facultative epiphytes (e.g. Werner et al., 2005; Francisco et al., 2023). Likewise excluded were all checklists that assigned all species to a single category (e.g. Freitas et al., 2016; Kipkoech et al., 2019; Jiménez López et al., 2023). Although one misses potentially valid information with this conservative approach, the inclusion of such studies would bias our data set to extreme values of habitat preference.
The final number of sources that was used for this study was 542 (Supplementary Data Table S1). All species names were standardized against the check list of Hassler (2004–2022), but the names used in the original publications can still be found in the Supplementary Data (Table S1).
A quantification of epiphytic, lithophytic or terrestrial occurrences of the individual plants of a given species in a study is exceedingly rare (e.g. Acuña-Tarazona et al., 2022). Thus, in almost all cases we had to translate literal descriptions of epiphytic, lithophytic or terrestrial occurrences of a given species in proportions, as detailed in Table 1.
Table 1.
Numerical translation of literal descriptions of species occurrences in the original sources as epiphytes (E), lithophytes (L) or terrestrials (T) used in the large majority of all sources. There are a few special cases, such as Bidin (1987), with explicit information on the habit of specimens, where we used the proportions, or Acuña-Tarazona et al. (2022), where the individual counts of a census were available.
| Verbal information | Percentage epiphytic occurrence (= EV) |
|---|---|
| Epiphyte and lithophyte (50 % to L)/epiphyte or terrestrial/facultative epiphyte (50 % to T) | 50 |
| Epiphyte, lithophyte or terrestrial (33/34 % to L and T) | 33 |
| Holoepiphyte/hemiepiphyte/epiphyte | 100 |
| Obligatory epiphyte | 100 |
| Rarely/seldom | 5 |
| Very rarely/accidental epiphyte/exceptionally | 1 |
| Also as/sometimes/occasionally/uncommonly | 15 |
| Commonly/generally/mainly/mostly/preferentially/primarily/usually | 85 |
| Often/frequently/most frequently | 67 |
| Less frequently | 33 |
This approach yielded three values per entry, an epiphyte value (EV), a lithophyte value (LV) and a terrestrial value (TV), which sum to unity (100 %). We are fully aware that it is unlikely that, e.g. the description ‘epiphytic and lithophytic’ is really meant to indicate the exact same number of 50 % epiphytic and 50 % lithophytic occurrences. Moreover, one cannot assume that different authors use a term such as ‘occasionally’ in a consistent way, and inevitably, the translation of vague descriptions such as ‘mostly terrestrial’ or ‘rarely epiphytic’ in a numerical system can only be arbitrary. However, our approach allows a coarse ranking of preferences for epiphytic, lithophytic and terrestrial growth and is a clear improvement on the typical practice of simple categorical assignments (e.g. Zotz et al., 2021b). Moreover, our approach is transparent and flexible, in that the criteria given in Table 1 can be adjusted in any future analysis by other researchers.
For the majority of species (about two-thirds), information from several sources was available. We considered all sources as equivalent and calculated the simple average of EV, LV and TV for each species (Supplementary Data Table S2). A large number of sources should improve the quality of the final estimate. This study provides data for 1475 species (~53–76 % per genus; see Supplementary Data Tables S1 and S2), with an average number of four sources with occurrence information per species (range: 1–54 entries per species).
As already discussed by Zotz and Einzmann (2023), any number can be seen only as a rough estimate, and the interpretation for a particular species is not straightforward. For example, an entry of 50 % epiphyte/50 % lithophyte for a species in the final results (Supplementary Data Table S2) can be for a number of reasons: (1) there might be a single source available that describes the species as, e.g. ‘growing on tree trunks and rocks’; or (2) there might be two or more studies, half of which describe the species as ‘growing epiphytically’, the others as ‘lithophyte’, or other possible combinations. Such differences could reflect true biological diversity when sources describe regional differences in occurrence patterns as reported, e.g. for the bromeliad Aechmea distichantha (Barberis et al., 2021), but there might also be erroneous entries in some sources. By presenting all this information in the Supplementary Data, we expose such differences to future analyses and provide a starting point to study the underlying reasons.
We performed three types of numerical analyses with our data set. First, we produced a histogram of epiphytic (EV) vs. non-epiphytic (LV + TV) occurrences and compared these distributions with uniform distributions with a χ2 test. A second type of analysis took advantage of the fact that the EV, LV and TV of each species sum to unity. This allows us to show the distribution of the species of the four genera in the epiphyte–lithophyte–terrestrial space (Zotz and Einzmann, 2023). For these ordinations, we used the plotrix library v.3.8-1 (Lemon, 2006) in R v.4.2.0 (R Core Team, 2022). In a third analysis, we quantified the number of species between two extremes (i.e. species located on the edges of the triangles) by, e.g. counting the number of facultative epiphyte/lithophyte species, which are defined by TV < 1 %, LV < 95 % and EV < 95 %. In an analogous way, we determined the number of facultative epiphyte/terrestrials and facultative lithophyte/terrestrials.
