Abstract
Background and Aims
Whole-plant performance in water-stressed and disturbance-prone environments depends on a suitable supply of water from the roots to the leaves, storage of reserves during periods of shortage, and a morphological arrangement that guarantees the maintenance of the plants anchored to the soil. All these functions are performed by the secondary xylem of roots. Here, we investigate whether different growth forms of Fabaceae species from the seasonally dry Neotropical environment have distinct strategies for water transport, mechanical support and non-structural carbon and water storage in the root secondary xylem.
Methods
We evaluated cross-sections of root secondary xylem from species of trees, shrubs and subshrubs. We applied linear models to verify the variability in secondary xylem anatomical traits among growth forms.
Key Results
Secondary xylem with larger vessels and lower vessel density was observed in tree species. Vessel wall thickness, vessel grouping index, potential hydraulic conductivity and cell fractions (vessels, fibres, rays and axial parenchyma) were not statistically different between growth forms, owing to the high interspecific variation within the groups studied.
Conclusion
Our results showed that the variability in anatomical traits of the secondary xylem of the root is species specific. In summary, the cellular complexity of the secondary xylem ensures multiple functional strategies in species with distinct growth forms, a key trait for resource use in an environment with strong water seasonality.
Keywords: Chaco vegetation, Leguminosae, shrub, subshrub, tree, wood anatomy
INTRODUCTION
Woody plants of different growth forms coexist in seasonally dry tropical forests, where water availability is the major limiting resource for the composition and structure of these plant communities (Chaturvedi et al., 2011). The coexistence of plants with different growth forms is associated with a complex set of functional strategies that enable partitioning in the use of limited resources in environments with strong water seasonality (Rossatto and Franco, 2017). The different xylem cell types (water-conducting cells, fibres, and axial parenchyma and rays) provide numerous cellular arrangements that can result in the development of different ecological strategies to ensure simultaneous longitudinal water transport, mechanical support and storage of carbohydrates and water throughout the plant body (Baas et al., 2004). Water transport from roots to leaves and the storage of water and non-structural carbohydrates are primary functions performed by the secondary xylem of roots in woody species (Carlquist, 2001). In addition, the secondary xylem provides mechanical support for the aerial organs and anchors the plants to the soil (Meijer, 2021). However, despite the numerous functions performed by the secondary xylem of roots, little is known about their functional strategies in woody species of different growth forms in environments with strong water seasonality.
Tree and shrub species, in general, develop an extensive root system, with vertically oriented taproots in the soil reaching many metres in length and with lateral roots distributed on the soil surface (Villagra et al., 2009). Despite the morphological similarity in the root system, trees and shrubs can differ in the functional traits of the secondary xylem (Martínez-Cabrera et al., 2011). Generally, trees have wider and less dense vessels than shrubs (Carlquist and Hoekman, 1985; Martínez-Cabrera et al., 2011). Given that the potential hydraulic conductivity of a vessel increases to the fourth power of its diameter (Hagen–Poiseuille laminar flow equation; Tyree and Zimmermann, 2002), wide vessels in trees have the potential to conduct a larger volume of water per unit of time and offer low resistance to water flow (Hacke et al., 2006). However, wide vessels in the secondary xylem decrease its mechanical resistance, and the formation of a larger area of thick-walled fibres would be an important strategy to increase the mechanical support to the vessels and, consequently, to the organ as a whole (Hacke and Sperry, 2001; Jacobsen et al., 2007). In contrast, narrower and more numerous vessels in shrubs have less water-conducting potential but are considered to be important for water security in environments with strong water seasonality (Dória et al., 2016). The greater number of vessels per millimetre cubed increases the possibility of pathways for water flow in the event of embolisms (Hacke and Sperry, 2001), while at the same time increasing the water-conducting area (Tyree et al., 1994). As the number of vessels increases, so does the likelihood of an organ reaching maximum water transport safety, which is an essential requirement for the survival of plants from water-limited environments (Ewers et al., 2007, 2023).
Subshrub species, in contrast, have shallow, thickened or non-thickened taproots vertically oriented in the ground, with slender lateral roots near the soil surface (Schenk and Jackson, 2002; Silva et al., 2020). Overgrazing, trampling by cattle, severe droughts and frosts are frequent causes of above-ground biomass loss in subshrub species distributed in seasonally dry environments (Silva and Rossatto, 2019; Silva et al., 2020). After such disturbances, resprouting from buds in roots or root crowns is an important functional trait to increase above-ground branching (Appezzato-da-Glória, 2015). This strategy requires secondary xylem with a large area of parenchyma cells, with storage of non-structural carbohydrates and water to support the formation of new buds and growth of new stems (Chapin et al., 1990). Furthermore, water and carbohydrates stored in underground organs are a key resource to buffer the effects of low water availability during drought (Borchert, 1994).
Here, we examined the secondary xylem traits in tree, shrub and subshrub Fabaceae species from seasonally dry vegetation to test whether the secondary xylem of roots in plants of different growth forms is associated with distinct strategies for water transport, mechanical support, and carbon and water storage. We hypothesized that tree species would have larger vessels that contribute more efficient water transport than shrub and subshrub species. Also, we expected that tree species would have secondary xylem with a larger fraction of fibre to provide more mechanical support. In contrast, because of their ability to resprout (Silva et al., 2020), we hypothesized that subshrub species would have a higher percentage of parenchyma cells as a key ecological strategy to store carbohydrates and water.
In order to test our hypothesis, we collected roots of Fabaceae species from seasonally dry environments of the Neotropics, specifically in the South American Chaco. Chaco vegetation covers areas of woodlands and xeromorphic forests in central South America (Pennington et al., 2000) and supports a rich assemblage of Fabaceae species that experience regular annual droughts and frosts (Lima et al., 2015; Baptista et al., 2020). Fabaceae species are particularly important in dry environments owing to their capacity to fix nitrogen through nodulation, increasing soil fertility (Baptista et al., 2020). Moreover, native Fabaceae species are important food sources for wild and domestic animals and economic resources for the local people in the Chaco area (Felker et al., 2003). The coexistence of several species of Fabaceae in Chaco vegetation makes this a good opportunity to test whether different growth forms submitted to similar climatic and edaphic conditions show distinct strategies in the root secondary xylem.
MATERIALS AND METHODS
Study area
We performed this study in a wet Chaco remnant located in the municipality of Porto Murtinho (21°41ʹ0.50″S, 57°46ʹ42.90″W), State of Mato Grosso do Sul, Brazil. Brazilian Chaco remnants are restricted to the far southern region of the Pantanal wetland. The vegetation of the study area is classified as a ‘thorn forest’ (UNESCO, 1973) characterized by shrubby-arboreal strata, interspersed with herbaceous plants and mainly composed of microphyll, deciduous and thorny species.
The Brazilian Chaco remnant experiences marked seasonality, with a dry season occurring from May to September and a rainy season from November to March (Fig. 1). The climate is hot and dry, with an average annual rainfall of 970 mm and a mean temperature of 25.1 °C. During the rainy season, temperatures can exceed 40 °C, and temporary floods occur owing to the poor drainage of the compacted soil. In the dry season, frosts are frequent during the colder months (from June to August), when temperatures may drop below 5 °C. Meteorological data were provided by the CEMTEC-Monitoring Centre for Weather, Climate and Water Resources of Mato Grosso do Sul State, Brazil. The soil in the area is classified as natric planosol with a clay texture, dystrophic, saline and compacted, with slow drainage (Couto and Oliveira, 2014; Assunção et al., 2021).
Fig. 1.
