Abstract
Background and Aims
Phenotypic plasticity allows plants to cope with environmental variability. Plastic responses to the environment have mostly been investigated at the level of individuals (plants) but can also occur within leaves. Yet the latter have been underexplored, as leaves are often treated as functional units with no spatial structure. We investigated the effect of a strong light gradient on plant and leaf traits and examined whether different portions of a leaf show similar or differential responses to light intensity.
Methods
We measured variation in 27 morpho-anatomical and physiological traits of the rosette and leaf portions (i.e. base and apex) of the tank bromeliad Aechmea aquilega (Bromeliaceae) when naturally exposed to a marked gradient of light intensity.
Key Results
The light intensity received by A. aquilega had a strong effect on the structural, biochemical and physiological traits of the entire rosette. Plants exposed to high light intensity were smaller and had wider, shorter, more rigid and more vertical leaves. They also had lower photosynthetic performance and nutrient levels. We found significant differences between the apex and basal portions of the leaf under low-light conditions, and the differences declined or disappeared for most of the traits as light intensity increased (i.e. leaf thickness, adaxial trichome density, abaxial and adaxial trichome surface, and vascular bundle surface and density).
Conclusions
Our results reveal a strong phenotypic plasticity in A. aquilega, particularly in the form of a steep functional gradient within the leaf under low-light conditions. Under high-light conditions, trait values were relatively uniform along the leaf. This study sheds interesting new light on the functional complexity of tank bromeliad leaves, and on the effect of environmental conditions on leaf trait regionalization.
Keywords: Aechmea aquilega, functional traits, leaf anatomy, leaf morphology, leaf regionalisation, light intensity gradient, phenotypic plasticity, tank bromeliad
INTRODUCTION
Phenotypic plasticity, sensu stricto, is the ability of a genotype to display different phenotypes according to the environment while the response of different species and populations in their ecological context is referred to as phenotypic plasticity sensu lato (Valladares et al., 2006). Sessile organisms, such as plants, cannot escape adverse environmental conditions and thus rely heavily on phenotypic plasticity sensu lato to cope with variability in light, temperature, water and nutrient availability, and wind or pollutant exposure (e.g. Audet and Charest, 2008; Bossdorf and Pigliucci, 2009; Fromm, 2019). Among these factors, light has a major influence on plant life because it provides energy for photosynthesis and controls individual growth and development. The heterogeneous light environment in many ecosystems requires acclimation to different light regimes, achieved through adjustments at both the whole-plant and the leaf level (Givnish, 1988). However, phenotypic plasticity is not necessarily a whole-plant response (De Kroon et al., 2005), but rather a property of individual meristems, leaves, branches and roots, triggered by local environmental conditions.
Leaf traits display extraordinary plasticity in response to varying environmental conditions (Sultan, 2000; Gratani, 2014). Hence, leaves play important roles in plant life strategies (Wright et al., 2004; Shi et al., 2020) especially because they are directly involved in photosynthetic processes (Mathur et al., 2018). This has led to numerous studies on leaf trait responses to light and researchers have distinguished sun-exposed from shaded species based on well-documented leaf traits in a wide range of plant taxa (e.g. Niinemets and Valladares, 2004; Rozendaal et al., 2006). This holds at the individual scale with the modification of morpho-anatomical and physiological leaf traits in response to the light gradient formed as light penetrates the foliage. According to their position and exposure to light, leaves of the same plant can display shade- or sun-associated traits (Niinemets, 2010; Niinemets et al., 2015). Sun and shade leaves differ predictably in several functional traits (Popma and Bongers, 1988). Typically, sun leaves are smaller and thicker, have higher leaf dry mass per surface area (LMA), have a thicker palisade parenchyma, epidermis and cuticle, and may also have greater stomata density compared with shade leaves from the same individual (Givnish, 1988; Terashima et al., 2001; Dörken and Lepetit, 2018). Furthermore, in sun leaves the photosynthetic rate and total nitrogen are higher and chlorophyll content is lower than in shade leaves (Gratani et al., 2006; Niinemets, 2010).
Bromeliads (Bromeliaceae) are a family of flowering plants native to the Neotropics and representing some 3755 species (Gouda and Butcher, 2023). Bromeliads are either epiphytes or lithophytes or are rooted on the soil. The leaves of tank-forming bromeliads are tightly interlocking, forming wells that collect rainwater and detritus. Tank bromeliads span a broad range of habitat and understorey conditions, leading to a wide range of structural and functional adaptations (Benzing, 2000). They are therefore relevant model species to evaluate the effects of multivariate environmental change on phenotypic plasticity. Some bromeliad species are found either in sun-exposed or shaded areas under sun or shade plants while others experience very heterogeneous light environments (Barberis et al., 2017). Light exposure can affect whole-plant characteristics with variations in the shape of the rosette, leaf colour and leaf size indicative of more compact rosettes in high light compared with low light level (Cavallero et al., 2009, 2011; Rodrigues Pereira et al., 2013; Barberis et al., 2017). Light can also affect morpho-anatomical, biochemical and physiological leaf traits (e.g. Medina et al., 1986; Ceusters et al., 2011; Rodrigues Pereira et al., 2013; North et al., 2016). Rodrigues Pereira et al. (2013) found that trichome density was higher in both Billbergia elegans and Neoregelia mucugensis when exposed to high light. These authors also found that the thickness of the leaf blade (along with water-storage parenchyma and chlorenchyma) and stomatal density were higher in individuals exposed to low light whereas Leroy et al. (2019) found the opposite for Aechmea mertensii. Previous investigations have established for Guzmania monostachia a strong photoinhibition and a decrease by ~50 % in total chlorophyll with increasing light condition (Maxwell et al., 1995). Finally, North et al. (2016), found that the effects of different light conditions on leaf hydraulic conductances were divergent for G. monostachia and G. lingulata.