RESULTS
Our analyses are based on >6000 entries, covering almost 1500 species (Table 2). In a first analysis, we lumped terrestrials and lithophytes as non-epiphytes and quantified the degree of epiphytism of each species. The bins of the histogram should not differ much in frequency if the propensity to grow as an epiphyte were a continuous trait (scenario 1). Alternatively, if there were strong preferences for growth on trees, or not, we would expect a pronounced bimodal distribution (scenario 2). Support for the scenario 1 was not found in any case (Fig. 1). Invariably, the distribution deviated significantly from uniformity (χ2 tests, d.f. = 9, P < 0.001). Scenario 2 was observed only in Phlegmariurus, where the two extreme bins held >80 % of all species. In the other genera, the terrestrial/lithophytic extreme (Asplenium) or the epiphytic extreme (Elaphoglossum and Hymenophyllum) held the highest number of species. The existence of a possible third peak of ‘facultative’ epiphytes in Hymenophyllum and Elaphoglossum is doubtful, because a large proportion of the species that fell into the category of even probability to occur epiphytically or not were single-entry cases.
Table 2.
Proportion of species in the four genera with a preference (EV ≥ 50 %) or specialization (EV > 95 %) for epiphytic growth, the number of species in the present analysis, and the proportion of epiphyte species per genus in EpiList 1.0 (Zotz et al., 2021b) and total number of species given by Hassler (2004–2022). Life form description follows Kubitzki (1990) for Asplenium, Elaphoglossum and Phlegmariurus and Ebihara et al. (2006) for Hymenophyllum. Note that the values of the first three data columns are derived from the set of sampled species in this study (number of species), whereas the percentage of epiphytes in Epilist 1.0 was calculated relative to the total number of species per genus in world flora online.
| Genus | Life forms | EV ≥ 50 % | EV > 95 % | Number of species | Percentage of epiphytes in EpiList | Number of species |
|---|---|---|---|---|---|---|
| Asplenium | Terrestrial, epilithic or epiphytic | 30 | 9 | 440 | 36 | 830 |
| Elaphoglossum | Epiphytic or epilithic, less often terrestrial | 65 | 36 | 584 | 57 | 764 |
| Hymenophyllum | Usually, low to middle epiphytes on tree trunks; sometimes, canopy sun epiphytes; occasionally, epilithic or terrestrial | 81 | 38 | 237 | 89 | 319 |
| Phlegmariurus | Epiphytic or terrestrial (Huperzia) | 51 | 35 | 214 | 35 | 308 |
Fig. 1.

Histogram showing the tendency of Asplenium, Elaphoglossum, Hymenophyllum and Phlegmariurus species to occur epiphytically.
Triangular ordinations (Fig. 2) allowed us to include preferences for epiphytic, lithophytic and terrestrial growth in a single analysis. There were clear differences between the genera in terms of habitat preferences. Specialists, i.e. species that occur (almost) exclusively in one habitat (species in which EV, LV or TV was >95 %) were a minority in all genera but Phlegmariurus. In contrast, a preference (species in which EV, LV or TV was >50 %) was found in the large majority of species in all genera.
Fig. 2.
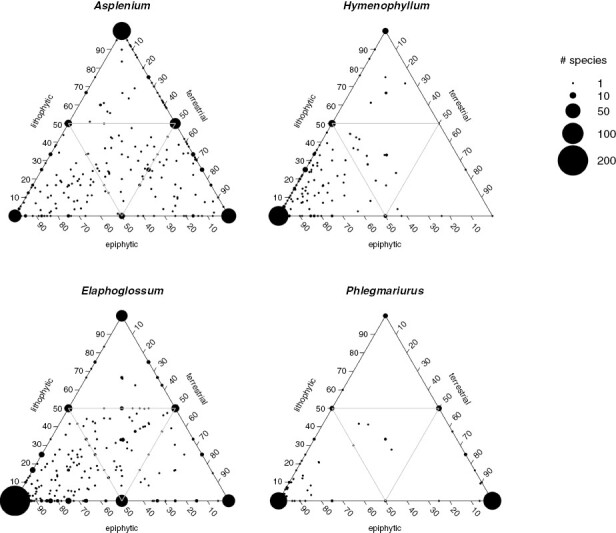
Distribution of 440 Asplenium, 584 Elaphoglossum, 237 Hymenophyllum and 214 Phlegmariurus species in the epiphyte–lithophyte–terrestrial space. Symbol size varies according to the number of species with the same values of EV, LV and TV. The thin grey lines separate three zones of preference for epiphytic, lithophytic or terrestrial growth, with the central triangle being occupied by generalists. Individual species values are based on 1–54 sources. The full data set is given in the Supplementary Data (Tables S1 and S2).
Habitat specialists were most common in Phlegmariurus, where 67 % of all taxa occurred almost exclusively as epiphytes, lithophytes or terrestrials, whereas the proportion of such specialists was lowest in Asplenium, where only about half that value (36 %) was reached. In Elaphoglossum, most of the 46 % specialists were restricted to growth on trees, although by far the highest proportion of obligatory epiphytes was found in Hymenophyllum.