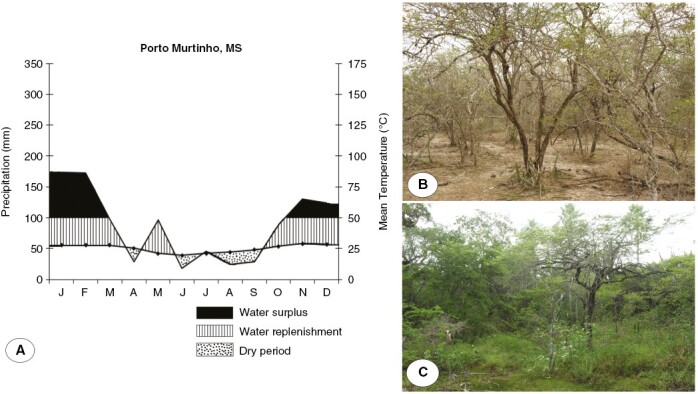
(A) Climatic diagrams with precipitation and mean temperature. (B, C) Brazilian Chaco remnants in the dry season (B) and the rainy season (C).
Studied species and sampling
We selected 14 co-occurring woody Fabaceae species considered more abundant in areas of Brazilian Chaco remanent. The species selection was based on a previous phytosociological and floristic survey by Baptista et al. (2020), Carvalho and Sartori (2015), Alves and Sartori (2009) and Noguchi et al. (2009). The species studied were divided into three functional groups based on their growth form: trees, shrubs and subshrubs. We consider as trees those woody species that have only one main stem supporting the branches; as shrubs, plants with numerous stems that are entirely woody and branched from the stem base; and as subshrubs those plants with multiple, ascending, woody stems, except in the apical portion of the branches (Pérez-Harguindeguy et al., 2016). The species studied are described in Table 1.
Table 1.
Root length and diameter and plant height of Fabaceae species sampled. The values are shown as the mean ± s.d.
| Species | Root length (cm) |
Root diameter (cm) |
Plant height (m) |
|---|---|---|---|
| Tree | |||
| Mimosa glutinosa Malme | 26.3 ± 1.5 | 1.5 ± 0.1 | 1.1 ± 0.05 |
| Mimosa hexandra Micheli | 33.6 ± 2.1 | 2.0 ± 0.2 | 1.2 ± 0.05 |
| Parkinsonia praecox (Ruiz & Pavon ex. Hook.) J. Hawkins | 30.0 ± 0.3 | 1.7 ± 0.1 | 0.6 ± 0.2 |
| Prosopis rubriflora Hassl. | 36.0 ± 4.0 | 2.0 ± 0.1 | 1.0 ± 0.2 |
| Prosopis ruscifolia Griseb | 33.5 ± 1.5 | 1.6 ± 0.1 | 1.1 ± 0.1 |
| Shrub | |||
| Bauhinia hagenbeckii Harms | 24.0 ± 3.6 | 4.0 ± 2.0 | 0.90 ± 0.07 |
| Mimosa polycarpa Kunth. | 14.0 ± 1.0 | 0.6 ± 0.1 | 0.40 ± 0.01 |
| Mimosa sensibilis var. urucumensis Barneby | 20.3 ± 1.5 | 1.6 ± 0.1 | 0.85 ± 0.05 |
| Subshrub | |||
| Arachis lignosa (Chodat & Hassl.) Krapov. & W.C. Gregory | 15.0 ± 1.3 | 1.2 ± 0.3 | 0.27 ± 0.03 |
| Galactia paraguariensis Chodat & Hassl. | 17.2 ± 0.2 | 0.7 ± 0.17 | 0.40 ± 0.01 |
| Neptunia pubescens Benth. | 22.3 ± 2.5 | 1.1 ± 0.15 | 0.32 ± 0.04 |
| Senna pilifera (Vogel) H.S. Irwin & Barneby | 14.6 ± 1.5 | 0.7 ± 0.12 | 0.29 ± 0.01 |
| Stylosanthes hamata (L.) Taub. | 23.0 ± 3.0 | 0.9 ± 0.15 | 0.37 ± 0.01 |
| Tephrosia chaquenha R. T. Queiroz & A. M. G. Azevedo | 20.0 ± 2.6 | 0.8 ± 0.1 | 0.40 ± 0.02 |
To determine the variation in secondary xylem traits among the different plant functional groups (trees, shrubs and subshrubs), we excavated trenches ≥50 cm deep and collected taproots from three individuals of each species studied. For botanical identification, we collected and herborized the branches with flowers and/or fruits, in addition to those with vegetative parts. The exsiccates were deposited at the CGMS Herbarium, Federal University of Mato Grosso do Sul, Campo Grande, Brazil.
Wood anatomical study
We fixed the root samples in FAA50 (10 % formaldehyde, 5 % glacial acetic acid and 50 % ethanol) for 48 h, then stored them in 70 % ethanol (Johansen, 1940). We cut transverse and longitudinal (tangential and radial) histological sections of the secondary xylem at a thickness of 14–18 µm using a Leica SM2000R sliding microtome. The histological sections were stained with aqueous 1 % Astra Blue (Roeser, 1972) and aqueous 1 % Safranin (Bukatsch, 1972) and mounted in glycerin and water solution (1:1 v:v). We also made histological sections of root samples, embedded them in plastic resin (Bennett et al., 1976) and sectioned them using a Leica RM2145 rotary microtome at 5–10 μm thickness. The histological sections were stained in 0.1 % Toluidine Blue at pH 4.7 (O’Brien et al., 1964). To dissociate the cellular elements of the xylem, we placed small fragments of secondary xylem in a solution of glacial acetic acid and hydrogen peroxide (1:1 v:v) at 60 °C for 24 h, rinsed them in water, stained them with aqueous 1 % Safranin, and mounted semi-permanent slides in a glycerin and water solution (1:1 v:v) (Franklin, 1945 modified by Kraus and Arduin, 1997). We used the dissociated cells of the secondary xylem for description of the following qualitative features: perforation plate type, presence or absence of vasicentric tracheids and fibre morphology.
We analysed the root secondary xylem slides under a light microscope equipped with a Moticam Pro 252B digital camera. We followed the recommendations of the International Association of Wood Anatomists (IAWA) Committee (1989) for anatomical description of the secondary xylem. Based on the IAWA list (1989), we wrote an anatomical description of the following secondary xylem features in transverse sections: growth rings; porosity, arrangement and grouping of vessels; fibre wall thickness; presence of gelatinous fibres; and arrangement of axial parenchyma. We used longitudinal radial sections to describe the cellular composition of rays. Longitudinal tangential sections were used to describe the arrangement of intervessel pits and vessel-ray pitting, strand length of the axial parenchyma, and the width and height of rays (number of cells).
We performed scanning electron microscopy to observe the features of intervessel pits. Secondary xylem samples 3 mm in length fixed in FAA50 were split in a tangential plane, dehydrated in an ethanol series from 50 to 100 %, then air dried for 24 h. The split wood samples were fixed to stubs using an electron-conductive carbon sticker and gold coated in a Baltec SCD 050 sputter coater for 3 min. We observed the intervessel pits using a Jeol JSM-6380LV scanning electron microscope at an accelerating voltage of 15 kV. We used scanning electron microscopy to confirm the presence of vestured pits in the vessel walls.
Wood data collection
We evaluated the following wood features: vessel diameter (in micrometres); vessel wall thickness (in micrometres); vessel density (number per millimetre squared); vessel grouping index; fractions of vessel (as a percentage), fibre (as a percentage), ray (as a percentage) and axial parenchyma (as a percentage) in transverse section; and potential hydraulic conductivity (in kilograms per metre per megapascal per second). We performed 30 measurements of vessel lumen area and vessel wall thickness per sampled individual of each species studied. We used the vessel lumen area to calculate the vessel diameter (D) based on the following equation:
where A is the vessel lumen area in micrometres (Scholz et al., 2013).