Most of the above-mentioned studies have examined trait variation by sampling and comparing the intermediate portion of leaves across environments. Indeed, leaves are often treated as single functional units with no spatial structure. However, it has been shown for both dicotyledonous and monocotyledonous species that structural and functional traits are heterogeneous and can vary across leaf portions (Nardini et al., 2008; Li et al., 2013). This is particularly true of tank bromeliads, where the basal leaf portions are in direct contact with the water and organic debris that accumulates in the tank, whereas the apical area is aerial and receives higher amounts of light. The plant obtains nutrients through the absorbing trichomes on the surface of the basal part of the leaf (Leroy et al., 2016; Kleingesinds et al., 2018). The intermediate and apical portions of a leaf capture light and ensure photosynthesis (Pikart et al., 2018). This functional duality implies morpho-anatomical, biochemical or physiological differences between the different portions of the same leaf. Trichomes and stomata densities show a clear inverse relationship, with more stomata in the apical portion and more trichomes in the basal portion (Freschi et al., 2010; Rodrigues et al., 2016; Kleingesinds et al., 2018). Nitrogen metabolism is also partitioned along the leaf lamina. The basal portion is preferentially involved in nitrogen absorption (e.g. nitrate, ammonium, urea), nitrate reduction and urea hydrolysis, while the apical portion could be the main area responsible for ammonium assimilation into glutamine through the action of glutamine synthetase and glutamate dehydrogenase (Takahashi et al., 2007; Takahashi and Mercier, 2011; Gonçalves et al., 2020).
The objective of this study was to investigate whether different portions of the leaf have a similar or differential response to the environment for tank bromeliads experiencing a strong light gradient. We evaluated differences in several morpho-anatomical and physiological leaf traits of the tank bromeliad Aechmea aquilega along a marked gradient of light intensity. We focused on the whole-rosette and leaf portion levels. We hypothesized a gradual change in both structural and functional leaf traits at both rosette and leaf levels in response to the gradient of light intensity. We also compared trait plasticity between the apex and basal portions of the leaf. Assuming that the basal portion is less exposed and functionally decoupled from light (i.e. nutrient acquisition), we expected higher plasticity in the apical portion of the leaf in response to the light intensity gradient due to the structural and functional relationship with light (i.e. photosynthesis) and its higher exposure.
MATERIALS AND METHODS
Study area and plant material
This study was conducted in October 2021 in a tropical rainforest near the Petit Saut hydroelectric dam, Sinnamary, French Guiana (05°04ʹ39″N, 052°59ʹ11″W). The climate is moist tropical, with 3000 mm of yearly precipitation in the study area. The dry season extends between September and November, and there is another shorter and more irregular dry period in March. The study site is located on the edge of the forest with a shift from a closed rainforest to an open area. Environmental HOBO sensors were used to measure the air relative humidity, air temperature and light intensity (model UA-002-64, HOBO Pendant Tem Light 64 k and model U23-001, HOBO Pro V2 Temp/RH Data Logger) from the forest to the open area.
From the low to the high light gradient, mean daily (from 0700 to 1900 h) relative humidity ranged from 97.55 ± 6.58 to 90.83 ± 12.07 %, mean daily temperature (T°) ranged from 25.87 ± 1.99 to 27.24 ± 3.42 °C, mean daily vapour pressure deficit (VPD) ranged from 0.01 ± 0.27 to 0.42 ± 0.66 kPa and light ranged from 4388 ± 8204 to 37 114 ± 515547 lux, which represents a range from 100.92 ± 188.69 to 853.62 ± 1185.58 µmol s−1 m−2 in terms of photon flux density.
Aechmea aquilega (Salisb.) Griseb., Bromelioideae, is a tank bromeliad with classical crassulacean acid metabolism (CAM) photosynthesis that can be found as epiphytic, lithophytic or terrestrial life forms (Leroy et al. 2013). In the study site, this species has a terrestrial life form and was found in different incident light conditions from a closed rainforest area to a full sun-exposed area. We selected 20 mature, non-flowering A. aquilega with non-damaged young leaves along this incident light gradient. We quantified the percentage of light intensity reaching each tank bromeliad rosette with a digital camera (Nikon Coolpix 4500) equipped with a Nikon Fisheye Converted lens (FC-E8) that provided a 180° canopy view. Hemispherical images were analysed using Gap Light Analyzer software v.2.0 (Frazer and Canham, 1999) to determine the amount of total incident radiation transmitted through gaps (hereafter light intensity). The light intensity ranged from 10.41 to 60.64 % from the closed rainforest to the open area.
Plant morphology
For all individuals, we counted the number of green leaves and we measured plant height (from the base of the tank to highest leaf tip; cm) and plant diameter as the maximum distance between the tips of the leaves (two measurements at 90°; cm). The length (cm) and mid-leaf width (cm) of the second youngest fully expanded leaf were measured.
Carbon metabolism traits
Chlorophyll fluorescence.
The maximum quantum yield of photosystem II (Fv/Fm) and the maximum electron transport rate (ETRmax, µmol photon m−2 s−1) were measured with a portable fluorometer (MINI-PAM II, Walz, Effeltrich, Germany). Measurements were done between 0900 and 1200 h on the adaxial surface of the first youngest fully expanded leaf.
To measure Fv/Fm (i.e. potential maximum photosynthetic capacity of plants) a portion of the leaf was acclimated to dark for 30 min with a dark leaf clip (DLC-8, Walz). The minimal leaf fluorescence F0 was measured at low intensity light (<0.1 µmol photon m−2 s−1), then a 0.8-s saturating pulse (5000 µmol photon m−2 s−1) was produced to assess maximal fluorescence. Fv/Fm was calculated as (Fm—F0)/Fm.
The same leaf was placed in an opaque plastic bag for quasi-dark acclimation for 30 s and maintained in the bag during a rapid light curve procedure (Rascher et al., 2000; Manzi et al., 2022). The leaf was exposed to 12 gradually increasing PAR values ranging from 50 to 3000 µmol photon m−2 s−1 for 30 s each. The ETR was calculated by the fluorometer and the WinControl-3 software (Walz, Effeltrich, Germany) according to the photoinhibition REG1 function of Platt et al. (1980). ETRmax was then extracted from the obtained curves as the highest measured ETR.
Chlorophyll content.
The leaf chlorophyll (a and b) content (Cchl, µg cm−2) was estimated on the same leaf with a chlorophyll metre SPAD-502 (Konica Minolta, USA). Ten measurements (distributed equally on the apical leaf portion) were averaged to obtain a SPAD (USPAD) value. Using the equation of Coste et al. (2010) [Cchl = (117.1 × USPAD)/(148.84 − USPAD)] and the leaf mass area (see Mass-related traits section), we obtained the chlorophyll content per unit of dry mass (mg g−1).