Apart from depicting specialists, the ordination could be used to visualize and quantify a preference to grow on trees, rocks or in soil. Although a position in the central small triangle of the ordination in Fig. 2 identifies generalists, the occurrence in one of the other three small triangles in Fig. 2 indicates a clear preference for one of the three habitat types (Fig. 3). In agreement with the large proportion of obligatory epiphytes in Hymenophyllum, >80 % of the filmy ferns grew primarily as epiphytes, i.e. species values were found in the lower left triangle of the epiphyte–lithophyte–terrestrial space, where EV was ≥50 %. A strong preference for epiphytic growth could also be observed in Elaphoglossum, whereas the genus Phlegmariurus was characterized by either epiphytic or terrestrial growth, with a similar number of species in both groups. The most even use of the epiphyte–lithophyte–terrestrial space was found among Asplenium species. The number of Asplenium species with a preference for each of the three habitats was virtually identical, with ~30 % of the total, with the remaining 8 % of species not showing a preference.
Fig. 3.
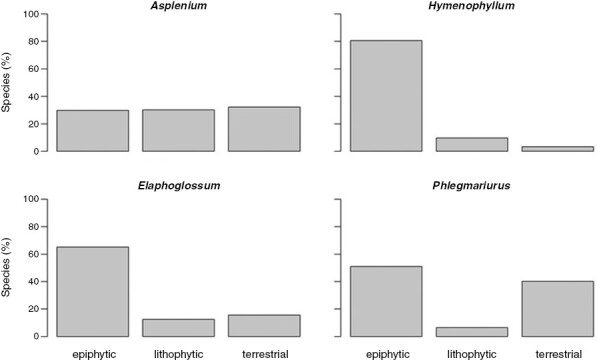
Preference for epiphytic, lithophytic or terrestrial growth in four genera of ferns and lycophytes. Preference is defined by species values of EV, LV or TV ≥ 50 %. For species at the border, e.g. EV = LV = 50 %, we assigned half of them to E and half to L. The numbers do not sum to 100 % because, in a small number of species, neither EV nor LV nor TV reaches 50 % (central triangle in Fig. 2).
From both ecological and evolutionary perspectives, it is instructive to analyse which of the three possible transitions (epiphyte–lithophyte, epiphyte–terrestrial or lithophyte–terrestrial) is more common. The four genera differ strongly in that regard. In Hymenophyllum, the genus with the strongest trend to epiphytic growth, the facultative epiphytic/lithophytic growth exceeds facultative epiphytic/terrestrial growth by a factor of three (48 species vs. 16 species). Only two species (Hymenophyllum macrosorum and Hymenophyllum nahuelhuapiense) seem to grow facultatively on rock or terrestrially. In contrast, in Elaphoglossum, which can also be described as a primarily epiphytic genus, the number of facultatively epiphytic/terrestrial species is twice as large as that of facultatively epiphytic/lithophytic species (91 vs. 44 species), with a smaller number of species along the lithophyte–terrestrial axis (31 species). In Asplenium, the number of facultatively epiphytic/lithophytic and facultatively epiphytic/terrestrial species was almost identical (37 vs. 34 species), whereas owing to the large proportion of habitat specialists in Phlegmariurus there are few facultative epiphyte/lithophyte/terrestrials in general.
DISCUSSION
How many ‘epiphyte species’ are there in the focal genera?
We compared the proportion of epiphytic species as listed in EpiList 1.0 (Zotz et al., 2021b) with the respective number of species with a preference for epiphytic growth (EV > 50 %) or the number of obligate epiphytes (EV > 95%) (Table 2). With the exception of Phlegmariurus, the number of ‘epiphyte species’ in EpiList 1.0 is always substantially higher than the estimated proportion of obligate epiphytes in the present study (EV > 95 %). This has a clear methodological reason. Epilist 1.0 included any species with at least one reference in the scientific literature describing it as epiphytic or primarily epiphytic. In contrast, the present study quantified habitat preference using as many sources as possible. For example, Elaphoglossum angulatum, Asplenium mucronatum or Hymenophyllum nephrophyllum were included in Epilist 1.0 because of their description as ‘epiphytes’ by Steyermark et al. (1995), Labiak and Prado (1998) and Hennequin et al. (2008), respectively. The present analysis, based on numerous sources, now suggests that their propensity for epiphytic growth varies substantially (EV = 67 %, EV = 99 % and EV = 43 %, respectively). However, this discrepancy between EpiList 1.0 and the present data set largely disappears in all genera when an ‘epiphyte species’ is defined as a species with a preference for epiphytic growth, i.e. where EV ≥ 50 % (Table 2).
Producing any such species list is ‘work in progress’. With the revision of additional literature for the present study, we found evidence for a number of species that certainly should have been included, but were missed, in EpiList 1.0. For example, Phlegmariurus balansae (Cerneaux et al., 1990), Phlegmariurus nutans (Cerneaux et al., 1990) and Phlegmariurus vanuatuensis (Field, 2018) or Elaphoglossum alvaradoanum (Matos et al., 2021) and Elaphoglossum moyeri (Tryon et al., 1991) all seem to be obligate epiphytes (Supplementary Data Table S1). Consequently, a future version of EpiList will both exclude now doubtful entries, such as H. nephrophyllum, and include additional species, such as those mentioned in the previous sentence.