To quantify the vessel density (number of vessels/area), we counted the number of vessels in three areas of 1 mm2 per sampled individual in each species studied. We decided to count the vessels in three areas per individual to avoid overlapping of vessel counting areas in those species with roots of narrow diameter. We estimated the fractions of vessels, fibres, rays and axial parenchyma in three areas of 1 mm2 in wood transverse sections of each sampled individual. We performed all wood measurements using ImageJ 1.6.0 (https://imagej.nih.gov/ij/download.html). Primary xylem vessels (protoxylem and metaxylem) and newly produced vessels were excluded from the measurements. We considered protoxylem vessels to be those with smaller diameters and thinner cell walls and the metaxylem vessels to be those with larger diameters and thicker walls (Evert, 2006). New vessels formed are those nearest the vascular cambium (Evert, 2006).
We calculated the vessel grouping index as the total number of vessels divided by the total number of vessel groupings (Carlquist, 1984; Scholz et al., 2013). For each sampled individual, we selected 25 vessel groups and counted the total number of vessels in these groups, then divided the total number of vessel groups by 25. We followed recommendations made by Carlquist (1984) and considered both solitary vessels and grouped vessels as vessel groups.
To determine the potential hydraulic conductivity, we first calculated the hydraulically weighed vessel diameter (Dh) for each sampled individual:
where D is the vessel diameter (in micrometres) and n is the number of vessels measured. Then we used the result of hydraulically weighed vessel diameter (Dh) and vessel density (VD) to calculate the potential hydraulic conductivity based on the Hagen–Poiseuille law (Poorter et al., 2010):
where Kp is the potential hydraulic conductivity (in kilograms per metre per megapascal per second), ρw is the density of water at 20 °C (998.2 kg m−3), η is the viscosity of water at 20 °C (1.002 × 10−3 Pa s), VD is the vessel density (number of vessels per millimetre squared) and Dh is the hydraulically weighed vessel diameter (in metres).
Data analyses
To test whether growth form differs in the anatomical traits of root secondary xylem, we performed mixed linear models, using groups (trees, shrub and subshrub) as a fixed factor and with species and individuals nested in species as a random factor for the following wood features: vessel diameter, density and wall thickness, and the fraction of vessels, fibres, rays and axial parenchyma. This design avoided inflating the variation of the fixed factors, because the variation of individuals is already nested within species by default (Schielzeth and Nakagawa, 2013). For vessel grouping index and potential hydraulic conductivity, we performed mixed linear models using groups (trees, shrubs and subshrubs) as a fixed factor and species as a random factor. To determine whether there were differences in wood traits between functional groups (trees, shrubs and subshrubs), we used one-way ANOVA. Tukey’s test was used as a post-hoc analysis to explore differences in the response variables among functional groups. To avoid multiple comparisons, we adjusted the P-value using the Bonferroni–Holm method, and we considered a significant level of 5 % (P < 0.05).
All the analyses were conducted by the R program (R Core Team, 2020), with the additional packages ‘lme4’ (Bates et al., 2015), ‘car’ (Fox and Weisberg, 2019) and ‘multcomp’ (Therneau et al., 2015).
RESULTS
The mixed model analysis revealed that the vessel diameter and density differed between tree, shrub and subshrub species. Tree species generally had larger vessels than shrub and subshrub species (Fig. 2; Tables 2 and 3). The average vessel diameter was 40 ± 15.6 µm in tree species, 25.7 ± 10.15 µm in shrub species and 32.1 ± 14.8 µm in subshrub species. The vessel diameter in shrub and subshrub species was statistically similar. Concerning vessel density, tree species had lower vessel density than shrub species. The vessel density was not statistically different between trees and subshrub species and between shrub and subshrub species. The vessel density in tree species ranged from 34.9 ± 5.2 vessels mm−2 in Parkinsonia praecox to 75.5 ± 36.4 vessels mm−2 in Prosopis ruscifolia, and the vessel density in shrub species ranged from 96 ± 15.6 vessels mm−2 in Bauhinia hagenbeckii to 302 ± 26 vessels mm−2 in Mimosa polycarpa.
Fig. 2.
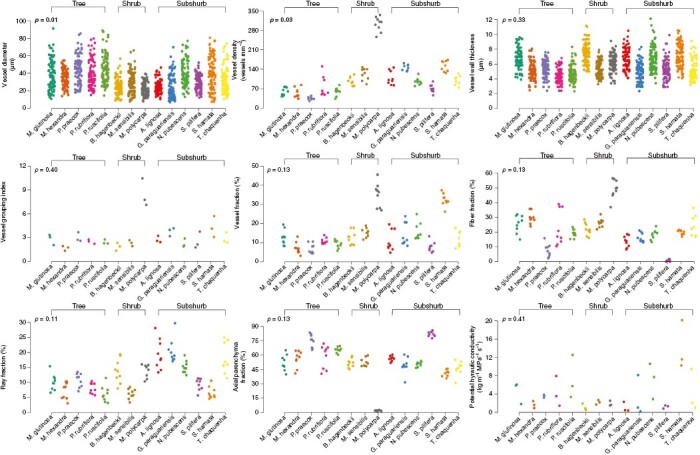
Stripchart showing wood features of woody Fabaceae species from a Brazilian Chaco remnant. A P-value in bold indicates that the secondary xylem trait differs statistically between growth forms (tree, shrub and subshrub).
Table 2.
Secondary xylem features of Fabaceae woody species from Brazilian Chaco remnants. The values are shown as the mean ± s.d.