Leaf biochemistry
The upper part of the first youngest fully expanded leaf of each tank bromeliad was harvested at dusk, corresponding to maximum storage of carbohydrates, to quantify metabolite content. The samples were immediately frozen in liquid nitrogen for 15 min and stored in a freezer until they were freeze-dried (Alpha 1-2 LD; Christ). Each sample was then ground to a fine powder in a Retsch MM301 Mixer Mill and stored in airtight vials in the dark until quantification of carbon (C), nitrogen (N) and phosphorus (P) and non-structural carbohydrates (soluble sugars and starch).
Carbon, nitrogen and phosphorus contents.
About 9–11 mg of leaf powder was used to quantify C (mg cm−2) and N (mg cm−2) contents (elemental analyser, Flash 2000 ThermoFisher, NF ISO 10694, NF ISO 13878, NF EN 13137). Three to four milligrams of leaf powder was used to quantify total P (mg cm−2) content (spectrometer, Uvi Light XT5 Secomam, spectrometric method with ammonium molybdate at 880 nm after H2SO4 acid hydrolysis and persulfate oxidation, adapted NF EN 6878).
Non-structural carbohydrate extraction.
Soluble sugars (mg g−1) were extracted from 10–15 mg powder mixed in 0.5 mL 80 % ethanol (v/v) and incubated for 20 min at 80 °C. Extraction was repeated twice and all three supernatants were collected in a tube and dried (Refrigerated CentriVap Vacuum Concentrator, Labconco). The resulting soluble sugar extract was solubilized in 1.5 mL ultrapure water by sonication and agitation and then stored at −20 °C. Starch was extracted from the dried pellets in 1.5 mL of 0.2 m KOH solution, incubated for 20 min at 80 °C and then stored at 4 °C. Total soluble sugar contents were determined by spectrophotometry at 620 nm (spectrophotometer UV-visible: UVmc2, SAFAS, Monaco) after heating at 100 °C for 10 min, using anthrone reagent (0.15 % (w/v) dissolved in 70 % H2SO4) and glucose as standard (Yemm and Willis, 1954); analyses were carried out in triplicate. Starch contents were determined after hydrolysis into glucose by amyloglucosidase (from Aspergillus niger, Sigma, EC 3.2.1.3) for 2 h at 50 °C. The obtained glucose was determined colorimetrically using an enzymatic reagent containing glucose oxidase (Type II from Aspergillus niger, Sigma, EC 1.1.3.4)/peroxidase (Type I from horseradish, Sigma, EC 1.11.1.7) and o-dianisidine dihydrochloride (Sigma, EC 243-737-5). After adding 6 N hydrochloric acid, absorbance was measured at 530 nm, using glucose as a standard (Chow and Landhausser, 2004). Soluble sugars and starch contents were expressed as milligrams (equivalent glucose) per gram dry mass (MD).
Leaf apex and base measurements
The second youngest fully expanded leaf was cautiously stripped off the plant and brought back to the laboratory in a cool box for subsequent measurements.
Mass-related traits.
Eight discs of 1 mm diameter were sampled in a 2.5 × 6-cm leaf rectangle in both basal and apical regions. The discs were immediately weighed using an electronic balance (AB204-S, Metler-Toledo) to determine the fresh mass (MF, g), stored in distilled water at 4 °C for 48 h to get the turgid mass (MT, g) and then dried at 60 °C for 48 h to get the dry mass (MD, g). Leaf mass area (LMA, g m−2) was calculated as MD/sum of leaf discs surface, leaf dry mass content (LDMC, mg g−1) as MD/MF, relative water content (RWC, %) as (MF − MD/MT − MD) × 100 and leaf succulence (gH2O m−2) as (MF − MD)/sum of leaf discs surface.
Stomatal and trichome density and size.
Two 1 × 4-cm leaf portions from the middle part of the apex and the base were fixed in FAA (5 % formalin, 5 % acetic acid and 90 % ethanol at 70 %) for 2 weeks, and then transferred to 70 % ethanol for storage. Then, the fixed leaf portions were removed from the 70 % ethanol and allowed to dry for a few minutes to eliminate the ethanol from the leaf surface. Stomata and trichomes were observed from imprints on the surface-dried fixed leaf samples using a thin layer of transparent nail varnish, followed by examination under an inverted microscope (Olympus BX51). Three pictures per imprint were made with a digital camera (Lumenera LW1135C-IO, Ottawa, Canada) and processed using ImageJ (NIH, USA, Schneider et al., 2012). The numbers of stomata and trichomes were counted and divided by the picture area (1.27 mm2) to estimate their density per square millimetre (average of the three pictures). As A. aquilega is hypostomatous and presents no stomata at the leaf base, stomatal density (Nb mm−2) is only available for the abaxial face of the leaf apex. Trichome density (Nb mm−2) and trichome area (mm2) were measured on both abaxial and adaxial faces for both leaf apex and base. The average area of the trichome was measured for 45 trichomes for each leaf portion using the FreeHandTools from ImageJ
Anatomical structure and measurements.
Handmade transverse sections (<0.5 mm thick) from the second 1 × 4-cm portion were made using a razor blade. One picture per location was taken using an inverted microscope (Olympus BX51-TF, Tokyo, Japan). Images were acquired with a digital camera (Lumenera LW1135C-IO, Ottawa, Canada) and measurements were made using the ‘straight line’ (thickness) and ‘freehand’ (surface) tools from the ImageJ software. The total leaf thickness (mm, mean of four measurements), abaxial and adaxial epidermal wall and cuticle thicknesses (mm, six measurements) and hydrenchyma thickness (mm, six measurements) were measured. Additionally, the density of fibres (Nb mm−2), the density of vascular bundles (Nb mm−2) and the mean surface of the vascular bundles (mm²) were measured. The interveinal distance (IVD, mm) was measured as the distance between the centre of two adjacent vascular bundles, and the vein–epidermis distance (VED, mm) as the distance from the centre of a vascular bundle to the stomatiferous abaxial epidermis to get the IVD/VED ratio.
Statistical analysis
All statistical analyses were carried out in R version 4.1.1 (R Core Team, 2021). Graphs were produced using the R package ggplot2 (Wickham, 2016). To study the response of leaf traits to light intensity, we fitted simple linear models with the light intensity (%) as the predictor (lm function, stats package, R Core Team, 2021). For traits measured at different positions on the leaf blade (apex or base) the position was added as a predictor with an interaction term with light. All variables were log-transformed to match conditions of linear models while all the figures display raw data and back-transformed model estimates. Complete model outputs are available as supplementary data (Supplementary Data Tables S1 and S2).