Interestingly, the problem with defining an ‘epiphytic species’ resembles that of defining a ‘CAM species’. Plants using crassulacean acid metabolism show a highly variable proportion of nocturnal and diurnal CO2 uptake (Zotz et al., 2023), and calling every species with some measurable nocturnal acidification a ‘CAM species’ would inflate their number in a similar fashion to calling any species with occasional epiphytic occurrence an ‘epiphyte species’. Although admittedly arbitrary, Winter (2019) defined ‘CAM species’ as those that obtain the majority of their carbon through the CAM pathway throughout their lives, typically deduced from a δ13C values of leaf tissue of >−20 ‰. Using this rationale as a model for how to deal with the observed continuous variation in habitat preference in vascular plants, we suggest that ‘epiphyte species’ should be defined as those species that occur primarily in tree crowns. Within the epiphyte–lithophyte–terrestrial space, this would be equivalent to all species in the lower left of the four triangles (Fig. 2).
Intraspecific variation in habitat preference: overestimated or underestimated?
A fundamental goal of this study was to capture natural intraspecific and interspecific variation in habitat use in four large fern and lycophyte genera with a numerical approach. This goal faces many challenges. In many cases, information is very limited, e.g. when data are available only from the type or from very few specimens from one or a few locations. This is true, e.g. for Asplenium dayi (Proctor, 1985), Asplenium merapohense (Jaman et al., 2017) or Hymenophyllum chamaecyparicola (Chang et al., 2022), which might potentially lead to an underestimation of intraspecific variation in such cases. In turn, lumping several taxa with distinct ecology in one species might lead to an overestimation of intraspecific variation. Recently, Gonzatti et al. (2023) analysed the Hymenophyllum polyanthos complex in the Brazilian Atlantic Forest Domain: taxa such as Hymenophyllum schomburgkii or Hymenophyllum viridissimum, which are treated as synonymous in the check list of Hassler (2004–2022), differ in their habitat preferences from H. polyanthos, which might increase the variation documented for that species. Similar problems are likely in the Asplenium nidus complex (Yatabe and Murakami, 2003). There are numerous other examples, in which different species concepts influence the outcome of such an analysis, and variation might thus be interpreted as either intraspecific or interspecific. For example, Kramer (1978) distinguishes Elaphoglossum schomburgkii and Elaphoglossum luridum (with different preferences: terrestrial and epiphytic vs. epiphytic), but Hassler (2004–2022) treats them as synonyms, or an epiphytic form of H. polyanthos with pendulous fronds has been treated as a species, Hymenophyllum blumeanum, or can be seen as a variety (Sledge, 1982). As a final example, variants of several Asplenium species vary in preferences, e.g. Asplenium radicans var. cirrhatum is ‘usually on mossy tree trunks and shaded banks’, whereas the three other variants occur only terrestrially (Proctor, 1985).
Besides taxonomic ambiguities, inconsistent use of terminology is another major problem. For example, in the species description of Phlegmariurus loefgrenianus, Øllgaard and Windisch (2019) initially described it as ‘epiphytic’, but later as ‘epiphytic or epilithic’. In the same paper, Phlegmariurus itambensis is initially described as ‘terrestrial’ and later as ‘epilith growing in some of the driest harshest quartzitic rock conditions’. The second example is probably indicative of a larger issue: many authors probably do not distinguish consistently between ‘terrestrial’ and ‘lithophytic’.
Apart from such inconsistencies, there seems to be a tendency among many researchers to prefer neat categories over ‘noise’. As a case in point, Mellado-Mansilla et al. (2018) state that at their site a large proportion of the species described as ‘epiphytes’ by Moreno et al. (2013) for the region were found primarily on rocks or slopes of river beds. Yet, they still classified them as epiphytes as ‘the most common growth form’ in the region. Such an approach produces neat categories but obviously conceals true biological variation. Being mentioned explicitly by Mellado-Mansilla et al. (2018), we could at least reject that data set for our analysis. An unknown number of studies might have taken a similar approach and included the ‘typical’ habit from other sources without mentioning it. There is no way to exclude this potential source of error from our analysis.
As discussed in detail by Zotz and Einzmann (2023), our numerical approach has some issues of its own. First, different researchers will not have had the same numerical equivalent in mind when using terms such as ‘commonly’, ‘less frequently’ or ‘rarely’. Second, to assign a 50 % preference for epiphytic and lithophytic growth for a statement such as ‘on trees and rocks’ vs. ‘on rocks and trees’ was simply the most parsimonious approach for want of a more convincing alternative; it remains unclear whether such an order reflects a real difference in preference. Third, we used a single species average for epiphytic, lithophytic or terrestrial growth in our analysis. Intermediate values can reflect local variation in substrate use or geographical variation of a rather strict growing site preference, as mentioned by Tryon et al. (1993) for numerous Asplenium species. It was outside the scope of the present study to tackle this question, but the detailed compilation of information in the Supplementary Data can be the starting point for future investigations.