| Species | Vessel diameter (µm) | Vessel density (number mm−2) | Vessel wall thickness (µm) | Vessel grouping index | Potential hydraulic conductivity (kg m−1 MPa−1 s−1) | Vessel fraction (%) | Fibre fraction (%) | Ray fraction (%) | Axial parenchyma fraction (%) |
|---|---|---|---|---|---|---|---|---|---|
| Tree species | |||||||||
| Mimosa glutinosa | 38.3 ± 16.9 | 59.4 ± 14.2 | 6.8 ± 1.3 | 2.8 ± 0.7 | 4.6 ± 2.4 | 12.6 ± 3.9 | 25.4 ± 5.9 | 10 ± 2.7 | 51.9 ± 8.1 |
| Mimosa hexandra | 34.4 ± 9.5 | 54.5 ± 17.2 | 5.1 ± 1.1 | 1.7 ± 0.3 | 1.7 ± 0.8 | 7.1 ± 3 | 29.7 ± 4 | 7.2 ± 2.7 | 56 ± 8.5 |
| Parkinsonia praecox | 43.8 ± 16.1 | 34.9 ± 5.2 | 5.3 ± 1 | 2.9 ± 0.6 | 3.6 ± 0.3 | 6.8 ± 2.5 | 7.6 ± 4.2 | 10.8 ± 2.3 | 74.8 ± 6.7 |
| Prosopis rubriflora | 38.6 ± 15.8 | 75.5 ± 36.4 | 4.4 ± 0.9 | 2.5 ± 0.3 | 4.3 ± 3.3 | 10.7 ± 1.6 | 22.5 ± 11.8 | 8.1 ± 1.8 | 58.6 ± 12 |
| Prosopis ruscifolia | 45.6 ± 15.9 | 59.7 ± 14.7 | 4.7 ± 1.1 | 2.4 ± 0.3 | 7.2 ± 4.8 | 9.2 ± 2 | 19.1 ± 2.8 | 6.2 ± 2.6 | 65.5 ± 2.9 |
| Shrub species | |||||||||
| Bauhinia hagenbeckii | 24.9 ± 9.1 | 96 ± 15.6 | 7.5 ± 1.3 | 1.8 ± 0.5 | 1.1 ± 0.7 | 12.6 ± 3.5 | 22 ± 4.1 | 13.8 ± 3.9 | 51.6 ± 4.7 |
| Mimosa polycarpa | 22.4 ± 7.2 | 302.2 ± 26 | 6.1 ± 1.1 | 8.4 ± 1.8 | 1.9 ± 0.5 | 34.9 ± 6.2 | 49.2 ± 6.2 | 14 ± 2.1 | 1.9 ± 0.4 |
| Mimosa sensibilis var. urucumensis | 29.5 ± 12.3 | 122.3 ± 21.3 | 5.3 ± 1 | 2.3 ± 0.4 | 2.2 ± 0.5 | 15.2 ± 2.4 | 26.1 ± 3 | 6.3 ± 1.6 | 52.4 ± 4.4 |
| Subshrub species | |||||||||
| Arachis lignosa | 24.4 ± 7.4 | 108.7 ± 24.6 | 6.6 ± 1.3 | 2.7 ± 0.41 | 1 ± 1.1 | 10.9 ± 5.5 | 13.6 ± 3.8 | 19.4 ± 4.9 | 56.1 ± 2.8 |
| Galactia paraguariensis | 25.5 ± 13.6 | 147.2 ± 10.5 | 4.8 ± 1.3 | 3.7 ± 0.5 | 3.1 ± 4.3 | 14.1 ± 5.8 | 16.2 ± 3 | 20.4 ± 3.9 | 49.3 ± 8.2 |
| Neptunia pubescens | 39.8 ± 15.1 | 95.7 ± 12.6 | 6.9 ± 1.6 | 2.2 ± 0.5 | 7.1 ± 3.9 | 15.8 ± 4.3 | 18.4 ± 3.2 | 15.2 ± 2 | 50.6 ± 2.5 |
| Senna pilifera | 29.7 ± 8.5 | 69.4 ± 14.6 | 5.4 ± 1.3 | 2.5 ± 1.1 | 1.2 ± 0.3 | 7.7 ± 2.6 | 0.6 ± 0.6 | 9.5 ± 1.9 | 82.2 ± 2.7 |
| Stylosanthes hamata | 40.9 ± 18.7 | 151.1 ± 18.6 | 7.1 ± 1.5 | 4.3 ± 1.3 | 13.9 ± 5.3 | 32.4 ± 3 | 19.9 ± 1.5 | 6.6 ± 1.9 | 41.1 ± 3.3 |
| Tephrosia chaquenha | 32.1 ± 14.1 | 100.5 ± 16.1 | 5.1 ± 1.2 | 2.8 ± 0.7 | 4.1 ± 4.6 | 12.3 ± 4.3 | 17.9 ± 4.9 | 23.5 ± 7.1 | 46.3 ± 7 |
Table 3.
Results of the mixed linear models used to test the difference between functional groups in woody Fabaceae species from Brazilian Chaco remnants. The values are shown as the mean ± s.d. *Significant difference (P < 0.05). Abbreviations: S, shrub species; Sb, subshrub species; T, tree species.
| MLM results: random effects | One-way ANOVA | Tukey’s test | |||||
|---|---|---|---|---|---|---|---|
| Wood features | Species | Species:individual | F-value | P-value | Sb vs. S | T vs. S | T vs. Sb |
| Vessel diameter | 18.98 ± 4.3 | 34.22 ± 5.8 | 6.52 | 0.01* | 0.10 | 0.001* | 0.03* |
| Vessel density | 2722.1 ± 52.1 | 232 ± 15 | 4.60 | 0.03* | 0.17 | 0.007* | 0.17 |
| Vessel wall thickness | 0.87 ± 0.9 | 0.26 ± 0.51 | 1.22 | 0.33 | 0.65 | 0.45 | 0.45 |
| Vessel grouping index | 2.63 ± 1.62 | – | 0.98 | 0.40 | 0.69 | 0.48 | 0.69 |
| Vessel fraction | 0.009 ± 0.09 | 0.002 ± 0.05 | 2.38 | 0.13 | 0.32 | 0.10 | 0.31 |
| Fibre fraction | 0.01 ± 0.14 | 0.003 ± 0.05 | 2.40 | 0.13 | 0.08 | 0.37 | 0.37 |
| Ray fraction | 0.004 ± 0.006 | 0.001 ± 0.03 | 2.67 | 0.11 | 0.60 | 0.60 | 0.06 |
| Axial parenchyma fraction | 0.04 ± 0.2 | 0.03 ± 0.05 | 2.41 | 0.13 | 0.16 | 0.09 | 0.56 |
| Potential hydraulic conductivity | 9.32 ± 3.05 | – | 0.94 | 0.41 | 0.51 | 0.64 | 0.69 |
Vessel wall thickness, vessel grouping index, potential hydraulic conductivity and fractions of secondary xylem cells (vessel, fibre, ray and axial parenchyma) were not statistically different between functional groups (Fig. 2; Tables 2 and 3). However, these wood traits varied substantially across tree, shrub and subshrub species. The largest variations in wood traits were observed across subshrub species: potential hydraulic conductivity varied 13.9-fold (from 1 kg m−1 MPa−1 s−1 in Arachis lignosa to 13.9 kg m−1 MPa−1 s−1 in Stylosanthes hamata), vessel fraction varied 4.2-fold (from 7.7 % in Senna pilifera to 32.4 % in Stylosanthes hamata), fibre fraction varied 33.1-fold (from 0.6 % in Senna pilifera to 19.9 % in Stylosanthes hamata), ray fraction varied 3.6-fold (from 6.6 % in Stylosanthes hamata to 23.5 % in Tephrosia chaquenha), and axial parenchyma fraction varied 2-fold (from 41.1 % in Stylosanthes hamata to 82.2 % in Senna pilifera). Vessel grouping index and axial parenchyma fraction also varied substantially across shrub species: vessel grouping index varied 4.6-fold (from 1.8 vessels per group in Bauhinia hagenbeckii to 8.4 vessels per group in Mimosa polycarpa) and axial parenchyma fraction varied 28-fold (from 1.9 % in Mimosa polycarpa to 52.4 % in Mimosa sensibilis var. urucumensis). Among tree species, potential hydraulic conductivity varied 4.2-fold (from 1.7 kg m−1 MPa−1 s−1 in Mimosa hexandra to 7.2 kg m−1 MPa−1 s−1 in Prosopis ruscifolia), and fibre fraction varied 3.9-fold (from 7.6 % in Parkinsonia praecox to 29.7 % in Mimosa hexandra).
The root wood anatomical features were similar among functional groups (tree, shrub and subshrub species), except for vestured pits, axial parenchyma arrangement and fibre type. Poorly defined growth rings were observed in all species studied (Fig. 3A, B). Vessels were diffuse-porous (Fig. 3A–I), solitary (Fig. 3A–D), and with two (Fig. 3C, D) to eight (Fig. 3F) vessels clustered; simple perforation plates (Fig. 4A), alternate intervessel pits (Fig. 4B) and vessel-ray pits with distinct borders, similar to intervessel pits in size and shape throughout the ray cell. Vessels with vestured pits were observed in all species (Fig. 4C), except the shrub species Bauhinia hagenbeckii (Fig. 4D) and subshrub species Senna pilifera. We observed gelatinous fibres in all species studied (Fig. 3A, D–I), except for the tree species Parkinsonia praecox and Prosopis rubriflora. In these species, we observed thin-walled libriform fibres in Parkinsonia praecox (Fig. 3B) and very thick-walled libriform fibres in Prosopis rubriflora (Fig. 3C). In all studied species, except the shrub species Mimosa polycarpa, the axial parenchyma was paratracheal in bands more than three cells wide (Fig. 3A–E, G–I). In the shrub species Mimosa polycarpa, the axial parenchyma was vasicentric (Fig. 3F). Non-lignified paratracheal parenchyma was observed in the shrub species Bauhinia hagenbeckii (Fig. 3D) and subshrub species Arachis lignosa, Galactia paraguariensis, Stylosanthes hamata (Fig. 3H) and Tephrosia chaquenha (Fig. 3I). We observed rays one to three cells wide (Fig. 4E, F) and the body with procumbent cells in all species (Fig. 4G). Detailed anatomical descriptions of the root secondary xylem of the studied species are provided in the Supplementary Data S1.