RESULTS
Effect of light intensity gradient on whole-rosette characteristics
The light intensity had a significant effect on the whole-rosette structure, photosynthesis and chemical contents with the exception of leaf starch content (Fig. 1). As light intensity increased, the number of leaves was significantly reduced and the diameter and height of the rosette decreased by 2-fold (Fig. 1A, C). The leaves were two to three times shorter and about two times wider at the highest light intensity compared with those at the lowest (Fig. 1D, E). Stomatal density, chlorophyll content and photosynthetic performance (ETRmax and Fv/Fm) significantly decreased as light intensity increased (Fig. 1F, I). On the other hand, leaf C and soluble sugars were significantly reduced with light intensity while leaf starch content was not affected by the light (Fig. 1J–L). Finally, leaf N and P contents were halved with increased light exposure (Fig. 1M, N).
Fig. 1.
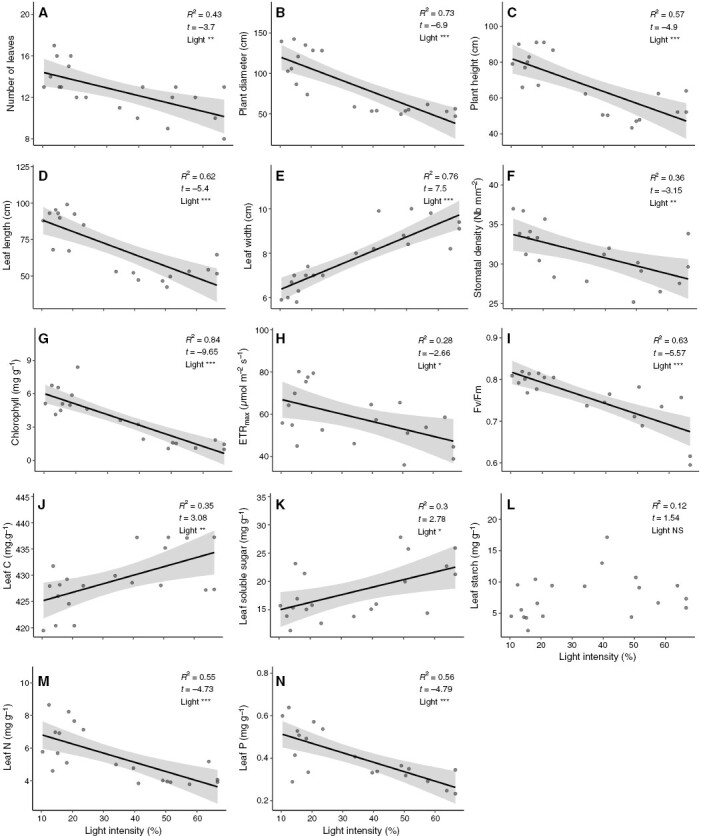
Effect of light intensity gradient on whole-rosette characteristics. Effects of light intensity (%) on (A) number of leaves, (B) plant diameter, (C) plant height, (D) leaf length, (E) leaf width, (F) stomatal density, (G) leaf chlorophyll content, (H) maximum electron transport rate (ETRmax), (I) maximum quantum yield of photosystem II (Fv/Fm), (J) leaf carbon content, (K) leaf soluble sugars content, (L) leaf starch content, (M) leaf nitrogen content and (N) leaf phosphorous content. Dots are observations. Solid lines represent the regression line of significant (α < 0.05) linear models and shaded in grey is the 0.95 confidence interval. At the top right corner of each plot is given the regression R², t value and the significance of the light effect. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Effect of light intensity gradient on apex and basal leaf portion characteristics
Relative water content and LMA were not affected by either the light intensity or portion of the leaf (Fig. 2A, B). As light intensity increased, LDMC increased at the base while it remained constant at the apex portion of the leaf (Fig. 2C). Plants under low light thus had leaves with high LDMC at the apex and low LMDC at the base, whereas it was the opposite for plants exposed to higher light. Leaf succulence and thickness decreased at the base and increased at the apex portion of the leaf with increasing light intensity (Fig. 2D, E). Hence, plants under low light had higher leaf succulence and thickness at the base compared with the apical portion of the leaf, a pattern that tended to disappear at higher light intensities.
Fig. 2.
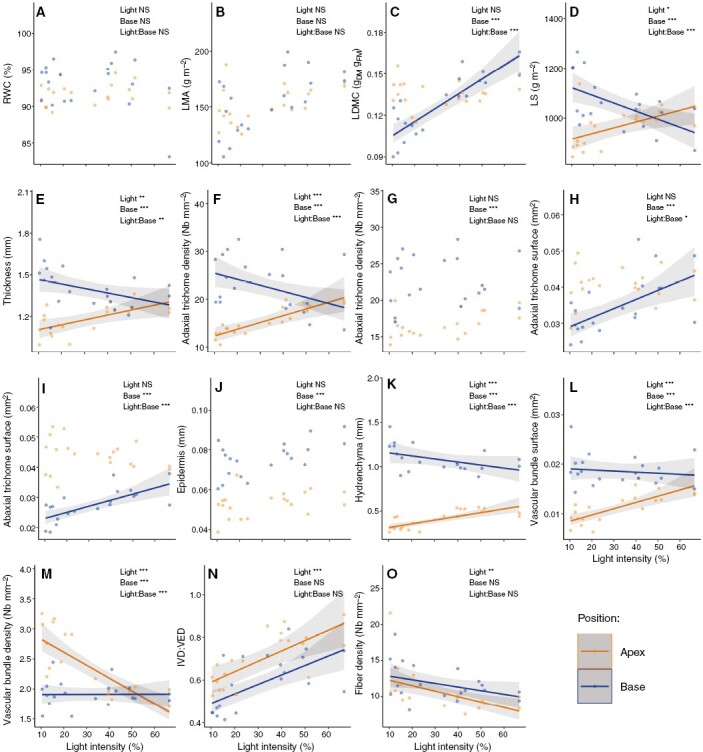
Effect of light intensity gradient on apex and basal leaf portion characteristics. Effects of light intensity (%) according to the leaf portion (apex and base) on (A) relative water content (RWC), (B) leaf mass area (LMA), (C) leaf dry mass content (LDMC), (D) leaf succulence (LS), (E) leaf thickness, (F) adaxial trichome density, (G) abaxial trichome density, (H) adaxial trichome surface, (I) abaxial trichome surface, (J) epidermis and cuticle thickness, (K) hydrenchyma thickness, (L) vascular bundle surface, (M) vascular bundle density, (N) interveinal distance/vein–epidermis distance ratio (IVD/VED) and (O) fibre density. Dots are observations, orange and blue colours correspond to the apex and basal portion of the leaf, respectively. Solid lines represent the regression line of significant (α < 0.05) linear models and shaded in grey is the 0.95 confidence interval. At the top right corner of each plot is given the significance of the effects of light intensity (Light), basal position compared to apex (Base) and their interactions (Light:Base). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Adaxial trichome densities increased at the apex and decreased at the basal portion of the leaf with increasing light, while the density of abaxial trichomes remained unchanged (Fig. 2F, G). The density of abaxial trichomes was higher at the base compared with the apex portion of the leaf, regardless of the light intensity. For adaxial trichomes, the density was similar for both leaf portions at high light. The surfaces of adaxial and abaxial trichomes were significantly affected by the leaf portion and the interaction of light intensity and leaf portion (Fig. 2H, I). In both cases, the surface of the trichomes was significantly higher at the apex compared with the basal portion of the leaf for plants under low light intensity, while the surface of the trichomes remained similar for both leaf portions in the most exposed plants.