Towards a more realistic view of habitat preferences?
The correct assessment of the growing sites of a species represents an important part of the description of its biology. It is even indispensable when trying to understand its ecology. Unfortunately, species are often assigned rather coarsely to one of several types, ignoring the true complexity as evidenced in the present study. The notion that lithophytic, terrestrial and epiphytic habitats are inherently distinct (e.g. Watts and Watkins, 2021) is clearly an oversimplification, because arguably, the growth conditions among ‘epiphytes’ or ‘lithophytes’ can vary almost as much as between them. For instance, compare the vastly different growth conditions of an epiphyte in the moist understorey of a rainforest with those of an epiphyte in the outermost parts of a tree crown in a dry forest or those of a lithophyte on a fully exposed boulder vs. those of one on a shaded rock near a creek. In contrast, the growth conditions on the lower portions of a moss-covered tree trunk might hardly differ from those on a moss-covered rock in the understorey; growth in thick moss cushions might be more important than the subjacent substrate rock, soil or bark. Likewise, we recently showed that an epiphyte growing in a detritus-filled crotch can enjoy similar or even better growth conditions than a terrestrial conspecific in the immediate vicinity on the ground (Hoeber and Zotz, 2021).
Overall, however, there is the frequent notion that lithophytic and epiphytic growth conditions are more similar than conditions of plants rooting in soil. This can be an implicit assumption, e.g. by Holtum and Winter (1999) or by Dittrich et al. (2005), or stated explicitly. For example, Seifriz (1943) states that ‘epiphytic aroids … on rocks … are still epiphytes, physiologically considered’, Tsutsumi and Kato (2006) claim that ‘obligate epiphytes also occur on rocks’, Weston et al. (2005) write that ‘many epiphytes also grow on rocks’ or Couto et al. (2023) stress that growth in trees and on rocks ‘requires similar adaptations’. The claimed ecological similarity also suggests evolutionary scenarios. With this in mind, Zotz (2016) speculated that evolutionary transitions from lithophyte to epiphyte and vice versa might have been more common than those from the terrestrial habit to either of the two. Our analysis does not provide strong support for this notion (Fig. 2). Although the facultative use of the epiphytic and lithophytic habitats was indeed much more common than the two alternatives in Hymenophyllum, this was not the case in Elaphoglossum: species of this genus that occur both terrestrially and epiphytically are much more common than facultative epiphyte/lithophytes. In the two other genera, we found no differences in the number of facultative epiphyte/lithophytes vs. epiphyte/terrestrials (Asplenium), or the total number of species with facultative habitat use was generally small (Phlegmariurus).
Acknowledging intraspecific variation is not restricted to the three categories of this study. Lagomarsino et al. (2012) have shown that at least in one Elaphoglossum species, Elaphoglossum amygdalifolium, spores consistently germinate on bark, producing epiphytic gametophytes, while sporophytes later establish root contact with the soil. This ontogenetic pattern, with an epiphytic and ground-rooted stage, characterizes so-called hemiepiphytes (Zotz et al., 2021a). Categorizing a species as ‘hemiepiphyte’ faces the same problem as outlined for ‘epiphytes’ or ‘lithophytes’. It should be highly instructive to study whether the establishment in this species follows this pattern without exception and whether this ontogenetic pattern might be found, at least occasionally, in related species.
Acknowledging intraspecific variation in substrate use is also crucial for analyses of trait or life-history evolution. When studying the evolution of epiphytism, many analyses use simple dichotomies of terrestrial vs. epiphytic species (e.g. Hennequin et al., 2008; Field et al., 2016; Bauret et al., 2018; Testo et al., 2018), although more elaborate schemes have been used in a few cases. For example, Lehnert and Krug (2019) differentiate between terrestrials, lithophytes and ‘low’ and ‘high’ epiphytes. As stated previously (Zotz and Einzmann, 2023), analyses of evolutionary transitions from, e.g. terrestrial/ lithophytic to epiphytic growth (Hennequin et al., 2008) or vice versa (Chen et al., 2023) would benefit from abandoning the typically adopted categorical approach. Ignoring intraspecific variation allows only step changes instead of a more realistic gradual change.
In summary, we adopt a recently introduced approach to quantify substrate preferences to four genera of ferns and lycophytes. A large majority of species show clear preferences for epiphytic, lithophytic or terrestrial growth, but strict specialization is found in only a minority of species. Phlegmariurus is exceptional in this regard, with almost 70 % of all species being apparently restricted to either epiphytic or terrestrial growth. Our approach should be very useful in phylogenetic and ecological studies, and we reiterate the plea by Ebihara et al. (2007) on the importance of field observations for evolutionary studies. Our ecological classification schemes have to embrace the complexity of the biology of these plants, but any such scheme will translate into real progress only with high-quality field data. We hope that this paper helps to motivate researchers to collect and publish such data.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: original data of habitat preferences in four genera of ferns and lycophytes from 542 sources. Table S2: mean substrate preference values for 1475 species in four genera of ferns and lycophytes for epiphytic (EV), lithophytic (LV) and terrestrial growth (TV).