Fig. 3.

Root secondary xylem cross-sections of Fabaceae species. (A–C) Cross-sections of the tree species Mimosa hexandra (A), Parkinsonia praecox (B) and Prosopis rubriflora (C). (D–F) Cross-sections of shrub species Bauhinia hagenbeckii (D), Mimosa sensibilis var. urucumensis (E) and Mimosa polycarpa (F). Note the non-lignified paratracheal parenchyma in Bauhinia hagenbeckii (D) and gelatinous fibres in Bauhinia hagenbeckii (D) and Mimosa sensibilis var. urucumensis (E). (G–I) Cross-sections of the subshrub species Senna pilifera (G), Stylosanthes hamata (H) and Tephrosia chaquenha (I). Note gelatinous fibres (*) and non-lignified paratracheal parenchyma (arrowheads) in Stylosanthes hamata (H) and Tephrosia chaquenha (I). Scale bars: 100 μm in A–I.
Fig. 4.
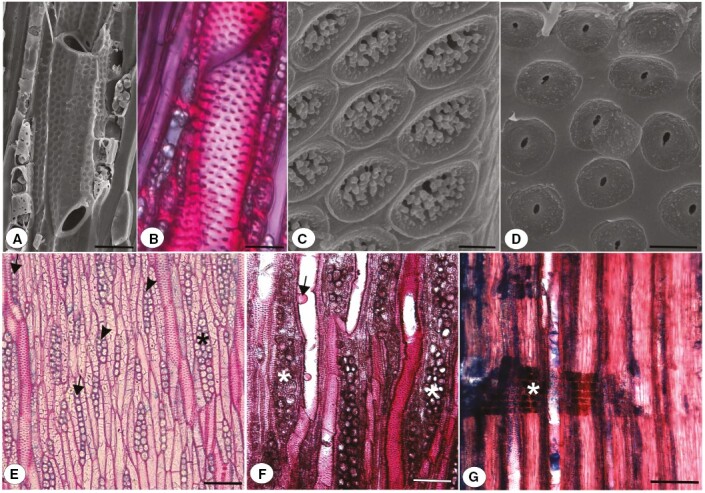
Details of the root secondary xylem of Fabaceae species. (A) Simple perforation plate in vessel of Bauhinia hagenbeckii, scanning electron micrograph. (B) Alternate intervessel pits Mimosa sensibilis var. urucumensis, tangential longitudinal section. (C) Vestured pits in scanning electron micrograph of the tree species Mimosa glutinosa. (D) Non-vestured pits in Bauhinia hagenbeckii. (E) Rays with a width of one (arrows), two (arrowheads) and three cells (*) in Parkinsonia praecox, tangential longitudinal section. (F) Rays with a width of three cells (*) and tyloses (arrow) in Mimosa polycarpa, tangential longitudinal section. (G) Procumbent ray cells (*) in Mimosa polycarpa, radial longitudinal section. Scale bars: 20 μm in A, B; 1 μm in C; 2 μm in D; 50 μm in E–G.
DISCUSSION
In this study, we investigated whether the secondary xylem traits of roots differ between growth forms (tree, shrub and subshrub) of Fabaceae species that co-occur in the seasonally dry Neotropical environment. The results showed that only vessel diameter and density varied among growth forms, with larger vessels being observed in tree species. Other vessel features (wall thickness, grouping index and potential hydraulic conductivity) and xylem cell fractions (vessels, fibres, rays and axial parenchyma) were not statistically different among the growth forms, indicating the wide range of variation in root secondary xylem traits within the functional groups studied.
As expected, tree species were found to have larger vessels than shrub and subshrub species. In general, our results confirm the findings of previous studies based on anatomical studies of the secondary xylem of roots (Carlquist and Hoekman, 1985; Martínez-Cabrera et al., 2011) and indicate that the vessel diameter varies as a function of plant size (Olson and Rosell, 2013) and probably also as a function of root size. Tree species also showed lower vessel density, highlighting the inversely proportional ratio of vessel diameter and density in wood (Baas et al., 2004). Despite the larger vessels in the tree species, potential hydraulic conductivity was not statistically different between growth forms. This is likely to be explained by the broad range of variation in vessel density within the studied functional groups.
Larger vessels in tree species enhance water transport efficiency, but they can also increase the vulnerability to drought-induced cavitation (Hacke and Sperry, 2001; Hacke et al., 2006). Taking into account that species occurring in the Brazilian Chaco are exposed to the stress conditions of drought in winter and flood in summer (Pott et al., 2011), both conductivity efficiency and safety are key traits to guarantee water transport. The species and groups presented high intra- and interspecific variation of wood traits. This could help to maintain the species in the environment, given that narrow vessels, with a minor tangential area, promote more tension to maintain the integrity of the water column under higher vapour pressure, which might cause cavitation and/or embolism events during the dry season (Hacke and Sperry, 2001; Bucci et al., 2004). This trait is common and expected in species inhabiting dry environments (Alves and Angyalossy-Alfonso, 2000; Sonsin et al., 2012). In contrast, wide vessels ensure efficiency during the hot and flooded season, when it is necessary to move a larger volume of water to maintain transport and transpiration. All of these vessels communicate and create a net, combining different vessel sizes and densities to ensure safety and efficiency in different environmental conditions (Zanne et al., 2010). In our study, most Fabaceae species, regardless of growth habit, showed vessels with different diameter classes (see Fig. 2; Table 2). The occurrence of different vessel diameter classes within the secondary xylem, known as diameter polymorphism, could be a valuable feature to provide both efficient and safe transport in a strongly seasonal environment (Jacobsen and Pratt, 2023).
In angiosperms, the intervessel pit features are important anatomical traits that can influence vessel function and minimize air-seeding dispersion (Lens et al., 2011). There are two hypotheses that suggest the function of vestured pits in the air-seeding mechanism: first, vestured pits could trap air bubbles during air seeding; and second, vestured pits could prevent the rupture of the pit membrane during its stretching in air seeding (Carlquist, 2001; Jansen et al., 2003). Despite limited knowledge of how vestured pits function to minimize air bubble dispersal during air seeding, such structures are common in species that evolved in warm and dry environments, such as most Fabaceae species.