Epidermis and cuticle were thicker at the base compared with the apex portion of the leaf, regardless of the light intensity and remained unchanged with increasing light (Fig. 2J). Hydrenchyma significantly increased at the apex and decreased at the basal portion of the leaf with increasing light intensity but remained significantly thicker at the base compared with the apical portion of the leaf, regardless of the light intensity (Fig. 2K). Vascular bundle surface significantly increased with increasing light intensity at the apex portion of the leaf while it remained unchanged at the basal portion (Fig. 2L). Hence, at low light intensity the surface of vascular bundles was 2-fold higher at the base compared with the apex portion of the leaf. Conversely, the density of vascular bundles significantly decreased with increasing light at the apex portion of the leaf, while it remained constant for the basal portion. At the lowest light intensity, the density of vascular bundles was thus higher in the apex portion of the leaf compared with the base (Fig. 2M). The IVD/VED ratio and the density of fibres significantly increased and decreased, respectively, with increasing light intensity but were not affected by the position on the leaf (Fig. 2N, O).
DISCUSSION
Plasticity of the rosette in response to light intensity
Light intensity had a strong effect on the rosette shape, size and leaf colour. Plants in the high-light end of the gradient were smaller, and characterized by wider, shorter and more vertical leaves. Similar responses to light intensity were reported for other bromeliad species (e.g. Cavallero et al., 2009, 2011; Leroy et al., 2009; Rodrigues Pereira et al., 2013; Barberis et al., 2017). These architectural differences enable higher exposure to light by limiting self-shading due to narrower and longer leaves in shaded areas, and lower exposure to excessive radiation through wider and smaller leaves in sun-exposed areas. The linear shape of the leaves under low light intensity suggests a trade-off between C gain and mechanical support (Read and Stokes, 2006; Cavallero et al., 2011). In addition, we found that shaded leaves had a higher density of fibres compared with sun-exposed leaves, which would increase their flexural stiffness and reduce bending loads (Oliveira et al., 2008; Cavallero et al., 2011; Onoda et al., 2011). The stomatal density was lower in the leaves of A. aquilega exposed to high light intensity, which contrasts with what is usually found in the literature. Exposure to high light levels cause higher stomatal density in other bromeliad species (e.g. Scarano et al., 2002; Cavallero et al., 2011; Petit et al., 2014; Leroy et al., 2017; but see Oliveira et al., 2008) and more generally in dicotyledonous plants (Bertolino et al., 2019). Species with higher stomatal density under high light have greater photosynthetic capacity (Tanaka et al., 2013) and transpiration rates, contributing to a cooling effect of the leaves (Rozendaal et al., 2006). However, a reduction of stomatal density constrains both stomatal conductance and transpiration, allowing a more conservative use of water (Bertolino et al., 2019), in line with the CAM habit.
Aechmea aquilega is a classical CAM species that permits the net uptake of CO2 at night, thus improving the water-use efficiency of carbon assimilation under high solar radiation (Pierce et al., 2002). In our study, the light intensity did not modify the type of CAM photosynthesis (e.g. from classical to CAM idling) as gas exchange and stomatal conductance occurred in both high and low-light environments (Supplementary Data Fig. S1). Instead, we observed differences in metabolic and biochemical processes that may underlie the response to varying levels of light intensity. The higher chlorophyll concentration under low light compared with high light intensity generally confers better light-capturing ability on A. aquilega leaves (Lee et al., 1989; Fetene et al., 1990). In parallel, low chlorophyll content under high light intensity can also result from damage caused by reactive oxygen species (Nishiyama et al., 2006; Pospíšil, 2016). Reductions in Fv/Fm (below 0.8) and ETRmax with increasing light intensity are common in sun-exposed plants (e.g. He et al., 1996; Valladares and Pearcy, 1997) and can result from multiple processes, such as the degradation of the photosynthetic apparatus (Takahashi and Badger, 2011) or investment in photoprotective pigments (Young, 1991; Kumar and Pal, 2022). Nutrient deficiency, as suggested by the lower leaf N and P contents in high-light-exposed A. aquilega, might also contribute to the lower Fv/Fm (Wu et al., 2008; Kalaji et al., 2014, 2018) and chlorophyll content (Evans, 1989). Despite having low leaf N content, Fv/Fm and ETRmax in sun-exposed A. aquilega, we found higher leaf C and soluble sugar contents than in the shaded plants. This indicates that photosynthetic C fixation was sufficient for carbohydrate reserve production (i.e. starch) and did not differ from that of plants in low light intensity. A higher leaf C/N ratio can result from both increasing light and N scarcity (Grechi et al., 2007) and is associated with higher nitrogen use efficiency (Zhang et al., 2020). On the other hand, differences in C/N ratio might also be due preferential allocation of N to the leaf in order to maximize growth under low-light conditions (Makino et al., 1997).