Contributor Information
Gerhard Zotz, Institute for Biology and Environmental Sciences, Functional Ecology, Carl von Ossietzky University Oldenburg, Box 2503, D-26111 Oldenburg, Germany; Smithsonian Tropical Research Institute, Balboa, Ancon, Panama City 0843-03092, Panama.
Lisa Armenia, Institute for Biology and Environmental Sciences, Functional Ecology, Carl von Ossietzky University Oldenburg, Box 2503, D-26111 Oldenburg, Germany.
Helena J R Einzmann, Institute for Biology and Environmental Sciences, Functional Ecology, Carl von Ossietzky University Oldenburg, Box 2503, D-26111 Oldenburg, Germany.
FUNDING
No funding was received for this research.
CONFLICTS OF INTERESTS
The authors have no conflicts of interest to declare.
LITERATURE CITED
- Acuña-Tarazona M, Mehltreter K, Toledo-Aceves T, Sosa VJ, Flores-Palacios A, Kessler M.. 2022. Effects of microenvironmental factors on the diversity and composition of fern and orchid assemblages in an Andean paramo in Peru. Flora 293: 152107. doi: 10.1016/j.flora.2022.152107. [DOI] [Google Scholar]
- Barberis IM, Mogni VY, Oakley LJ, Vogt C, Prado DE.. 2021. Biogeography of different life forms of the southernmost neotropical tank bromeliad. Journal of Biogeography 48: 2085–2097. doi: 10.1111/jbi.14137. [DOI] [Google Scholar]
- Bauret L, Field AR, Gaudeul M, Selosse M-A, Rouhan G.. 2018. First insights on the biogeographical history of Phlegmariurus (Lycopodiaceae), with a focus on Madagascar. Molecular Phylogenetics and Evolution 127: 488–501. doi: 10.1016/j.ympev.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Benavides AM, Duque AJ, Duivenvoorden JF, Vasco GA, Callejas R.. 2005. A first quantitative census of vascular epiphytes in rain forests of Colombian Amazonia. Biodiversity and Conservation 14: 739–758. [Google Scholar]
- Bidin A. 1987. A preliminary survey of the fern flora of Langkawi Islands. The Gardens’ bulletin, Singapore 40: 77–102. [Google Scholar]
- Brazilian Flora 2020 in Construction. continuously updated. Rio de Janeiro Botanical Garden. http://floradobrasil.jbrj.gov.br/ (March 2023, date last accessed).
- Cerneaux L, Jaffré T, Lescot M, Morat P, Veillon JM.. 1990. Flore de Nouvelle Calédonie. Familles revues (nomenclature et écologie). Nouméa: Orstom. [Google Scholar]
- Chang Z-X, Hsu T-C, Kuo L-Y.. 2022. Hymenophyllum chamaecyparicola (Hymenophyllaceae), a new filmy fern species from Taiwan. PhytoKeys 204: 23–34. doi: 10.3897/phytokeys.204.86045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Hyvönen J, Schneider H.. 2023. Re-terrestrialization in the phylogeny of epiphytic plant lineages: microsoroid ferns as a case study. Journal of Systematics and Evolution 61: 613–626. [Google Scholar]
- Couto DR, Porembski S, Barthlott W, de Paula LFA.. 2023. Hyperepilithics—an overlooked life form of vascular plants on tropical vertical rock walls. Austral Ecology 48: 1047–1222. [Google Scholar]
- Dittrich VAO, Waechter JL, Salino A.. 2005. Species richness of pteridophytes in a montane Atlantic rain forest plot of Southern Brazil. Acta Botanica Brasilica 19: 519–525. doi: 10.1590/s0102-33062005000300013. [DOI] [Google Scholar]
- Ebihara A, Dubuisson J-Y, Iwatsuk K, Hennequin S, Ito M.. 2006. A taxonomic revision of Hymenophyllaceae. Blumea 51: 221–280. [Google Scholar]
- Ebihara A, Dubuisson J-Y, Iwatsuki K, Ito M.. 2007. Systematics of Trichomanes (Hymenophyllaceae: Pteridophyta), progress and future interests. Fern Gazette 18: 53–58. [Google Scholar]
- Einzmann HJR, Beyschlag J, Hofhansl F, Wanek W, Zotz G.. 2015. Host tree phenology affects vascular epiphytes at the physiological, demographic and community level. AoB Plants 7: plu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett S, Agosti D, Cole SR, Wright DF.. 2022. Digital accessible knowledge: mobilizing legacy data and the future of taxonomic publishing. Bulletin of the Society of Systematic Biologists 1: 1–12. [Google Scholar]
- Field AR. 2018. Phlegmariurus vanuatuensis (Huperzioideae, Lycopodiaceae) a new species from Vanuatu, re-circumscription of P. nummulariifolius and new combinations in Phlegmariurus. PhytoKeys 109: 53–66. doi: 10.3897/phytokeys.109.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AR, Testo W, Bostock PD, Holtum JAM, Waycott M.. 2016. Molecular phylogenetics and the morphology of the Lycopodiaceae subfamily Huperzioideae supports three genera: Huperzia, Phlegmariurus and Phylloglossum. Molecular Phylogenetics and Evolution 94: 635–657. doi: 10.1016/j.ympev.2015.09.024. [DOI] [PubMed] [Google Scholar]