We hypothesized that tree species would have a larger fibre cross-sectional area than shrub and subshrub species. Contrary to our expectation, the amount of fibre was not statistically different among the growth forms studied. This result can be explained, in part, by the wide variation in fibre cross-sectional area within the groups studied. Fibre fraction varied 3.9-fold in tree species (from 7.6 % in Parkinsonia praecox to 29.7 % in Mimosa hexandra), 2.2-fold in shrub species (from 22 % in Bauhinia hagenbeckii to 49.2 % in Mimosa polycarpa) and 33.1-fold in subshrub species (from 0.6 % in Senna pilifera to 19.9 % in Stylosanthes hamata). Fibres are specialized cells that provide mechanical strength to support the plant body against gravity and provide mechanical stability (Ennos, 1993). In the root, the surrounding soil matrix and soil physical properties also contribute to mechanical support and maintenance of plants anchored to the soil (Pratt et al., 2007; Fortunel et al., 2014). For example, soil moisture content influences plant stability, because the decrease in the surrounding soil moisture caused by root water uptake leads to an increase in matrix suction and soil mechanical strength (Easson et al., 1995; Schwarz et al., 2010). Clayey soils also provide greater resilience to plant movement than other soil types (Ennos, 2000). Thus, in environments with clay soils and prolonged periods of drought, such as the Brazilian Chaco, low soil moisture and the soil physical properties would act as major drivers for plant anchorage.
We observed gelatinous fibres in the root secondary xylem of the studied species, except in the tree species Parkinsonia praecox and Prosopis rubriflora. Gelatinous fibres are characterized by the presence of an inner cell wall with concentric cellulose layers and other polysaccharides, such as hemicelluloses, pectins or arabinogalactan-proteins (Evert, 2006; Bowling and Vaughn, 2008). Gelatinous fibres are related to organ movement, providing a strong tensile force that enables plant organs to bend, straighten or remain upright (Chery et al., 2022). The unique cell wall of gelatinous fibres could increase the mechanical resistance of roots against bending stress during external events, such as wind, and mitigate rotational effects when the plant is pulled from the soil by large mammals in grazing. In addition, the cellulose-rich inner layer of gelatinous fibres has a high water-absorption capacity (Carlquist, 2001), which could act as an important source of water storage in the roots of the studied species. However, experimental studies are necessary to understand the function of gelatinous fibres in water storage in plant organs.
It was expected that there would be a larger parenchyma cell fraction (rays and axial parenchyma) in subshrub species, because these species have resprouting potential from the root crown and roots. However, we did not observe a statistically different amount of rays and axial parenchyma among the functional groups studied. Paratracheal parenchyma occupying a large cross-sectional area of the secondary xylem is a common feature in Fabaceae species (Dória et al., 2022) and, except for the subshrub species Mimosa polycarpa, the species studied had >40 % of the area of secondary xylem of the roots occupied by parenchyma tissue, highlighting the storage function of this organ. Parenchymal cells act in the storage and transport of non-structural carbohydrates (Morris and Jansen 2016; Plavcová et al., 2016) that support the growth of new stems after overgrazing, trampling by cattle, droughts and frosts, which are recurring events in Chaco vegetation. Furthermore, it is essential to emphasize that in Brazil, the remaining Chaco vegetation is located in the Pantanal wetlands (Pott et al., 2011), which are exposed to a high frequency of fire in the dry season, especially in recent years (Kumar et al., 2022), and a high proportion of parenchymal cells could be a key trait for post-fire plant persistence (Clarke et al., 2013).
A high fraction of parenchyma in roots could provide an extra area to store carbohydrates synthesized in green stems, a common feature in the studied tree species. Chloroplasts were observed in branches of all the tree species, and in Parkinsonia praecox chloroplasts were also found in the bark and wood tissues of the main stem and roots when exposed to light. Photosynthesis in bark and wood tissue is considered an additional strategy for whole-plant carbon gain and water economy in species with microphyllous or absent leaves during dry conditions and high temperatures in a seasonally dry environment (Aschan and Pfanz, 2003). Furthermore, photosynthesis in bark and wood tissue plays a role in repair of xylem embolism, contributing to the maintenance of hydraulic function in periods of reduced water availability (Bloemen et al., 2016).
In general, distinct growth forms occupy different niches within and across the environments, hence they exhibit different ecological strategies for the use and conservation of the major resources (water, light and soil nutrients; Martínez-Cabrera et al., 2011; Rossatto and Franco, 2017). Our findings, however, showed a high interspecific variation of root secondary xylem traits, regardless of growth form. Interspecific variability provides diversification of functional strategies in plants to cope with limited resources in seasonal environments, thus enabling their coexistence in the environment and reducing competitive interaction between species (Silva and Batalha, 2011).
Secondary xylem is a vascular tissue that exhibits high plasticity in the dimensions and number of its cells, features that have been used to differentiate growth forms in ecological anatomy studies. In our study, the similarity of secondary xylem trait estimates among growth forms might have been limited by our sample size. The inclusion of further specimens (or individuals), even if it does not change the estimates found, could enhance our understanding of these traits in the diverse spectrum of growth forms. Furthermore, we highlight that the estimates presented here could also be used as support for a broader study.
In this study, we measured root secondary xylem anatomical traits in 14 Fabaceae species from the seasonally dry Neotropical environment to assess whether the growth forms (tree, shrub and subshrub) show distinct strategies for water transport, mechanical support and non-structural carbon and water storage. Larger vessels in tree species suggest an effect of organ size on vessel diameter. Our results also highlighted high interspecific variability in potential hydraulic conductivity and secondary xylem cell fractions (vessels, fibres, rays and axial parenchyma) within growth forms. Altogether, our results suggest that the high variability in secondary xylem traits at the species level allows multiple strategies for water transport, mechanical support and non-structural carbon storage by species of different growth forms in environments with strong seasonal variation in water availability.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Data S1: anatomical descriptions of secondary xylem.
ACKNOWLEDGEMENTS
The authors declare no conflict of interest.
Contributor Information
Jane Rodrigues da Silva, Laboratório de Anatomia Vegetal, Instituto de Biociências (Inbio), Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, Mato Grosso do Sul 79070-900, Brazil.
Tamires Soares Yule, Laboratório de Anatomia Vegetal, Instituto de Biociências (Inbio), Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, Mato Grosso do Sul 79070-900, Brazil.
Augusto Cesar de Aquino Ribas, Agência de Tecnologia da Informação e Comunicação, Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, Mato Grosso do Sul 79070-900, Brazil.
Edna Scremin-Dias, Laboratório de Anatomia Vegetal, Instituto de Biociências (Inbio), Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, Mato Grosso do Sul 79070-900, Brazil.
AUTHOR CONTRIBUTIONS
J.R.S., T.S.Y., and E.S.-D. conceived and designed the study. J.R.S. and T.S.Y. collected and prepared the samples and conducted the anatomical analyses. J.R.S., and A.C.A.R. analysed the data. J.R.S., T.S.Y., A.C.A.R. and E.S.-D. wrote the manuscript.
DATA AVAILABILITY
The authors declare that all the data is available in the tables in the manuscript and as supplementary material.
FUNDING
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for funding the first and second authors’ graduate research; to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support (project number 620176/2008-3), to Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul—FUNDECT for financial support (project number 71/031.968/2022); and to the Postgraduate Program in Biologia Vegetal of the Federal University of Mato Grosso do Sul.