Structural and anatomical modifications of apex and basal portions of the leaf in response to light intensity
Leaf mass area and LDMC are two structural traits that indicate adjustments to light conditions (Poorter et al., 2009, 2010). By increasing the surface area relative to the leaf biomass (i.e. low LMA), the interception of light is increased under low light conditions (Poorter et al., 2009). Leaf dry mass content is often correlated with LMA and is considered an alternative predictor of plant resource capture (Wilson et al., 1999). In A. aquilega, only LDMC was affected by light; however, unexpectedly, this effect was observed only for the basal portion of the leaf. As we found significant differences in leaf thickness and anatomical traits with increasing light intensity, our unexpected results for LMA and LDMC may be due to other factors, such as soil fertility (Hodgson et al., 2011), air temperature (Zhu et al., 2020) or soil nutrient levels (Zheng et al., 2017). Regardless of the light intensity, the leaf water status (i.e. RWC) remained constant for both leaf portions, suggesting that A. aquilega was well supplied with water and did not experience drought under high light conditions. Conversely, leaf succulence increased at the apex and decreased at the basal portion of the leaf with increasing light intensity. Leaf succulence reflects the water storage capacity of the leaf and is mainly attributed to hydrenchyma tissue (Winter et al., 1983). In A. aquilega, we also observed an increase in hydrenchyma tissue thickness at the apical part of the leaf, which likely contributes to the overall increase in leaf succulence with higher light intensity. This increased succulence at the apical portion of the leaf under higher light intensity may provide a greater capacity for photoprotection (Graham and Andrade, 2004).
Water and nutrient absorption are mainly mediated by absorbing trichomes that cover both sides of the leaf surface. The higher trichome density in the basal portion of the leaf in A. aquilega supports previous studies conducted on various tank bromeliad species (e.g. Freschi et al., 2010; Rodrigues et al., 2016; Kleingesinds et al., 2018). However, when considering the adaxial and abaxial sides of the leaf, no consistent pattern of trichome density was observed across different bromeliad species (Adams and Martin, 1986; Cach-Pérez et al., 2016; Kleingesinds et al., 2018). Under low light intensity, we observed smaller trichomes occurring in higher density at the base of the leaf and larger trichomes occurring in lower density at the apex, potentially facilitating enhanced resource uptake at the basal portion of the leaf (Benzing, 2000). Conversely, under high light intensity both leaf portions exhibited approximately similar trichome density and size, similar to findings in different species of atmospheric tillandsioids (Cach-Pérez et al., 2016).
In contrast, leaf thickness, and surface and density of vascular bundles showed a different response of the two leaf portions to light. These results may be due to the different functional roles of the two leaf portions. Additionally, we found that the vascular bundle density at the apex portion of the leaf decreased with light intensity, along with a decrease in stomatal density, suggesting a coordinated response between water demand (e.g. leaf gas exchange) and water supply (transport) capacity (Sack and Scoffoni, 2013). Across diverse angiosperm species, IVD and VED are approximately equal, indicating an optimal arrangement of the vascular bundles for hydraulic efficiency (Zwieniecki and Boyce, 2014). In our study, we found an IVD/VED ratio <1 for both leaf portions, with no significant difference between them. With IVD < VED, A. aquilega is a species that ‘overinvests’ in veins, a feature that is physiologically disadvantageous since it involves the replacement of photosynthetic mesophyll cells with hydraulically redundant vascular bundles (Zwieniecki and Boyce, 2014; Males, 2017). Similar vascular overinvestment has been observed in other succulent and CAM taxa and was associated with thicker leaves in water-limited environments (Males, 2017; Leverett et al., 2023). This low ratio can facilitate hydraulic recharge to efficiently provide water to the mesophyll and resistance to transpirational water loss (de Boer et al., 2016; Males, 2017; Leverett et al., 2023).
Attenuation of the longitudinal degree of differences between apex and basal portions of the leaf with increasing light intensity
Overall, we found strong structural and anatomical differences along the leaf of A. aquilega growing in low light compared with individuals growing in a high-light environment. Most of the measured traits showed high longitudinal degree of difference between the apex and basal portions of the leaf in low light, and the differences were reduced or disappeared with increasing light intensity for most of the traits (i.e. thickness, adaxial trichome density, abaxial and adaxial trichome surface, vascular bundle surface and density). In a few other traits (i.e. LDMC, leaf succulence), we found a reverse pattern. These results suggest that the structural and functional regionalization were reduced in leaves from high light compared with leaves from low light intensity. The positive or negative co-variations of the two leaf portions may be due to mechanical and/or physiological adjustments at the leaf scale in response to light intensity and/or other strongly linked confounding factors, such as gradient of leaf litter/nutrient supplies. However, it is unlikely that drought is the driving factor behind these leaf adjustments, as the tanks in high light intensity were consistently filled with rainwater throughout the year (pers. obs., T. Lafont Rapnouil and C. Leroy). Regardless of the underlying mechanism, the loss of longitudinal differentiation between the apical and basal portions of the leaf in sun-exposed environments may have strong ecological and physiological implications, particularly in terms of nitrogen acquisition and photosynthetic performance.
The greater structural and anatomical differences between the two leaf portions under low light intensity, compared with leaf in high-light environments, may be attributed to the leaf base-to-tip developmental gradient. In monocotyledonous plants, cell divisions primarily occur in the basal meristem of the leaf, followed by cell elongation, and finally, the oldest and most mature cells are present at the tip (Fournier et al., 2005). Consequently, a positional gradient of cell ages is formed along the leaves during their growth, with the youngest cells located at the base and the oldest at the apex of the leaf (Sharman, 1942). Thus, it is likely that the cell age of the apical portion of the leaf under low light intensity is higher than that of the leaf under high light intensity, due to a greater number of cell divisions occurring in the longer leaf. It has been shown that shade can facilitate higher levels of cell division, cell elongation, or both, depending on the species (Rahim and Fordham, 1991). Nevertheless, if cell age was the only factor explaining the differences between leaves in low-light and high-light environments, it should have only affected the values of traits in the apical portion of the leaves.