- eFloras. 2020. Flora of China. Cambridge, MA: Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria. http://www.efloras.org (January 2023, date last accessed). [Google Scholar]
- Francisco TM, Couto DR, Moreira MM, Fontana AP, de Fraga CN.. 2023. Inselbergs from Brazilian Atlantic Forest: high biodiversity refuges of vascular epiphytes from Espirito Santo. Biodiversity and Conservation 32: 2561–2584. doi: 10.1007/s10531-023-02618-7. [DOI] [Google Scholar]
- Freitas L, Salino A, Menini Neto L, et al. 2016. A comprehensive checklist of vascular epiphytes of the Atlantic Forest reveals outstanding endemic rates. PhytoKeys 58: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzatti F, Vasques DT, Windisch PG, Ritter MR, Ito M.. 2023. Systematics and taxonomy of the Hymenophyllum polyanthos complex in the Brazilian Atlantic Forest domain. Systematic Botany 48: 55–77. doi: 10.1600/036364423x16758873924108. [DOI] [Google Scholar]
- Griffiths H, Males J.. 2017. Succulent plants. Current Biology 27: R890–R896. doi: 10.1016/j.cub.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Hassler M. 2004–2022. World Ferns. Synonymic Checklist and Distribution of Ferns and Lycophytes of the World. Version 15.3; last updated 3 May 2023. www.worldplants.de/ferns/ (12 May 2023, date last accessed).
- Hennequin S, Schuettpelz E, Pryer KM, Ebihara A, Dubuisson JY.. 2008. Divergence times and the evolution of epiphytism in filmy ferns (Hymenophyllaceae) revisited. International Journal of Plant Sciences 169: 1278–1287. doi: 10.1086/591983. [DOI] [Google Scholar]
- Hietz P, Hietz-Seifert U.. 1995. Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. Journal of Vegetation Science 6: 487–498. doi: 10.2307/3236347. [DOI] [Google Scholar]
- Hoeber V, Zotz G.. 2021. Not so stressful after all: epiphytic individuals of accidental epiphytes experience more favourable abiotic conditions than terrestrial conspecifics. Forest Ecology and Management 479: 118529. doi: 10.1016/j.foreco.2020.118529. [DOI] [Google Scholar]
- Holtum JAM, Winter K.. 1999. Degrees of crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Australian Journal of Plant Physiology 26: 749–757. [Google Scholar]
- Jaman R, Kamin I, Kiew R.. 2017. Asplenium merapohense (Aspleniaceae), a new species from the Peninsular Malaysia. PhytoKeys 89: 85–90. doi: 10.3897/phytokeys.89.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez López D, Marcial N, Krömer T, González-Espinosa M.. 2023. Plant life-form distribution patterns in a tropical mountain region: effect of climate, topography, and human disturbance. Journal of Vegetation Science 34: e13184. [Google Scholar]
- Kipkoech S, Melly DK, Mwema BW, et al. 2019. Conservation priorities and distribution patterns of vascular plant species along environmental gradients in Aberdare ranges forest. PhytoKeys 131: 91–113. doi: 10.3897/phytokeys.131.38124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KU. 1978. The Pteridophytes of Suriname: an enumeration with keys of the ferns and fern-allies. Utrecht, Netherlands: University of Utrecht. [Google Scholar]
- Kubitzki K. 1990. The families and genera of vascular plants. I Pteridophytes and gymnosperms. Berlin: Springer. [Google Scholar]
- Labiak PH, Prado J.. 1998. Pteridófitas epífitas da Reserva Volta Velha, Itapoá - Santa Catarina, Brasil. Boletim do Instituto de Botânica 11: 1–79. [Google Scholar]
- Lagomarsino L, Grusz A, Moran RC.. 2012. Primary hemiepiphytism and gametophyte morphology in Elaphoglossum amygdalifolium (Dryopteridaceae). Brittonia 64: 226–235. [Google Scholar]
- Lehnert M, Krug M.. 2019. Evolution of substrate specificity and fungal symbiosis in filmy ferns (Hymenophyllaceae): a Bayesian approach for ambiguous character state reconstruction. Symbiosis 78: 141–147. doi: 10.1007/s13199-018-00594-z. [DOI] [Google Scholar]
- Lemon J. 2006. Plotrix: a package in the red light district of R. R-News 6: 8–12. [Google Scholar]
- Matos FB, Loriga J, Moran RC.. 2021. Monograph of Elaphoglossum sect. Polytrichia subsect. Apoda (Dryopteridaceae). Systematic Botany 46: 764–789. doi: 10.1600/036364421x16312067913462. [DOI] [Google Scholar]