LITERATURE CITED
- Alves ES, Angyalossy-Alfonso V.. 2000. Ecological trends in the wood anatomy of some Brazilian species. 1. Growth rings and vessels. IAWA Journal 21: 3–30. doi: 10.1163/22941932-90000233. [DOI] [Google Scholar]
- Alves FDM, Sartori ALB.. 2009. Caesalpinioideae (Leguminosae) de um remanescente de Chaco em Porto Murtinho, Mato Grosso do Sul, Brasil. Rodriguésia 60: 531–550. doi: 10.1590/2175-7860200960305. [DOI] [Google Scholar]
- Appezzato-da-Glória B. 2015. Morphology of plant underground systems, 3rd edn. Belo Horizonte: 3i Editora. [Google Scholar]
- Aschan G, Pfanz H.. 2003. Non-foliar photosynthesis – a strategy of additional carbon acquisition. Flora – Morphology, Distribution, Functional Ecology of Plants 198: 81–97. doi: 10.1078/0367-2530-00080. [DOI] [Google Scholar]
- Assunção VA, Silva DMD, Dalponti G, Sartori ALB, Casagrande JC, Mansano VDF.. 2021. Environmental filters structure plant communities in the Brazilian Chaco. Acta Botanica Brasilica 34: 746–754. doi: 10.1590/0102-33062020abb0205. [DOI] [Google Scholar]
- Baas P, Ewers FW, Davis SD, Wheeler EA.. 2004. Evolution of xylem physiology. In: Hemley AR, Poole I, eds. The evolution of plant physiology. London, San Diego: Elsevier Academic Press, 273–295. [Google Scholar]
- Baptista MSP, Assunção VA, Bueno ML, Casagrande JC, Sartori ALB.. 2020. Species representativeness of Fabaceae in restrictive soils explains the difference in structure of two types of chaco vegetation. Acta Botanica Brasilica 34: 559–569. doi: 10.1590/0102-33062020abb0064. [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker BM, Walker SC.. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bennett HS, Wyrick AD, Lee SW, McNeil JH.. 1976. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technology 51: 71–97. doi: 10.3109/10520297609116677. [DOI] [PubMed] [Google Scholar]
- Bloemen J, Vergeynst LL, Overlaet-Michiels L, Steppe K.. 2016. How important is woody tissue photosynthesis in poplar during drought stress? Trees 30: 63–72. doi: 10.1007/s00468-014-1132-9. [DOI] [Google Scholar]
- Borchert R. 1994. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75: 1437–1449. doi: 10.2307/1937467. [DOI] [Google Scholar]
- Bowling AJ, Vaughn KC.. 2008. Immunocytochemical characterization of tension wood: gelatinous fibers contain more than just cellulose. American Journal of Botany 95: 655–663. doi: 10.3732/ajb.2007368. [DOI] [PubMed] [Google Scholar]
- Bucci SJ, Goldstein G, Meinzer FC, Scholz FG, Franco AC, Bustamante M.. 2004. Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiology 24: 891–899. doi: 10.1093/treephys/24.8.891. [DOI] [PubMed] [Google Scholar]
- Bukatsch F. 1972. Bemerkungen zur Doppelfärbung Astrablau-Safranin. Mikrokosmos 61: 33–36. [Google Scholar]
- Carlquist S. 1984. Vessel grouping in dicotyledon wood: significance and relationship to imperforate trachery elements. Aliso 10: 505–525. doi: 10.5642/aliso.19841004.03. [DOI] [Google Scholar]
- Carlquist S. 2001. Comparative wood anatomy: systematic, ecological, and evolutionary aspects of dicotyledon wood, 2nd edn. New York: Springer. [Google Scholar]
- Carlquist S, Hoekman DA.. 1985. Ecological wood anatomy of the woody-Southern Californian flora. IAWA Bulletin 6: 19–347. [Google Scholar]
- Carvalho FS, Sartori ALB.. 2015. Reproductive phenology and seed dispersal syndromes of woody species in the Brazilian Chaco. Journal of Vegetation Science 26: 302–311. doi: 10.1111/jvs.12227. [DOI] [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA.. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. doi: 10.1146/annurev.es.21.110190.002231. [DOI] [Google Scholar]
- Chaturvedi RK, Raghubanshi AS, Singh JS.. 2011. Plant functional traits with particular reference to tropical deciduous forests: a review. Journal of Biosciences 36: 963–981. doi: 10.1007/s12038-011-9159-1. [DOI] [PubMed] [Google Scholar]
- Chery JG, Glos RA, Anderson CT.. 2022. Do woody vines use gelatinous fibers to climb? New Phytologist 233: 126–131. doi: 10.1111/nph.17576. [DOI] [PubMed] [Google Scholar]
- Clarke PJ, Lawes MJ, Midgley JJ, et al. 2013. Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytologist 197: 19–35. doi: 10.1111/nph.12001. [DOI] [PubMed] [Google Scholar]
- Couto EG, Oliveira VA.. 2014. The soil diversity of the Pantanal. In: Junk WJ, Silva CJ, Cunha CN, Wantzen KM. eds. The Pantanal: ecology, biodiversity and sustainable management of a large neotropical seasonal wetland. Sofia: Pensoft, 71–102. [Google Scholar]
- Dória LC, Podadera DS, Batalha MA, Lima RS, Marcati CR.. 2016. Do woody plants of the Caatinga show a higher degree of xeromorphism than in the Cerrado? Flora 224: 244–251. doi: 10.1016/j.flora.2016.09.002. [DOI] [Google Scholar]
- Dória LC, Sonsin-Oliveira J, Rossi S, Marcati CR.. 2022. Functional trade-offs in volume allocation to xylem cell types in 75 species from the Brazilian savanna Cerrado. Annals of Botany 130: 445–456. doi: 10.1093/aob/mcac095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easson DL, Pickles SJ, White EM.. 1995. A study of the tensile force required to pull wheat roots from soil. Annals of Applied Biology 127: 363–373. doi: 10.1111/j.1744-7348.1995.tb06680.x. [DOI] [Google Scholar]
- Ennos AR. 1993. The scaling of root anchorage. Journal of Theoretical Biology 161: 61–75. doi: 10.1006/jtbi.1993.1040. [DOI] [Google Scholar]
- Ennos AR. 2000. The mechanics of root anchorage. Advances in Botanical Research 33: 133–157. doi: 10.1016/S0065-2296(00)33042-7. [DOI] [Google Scholar]
- Evert R. 2006. Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development, 3rd edn. New Jersey: John Wiley & Sons. [Google Scholar]
- Ewers FW, Ewers JM, Jacobsen AL, López-Portillo J.. 2007. Vessel redundancy: modeling safety in numbers. IAWA Journal 28: 373–388. doi: 10.1163/22941932-90001650. [DOI] [Google Scholar]
- Ewers FW, Jacobsen AL, López-Portillo J.. 2023. Carlquist’s indices for vulnerability and mesomorphy of wood: are they relevant today? IAWA Journal 1: 1–13. doi: 10.1163/22941932-bja10113. [DOI] [Google Scholar]
- Felker P, Grados N, Cruz G, Prokopiuk D.. 2003. Economic assessment of production of flour from Prosopis alba and P. pallida pods for human food applications. Journal of Arid Environments 53: 517–528. doi: 10.1006/jare.2002.1064. [DOI] [Google Scholar]
- Fortunel C, Ruelle J, Beauchêne J, Fine PV, Baraloto C.. 2014. Wood specific gravity and anatomy of branches and roots in 113 Amazonian rainforest tree species across environmental gradients. New Phytologist 202: 79–94. doi: 10.1111/nph.12632. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S.. 2019. An R companion to applied regression, 3rd edn. Thousand Oaks: SAGE Publications Inc. [Google Scholar]
- Franklin G. 1945. Preparation of thin sections of synthetic resins and wood-resins composites, and a new macerating method for wood. Nature 155: 51. [Google Scholar]
- Hacke UG, Sperry JS.. 2001. Functional and ecological xylem anatomy. Perspectives in Plant Ecology, Evolution and Systematics 4: 97–115. doi: 10.1078/1433-8319-00017. [DOI] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L.. 2006. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology 26: 689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- IAWA Committee. 1989. List of microscopic features for hardwood identification. IAWA Bulletin 10: 220–332. [Google Scholar]