Conclusions
We found significant morphological and functional trait differences in A. aquilega bromeliads growing along a light intensity gradient. Interestingly, we showed that the light intensity had contrasting effects on the morpho-anatomy of leaf apical and basal portions. When growing in low-light conditions, leaves of A. aquilega exhibit a particularly steep within-leaf functional gradient, while in high-light conditions leaf traits become homogeneous throughout the length of the leaf. This contrasting pattern might be due to a combination of apparent plasticity and true plasticity that would be essential to disentangle. These results reinforce the remarkable complexity of tank bromeliad leaves and show how structural and functional traits within a single leaf can be either heterogeneous or uniform, depending on the light environment. Overall, this study highlights the significance of considering different portions of the leaf in order to gain a better understanding of how plants respond to environmental changes.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Table S1: table summary of linear models output for plant traits according to light intensity (%). Table S2: table summary of linear models output for plant traits according to light intensity (%) and position on the leaf blade. Figure S1: overnight CO2 assimilation and stomatal conductance curves for eight plants taken along the light intensity gradient (%).
ACKNOWLEDGEMENTS
The authors would like to thank SILVATECH (Silvatech, INRAE, 2018. Structural and functional analysis of tree and wood facility, doi: 10.15454/1.5572400113627854E12) from UMR 1434 SILVA, 1136 IAM, 1138 BEF and 4370 EA LERMAB from the research centre INRAE Grand-Est Nancy for its contribution to carbohydrate analysis. SILVATECH facility is supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01). We thank the two anonymous reviewers for their valuable comments.
Contributor Information
Tristan Lafont Rapnouil, AMAP, Université de Montpellier, CIRAD, CNRS, INRAE, IRD, France; EcoFoG, AgroParisTech, CIRAD, CNRS, INRAE, Université des Antilles, Université de Guyane, Campus agronomique, Kourou, France.
Matthieu Gallant Canguilhem, AMAP, Université de Montpellier, CIRAD, CNRS, INRAE, IRD, France; EcoFoG, AgroParisTech, CIRAD, CNRS, INRAE, Université des Antilles, Université de Guyane, Campus agronomique, Kourou, France.
Frédéric Julien, Laboratoire Écologie Fonctionnelle et Environnement, Université Paul Sabatier Toulouse 3, CNRS, Toulouse, France.
Régis Céréghino, Laboratoire Écologie Fonctionnelle et Environnement, Université Paul Sabatier Toulouse 3, CNRS, Toulouse, France.
Céline Leroy, AMAP, Université de Montpellier, CIRAD, CNRS, INRAE, IRD, France; EcoFoG, AgroParisTech, CIRAD, CNRS, INRAE, Université des Antilles, Université de Guyane, Campus agronomique, Kourou, France.
FUNDING
This work received financial support from an Investissement d’Avenir grant managed by the Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-25-01).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
- Adams WWA, Martin CE.. 1986. Morphological changes accompanying the transition from juvenile (atmospheric) to adult (tank) forms in the Mexican epiphyte Tillandsia deppeana (Bromeliaceae). American Journal of Botany 73: 1207–1214. [Google Scholar]
- Audet P, Charest C.. 2008. Allocation plasticity and plant–metal partitioning: meta-analytical perspectives in phytoremediation. Environmental Pollution 156: 290–296. doi: 10.1016/j.envpol.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Barberis IM, Cárcamo JM, Cárcamo JI, Albertengo J.. 2017. Phenotypic plasticity in Bromelia serra Griseb.: morphological variations due to plant size and habitats with contrasting light availability. Brazilian Journal of Biosciences 15: 8. [Google Scholar]
- Benzing DH. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge: Cambridge University Press. [Google Scholar]
- Bertolino LT, Caine RS, Gray JE.. 2019. Impact of stomatal density and morphology on water-use efficiency in a changing world. Frontiers in Plant Science 10: 225. doi: 10.3389/fpls.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer HJ, Drake PL, Wendt E, et al. 2016. Apparent overinvestment in leaf venation relaxes leaf morphological constraints on photosynthesis in arid habitats. Plant Physiology 172: 2286–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossdorf O, Pigliucci M.. 2009. Plasticity to wind is modular and genetically variable in Arabidopsis thaliana. Evolutionary Ecology 23: 669–685. doi: 10.1007/s10682-008-9263-3. [DOI] [Google Scholar]
- Cach-Pérez MJ, Andrade JL, Cetzal-Ix W, Reyes-García C.. 2016. Environmental influence on the inter- and intraspecific variation in the density and morphology of stomata and trichomes of epiphytic bromeliads of the Yucatan Peninsula. Botanical Journal of the Linnean Society 181: 441–458. doi: 10.1111/boj.12398. [DOI] [Google Scholar]
- Cavallero L, López D, Barberis IM.. 2009. Morphological variation of Aechmea distichantha (Bromeliaceae) in a Chaco forest: habitat and size-related effects. Plant Biology 11: 379–391. doi: 10.1111/j.1438-8677.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- Cavallero L, Galetti L, López D, McCargo J, Barberis IM.. 2011. Morphological variation of the leaves of Aechmea distichantha Lem. plants from contrasting habitats of a Chaco forest: a trade-off between leaf area and mechanical support. Revista Brasileira de Biociências 9: 455–464. [Google Scholar]
- Ceusters J, Borland AM, Godts C, et al. 2011. Crassulacean acid metabolism under severe light limitation: a matter of plasticity in the shadows? Journal of Experimental Botany 62: 283–291. doi: 10.1093/jxb/erq264. [DOI] [PubMed] [Google Scholar]
- Chow PS, Landhäusser SM. 2004. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology 24: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Coste S, Baraloto C, Leroy C, et al. 2010. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Annals of Forest Science 67: 607–607. doi: 10.1051/forest/2010020. [DOI] [Google Scholar]