- Mellado-Mansilla D, Díaz IA, Godoy-Güinao J, Ortega-Solís G, Moreno-Gonzalez R.. 2018. Bosque Pehuen Park’s Flora: a contribution to the knowledge of the Andean montane forests in the Araucania region, Chile. Natural Areas Journal 38: 298–311. doi: 10.3375/043.038.0410. [DOI] [Google Scholar]
- Moreno R, Le Quesne C, Díaz I, Rodríguez R.. 2013. Flora vascular del Parque Futangue, región de Los Ríos (Chile). Gayana Botánica 70: 121–135. doi: 10.4067/s0717-66432013000100013. [DOI] [Google Scholar]
- Ogburn RM, Edwards EJ.. 2010. The ecological water-use strategies of succulent plants. In: Kader JC, Delseny M. eds. Advances in botanical research. Burlington, VT: Academic Press, 179–225. [Google Scholar]
- Øllgaard B, Windisch PG.. 2019. Lycopodiaceae in Brazil. Conspectus of the family III. The genera Huperzia and Phlegmariurus. Rodriguésia 70: e01932017. [Google Scholar]
- Proctor GR. 1985. Ferns of Jamaica. London: British Museum (Natural History). [Google Scholar]
- R Core Team. 2022. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org [Google Scholar]
- Seifriz W. 1943. The plant life of Cuba. Ecological Monographs 13: 375–426. doi: 10.2307/1948590. [DOI] [Google Scholar]
- Sledge WA. 1982. An annotated check-list of the Pteridophyta of Ceylon. Botanical Journal of the Linnean Society 84: 1–30. doi: 10.1111/j.1095-8339.1982.tb00357.x. [DOI] [Google Scholar]
- Steyermark JA, Berry PE, Holst BK, Yatskievych K.. 1995. Flora of the Venezuelan Guayana, Volume 2: Pteridophytes, Spermatophytes Acanthaceae-Araceae. St. Louis, MO: Missouri Botanical Garden Press. [Google Scholar]
- Testo W, Øllgaard B, Field A, Almeida T, Kessler M, Barrington D.. 2018. Phylogenetic systematics, morphological evolution, and natural groups in neotropical Phlegmariurus (Lycopodiaceae). Molecular Phylogenetics and Evolution 125: 1–13. doi: 10.1016/j.ympev.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Barnhart RK. 2005. The American heritage science dictionary. Boston: Houghton Mifflin. [Google Scholar]
- Tryon RM, Stolze RG, Mickel JT, Moran RC.. 1991. Pteridophyta of Peru. Part IV. 17. Dryopteridaceae. Fieldiana Botany 27: 1–176. [Google Scholar]
- Tryon RM, Stolze RG, Leon B.. 1993. Pteridophyta of Peru - Part V: 18. Aspleniaceae - 21. Polypodiaceae. Fieldiana, Botany, new series 32: 1–190. [Google Scholar]
- Tsutsumi C, Kato M.. 2006. Evolution of epiphytes in Davalliaceae and related ferns. Botanical Journal of the Linnean Society 151: 495–510. doi: 10.1111/j.1095-8339.2006.00535.x. [DOI] [Google Scholar]
- Watts J, Watkins JE Jr. 2021. Crossing the divide: an exploration of functional traits in ferns that grow across terrestrial, epipetric and epiphytic habitats. American Fern Journal 111: 308–326. [Google Scholar]
- Werner F, Homeier J, Gradstein SR.. 2005. Diversity of vascular epiphytes on isolated remnant trees in the montane forest belt of southern Ecuador. Ecotropica 11: 21–40. [Google Scholar]
- Weston PH, Perkins AJ, Entwisle TJ.. 2005. More than symbioses: orchid ecology, with examples from the Sydney Region. Cunninghamia 9: 1–15. [Google Scholar]
- Winter K. 2019. Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70: 6495–6508. doi: 10.1093/jxb/erz002. [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Murakami N.. 2003. Recognition of cryptic species in the Asplenium nidus complex using molecular data: a progress report. Telopea 10: 487–496. doi: 10.7751/telopea20035625. [DOI] [Google Scholar]
- Zotz G. 2016. Plants on plants. The biology of vascular epiphytes. Cham: Springer International. [Google Scholar]
- Zotz G, Einzmann HJR.. 2023. How epiphytic are filmy ferns? A semi-quantitative approach. Diversity 15: 270. doi: 10.3390/d15020270. [DOI] [Google Scholar]
- Zotz G, Almeda F, Bautista-Bello AP, et al. 2021a. Hemiepiphytes revisited. Perspectives in Plant Ecology, Evolution and Systematics 51: 125620. doi: 10.1016/j.ppees.2021.125620. [DOI] [Google Scholar]
- Zotz G, Weigelt P, Kessler M, Kreft H, Taylor A.. 2021b. EpiList 1.0: a global checklist of vascular epiphytes. Ecology 102: e03326. [DOI] [PubMed] [Google Scholar]
- Zotz G, Andrade JL, Einzmann HJR. 2023. CAM plants: their importance in epiphyte communities and prospects with global change. Annals of Botany, in press. doi: 10.1093/aob/mcac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