- Jacobsen AL, Pratt RB.. 2023. Vessel diameter polymorphism determines vulnerability-to-embolism curve shape. IAWA Journal 1: 1–15. doi: 10.1163/22941932-bja10115. [DOI] [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD.. 2007. Cavitation resistance among 26 chaparral species of southern California. Ecological Monographs 77: 99–115. doi: 10.1890/05-1879. [DOI] [Google Scholar]
- Jansen S, Baas P, Gasson P, Smets E.. 2003. Vestured pits: do they promote safer water transport? International Journal of Plant Sciences 164: 405–413. doi: 10.1086/374369. [DOI] [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw-Hill Book Company. [Google Scholar]
- Kraus JE, Arduin M.. 1997. Manual básico de métodos em morfologia vegetal. Seropédica: EDUR. [Google Scholar]
- Kumar S, Getirana A, Libonati R, Hain C, Mahanama S, Andela N.. 2022. Changes in land use enhance the sensitivity of tropical ecosystems to fire‑climate extremes. Scientific Reports 12: 964. doi: 10.1038/s41598-022-05130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S.. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist 190: 709–723. doi: 10.1111/j.1469-8137.2010.03518.x. [DOI] [PubMed] [Google Scholar]
- Lima JR, Tozzi AMG, Mansano VF.. 2015. A checklist of woody Leguminosae in the South American corridor of dry vegetation. Phytotaxa 207: 1–38. doi: 10.11646/phytotaxa.207.1.1. [DOI] [Google Scholar]
- Martínez-Cabrera HI, Schenk HJ, Cevallos-Ferriz SRS, Jones CS.. 2011. Integration of vessel traits, wood density, and height in angiosperm shrubs and trees. American Journal of Botany 98: 915–922. doi: 10.3732/ajb.1000335. [DOI] [PubMed] [Google Scholar]
- Meijer GJ. 2021. A generic form of fibre bundle models for root reinforcement of soil. Plant and Soil 468: 45–65. doi: 10.1007/s11104-021-05039-z. [DOI] [Google Scholar]
- Morris H, Jansen S.. 2016. Secondary xylem parenchyma – from classical terminology to functional traits. IAWA Journal 37: 1–15. doi: 10.1163/22941932-20160117. [DOI] [Google Scholar]
- Noguchi DK, Nunes GP, Sartori ALB.. 2009. Florística e síndromes de dispersão de espécies arbóreas em remanescentes de Chaco de Porto Murtinho, Mato Grosso do Sul, Brasil. Rodriguésia 60: 353–365. doi: 10.1590/2175-7860200960208. [DOI] [Google Scholar]
- O’Brien TP, Feder N, McCully ME.. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368–373. doi: 10.1007/bf01248568. [DOI] [Google Scholar]
- Olson ME, Rosell JA.. 2013. Vessel diameter–stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytologist 197: 1204–1213. doi: 10.1111/nph.12097. [DOI] [PubMed] [Google Scholar]
- Pennington RT, Prado DE, Pendry CA.. 2000. Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography 27: 261–273. doi: 10.1046/j.1365-2699.2000.00397.x. [DOI] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2016. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 64: 715–716. doi: 10.1071/bt12225_co. [DOI] [Google Scholar]
- Plavcová L, Hoch G, Morris H, Ghiasi S, Jansen S.. 2016. The amount of parenchyma and living fibers affects storage of nonstructural carbohydrates in young stems and roots of temperate trees. American Journal of Botany 103: 603–612. doi: 10.3732/ajb.1500489. [DOI] [PubMed] [Google Scholar]
- Poorter L, McDonald I, Alarcón A, et al. 2010. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytologist 185: 481–492. doi: 10.1111/j.1469-8137.2009.03092.x. [DOI] [PubMed] [Google Scholar]
- Pott A, Oliveira AK, Damasceno-Junior GA, Silva JS.. 2011. Plant diversity of the Pantanal wetland. Brazilian Journal of Biology 71: 265–273. doi: 10.1590/s1519-69842011000200005. [DOI] [PubMed] [Google Scholar]
- Pratt RB, Jacobsen AL, Ewers FW, Davis SD.. 2007. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytologist 174: 787–798. doi: 10.1111/j.1469-8137.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Roeser K. 1972. Die Nadel der Schwarzkiefer - Massenprodukt und Kunstwerk der Natur. Mikrokosmos 61: 33–36. [Google Scholar]
- Rossatto DR, Franco AC.. 2017. Expanding our understanding of leaf functional syndromes in savanna systems: the role of plant growth form. Oecologia 183: 953–962. doi: 10.1007/s00442-017-3815-6. [DOI] [PubMed] [Google Scholar]
- Schenk HJ, Jackson RB.. 2002. The global biogeography of roots. Ecology Monographs 72: 311–328. doi: 10.1890/0012-9615(2002)072[0311:tgbor]2.0.co;2. [DOI] [Google Scholar]
- Schielzeth H, Nakagawa S.. 2013. Nested by design: model fitting and interpretation in a mixed model era. Methods in Ecology and Evolution 4: 14–24. doi: 10.1111/j.2041-210x.2012.00251.x. [DOI] [Google Scholar]
- Scholz A, Klepsch M, Karimi Z, Jansen S.. 2013. How to quantify conduits in wood? Frontiers in Plant Science 4: 56. doi: 10.3389/fpls.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Cohen D, Or D.. 2010. Root‐soil mechanical interactions during pullout and failure of root bundles. Journal of Geophysical Research, Earth Surface 115: F04035. [Google Scholar]
- Silva IA, Batalha MA.. 2011. Plant functional types in Brazilian savannas: the niche partitioning between herbaceous and woody species. Perspectives in Plant Ecology, Evolution and Systematics 13: 201–206. doi: 10.1016/j.ppees.2011.05.006. [DOI] [Google Scholar]
- Silva BHP, Rossatto DR.. 2019. Are underground organs able to store water and nutrients? A study case in non-arboreal species from the Brazilian Cerrado. Theoretical and Experimental Plant Physiology 9: 413–421. doi: 10.1007/s40626-019-00155-9. [DOI] [Google Scholar]
- Silva JR, Yule TS, Scremin-Dias E.. 2020. Structural features and contribution of belowground buds to conservation of Fabaceae species in a seasonally dry Neotropical environment. Flora 264: 151570. doi: 10.1016/j.flora.2020.151570. [DOI] [Google Scholar]
- Sonsin JO, Gasson PE, Barros CF, Marcati CR.. 2012. A comparison of the wood anatomy of 11 species from two cerrado habitats (cerrado s.s. and adjacent gallery forest). Botanical Journal of the Linnean Society 170: 257–276. doi: 10.1111/j.1095-8339.2012.01282.x. [DOI] [Google Scholar]
- Therneau T, Atkinson B, Ripley B.. 2015. Recursive Partitioning and Regression Trees. R Package Version 4.1-8. http://CRAN.R-project.org/package=rpart (13 January 2023, date last accessed).
- Tyree M, Zimmermann M.. 2002. Xylem structure and the ascent of sap, 2nd edn. New York: Springer. [Google Scholar]
- Tyree MT, Davis SD, Cochard H.. 1994. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA Journal 15: 335–360. doi: 10.1163/22941932-90001369. [DOI] [Google Scholar]
- UNESCO. 1973. International classification and mapping of vegetation. Paris: Units Nations Educational Scientific and Cultural Organization. [Google Scholar]
- Villagra PE, Vilela A, Giordano C, Alvarez JÁ.. 2009. Ecophysiology of Prosopis species from the arid lands of Argentina: what do we know about adaptation to stressful environments? In: Ramawat K, ed. Desert plants: biology and biotechnology. New York: Springer, 321–340. [Google Scholar]
- Zanne AE, Westoby M, Falster DS, et al. 2010. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. American Journal of Botany 97: 207–215. doi: 10.3732/ajb.0900178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data is available in the tables in the manuscript and as supplementary material.