- Dörken VM, Lepetit B.. 2018. Morpho-anatomical and physiological differences between sun and shade leaves in Abies alba Mill. (Pinaceae, Coniferales): a combined approach. Plant, Cell & Environment 41: 1683–1697. doi: 10.1111/pce.13213. [DOI] [PubMed] [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- Fetene M, Lee HSJ, Lüttge U.. 1990. Photosynthetic acclimation in a terrestrial CAM bromeliad, Bromelia humilis Jacq. New Phytologist 114: 399–406. doi: 10.1111/j.1469-8137.1990.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Fournier C, Durand JL, Ljutovac S, Schäufele R, Gastal F, Andrieu B.. 2005. A functional–structural model of elongation of the grass leaf and its relationships with the phyllochron. New Phytologist 166: 881–894. doi: 10.1111/j.1469-8137.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- Frazer GW, Canham CD.. 1999. Modelling and application design. In: Gap Light Analyzer (GLA), version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, 40. https://www.caryinstitute.org/science/our-scientists/dr-charles-d-canham/gap-light-analyzer-gla (7 September2023, date last accessed) [Google Scholar]
- Freschi L, Takahashi CA, Cambui CA, et al. 2010. Specific leaf areas of the tank bromeliad Guzmania monostachia perform distinct functions in response to water shortage. Journal of Plant Physiology 167: 526–533. doi: 10.1016/j.jplph.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Fromm H. 2019. Root plasticity in the pursuit of water. Plants 8: 236. doi: 10.3390/plants8070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish T. 1988. Adaptation to sun and shade: a whole-plant perspective. Functional Plant Biology 15: 63. doi: 10.1071/pp9880063. [DOI] [Google Scholar]
- Gonçalves AZ, Latsanio S, Detmann KC, et al. 2020. What does the RuBisCO activity tell us about a C-3-CAM plant? Plant Physiology and Biochemistry 147: 172–180. [DOI] [PubMed] [Google Scholar]
- Gouda EJ, Butcher D. (cont.updated)The New Bromeliad Taxon List, version 4. University Botanic Gardens, Utrecht. https://bromeliad.nl/taxonlist/ (7 September2023, date last accessed). [Google Scholar]
- Graham EA, Andrade JL.. 2004. Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. American Journal of Botany 91: 699–706. doi: 10.3732/ajb.91.5.699. [DOI] [PubMed] [Google Scholar]
- Gratani L. 2014. Plant phenotypic plasticity in response to environmental factors. Advances in Botany 2014: 1–17. doi: 10.1155/2014/208747. [DOI] [Google Scholar]
- Gratani L, Covone F, Larcher W.. 2006. Leaf plasticity in response to light of three evergreen species of the Mediterranean maquis. Trees 20: 549–558. doi: 10.1007/s00468-006-0070-6. [DOI] [Google Scholar]
- Grechi I, Vivin P, Hilbert G, Milin S, Robert T, Gaudillère J-P.. 2007. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environmental and Experimental Botany 59: 139–149. doi: 10.1016/j.envexpbot.2005.11.002. [DOI] [Google Scholar]
- He J, Chee CW, Goh CJ.. 1996. ‘Photoinhibition’ of Heliconia under natural tropical conditions: the importance of leaf orientation for light interception and leaf temperature. Plant, Cell & Environment 19: 1238–1248. [Google Scholar]
- Hodgson JG, Montserrat-Martí G, Charles M, et al. 2011. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Annals of Botany 108: 1337–1345. doi: 10.1093/aob/mcr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaji HM, Oukarroum A, Alexandrov V, et al. 2014. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiology and Biochemistry 81: 16–25. doi: 10.1016/j.plaphy.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Kalaji HM, Bąba W, Gediga K, et al. 2018. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynthesis Research 136: 329–343. doi: 10.1007/s11120-017-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleingesinds CK, Gobara BNK, Mancilha D, Rodrigues MA, Demarco D, Mercier H.. 2018. Impact of tank formation on distribution and cellular organization of trichomes within Guzmania monostachia rosette. Flora 243: 11–18. doi: 10.1016/j.flora.2018.03.013. [DOI] [Google Scholar]
- De Kroon H, Huber H, Stuefer JF, Van Groenendael JM.. 2005. A modular concept of phenotypic plasticity in plants. New Phytologist 166: 73–82. [DOI] [PubMed] [Google Scholar]
- Kumar P, Pal M.. 2022. Comparative analysis of photoprotection mediated by photosynthetic pigments during summer midday heat stress in rice and wheat. Vegetos 35: 1165–1171. doi: 10.1007/s42535-022-00380-9. [DOI] [Google Scholar]
- Lee HSJ, Lüttge U, Medina E, et al. 1989. Ecophysiology of xerophytic and halophytic vegetation of a coastal alluvial plain in northern Venezuela. New Phytologist 111: 253–271. doi: 10.1111/j.1469-8137.1989.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Leroy C, Corbara B, Dejean A, Céréghino R.. 2009. Ants mediate foliar structure and nitrogen acquisition in a tank-bromeliad. New Phytologist 183: 1124–1133. doi: 10.1111/j.1469-8137.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- Leroy C, Carrias J-F, Corbara B, et al. 2013. Mutualistic ants contribute to tank-bromeliad nutrition. Annals of Botany 112: 919–926. doi: 10.1093/aob/mct147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Carrias J-F, Céréghino R, Corbara B.. 2016. The contribution of microorganisms and metazoans to mineral nutrition in bromeliads. Journal of Plant Ecology 9: 241–255. doi: 10.1093/jpe/rtv052. [DOI] [Google Scholar]
- Leroy C, Petitclerc F, Orivel J, et al. 2017. The influence of light, substrate and seed origin on the germination and establishment of an ant-garden bromeliad. Plant Biology 19: 70–78. doi: 10.1111/plb.12452. [DOI] [PubMed] [Google Scholar]
- Leroy C, Gril E, Si Ouali L, et al. 2019. Water and nutrient uptake capacity of leaf-absorbing trichomes vs. roots in epiphytic tank bromeliads. Environmental and Experimental Botany 163: 112–123. doi: 10.1016/j.envexpbot.2019.04.012. [DOI] [Google Scholar]
- Leverett A, Ferguson K, Winter K, Borland AM. 2023. Leaf vein density correlates with Crassulacean acid metabolism, but not hydraulic capacitance, in the genus Clusia. Annals of Botany mcad035. [DOI] [PMC free article] [PubMed]
- Li S, Zhang Y-J, Sack L, et al. 2013. The heterogeneity and spatial patterning of structure and physiology across the leaf surface in giant leaves of Alocasia macrorrhiza. PLoS One 8: e66016. doi: 10.1371/journal.pone.0066016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Sato T, Nakano H, Mae T.. 1997. Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta 203: 390–398. doi: 10.1007/s004250050205. [DOI] [Google Scholar]
- Males J. 2017. Adaptive variation in vein placement underpins diversity in a major Neotropical plant radiation. Oecologia 185: 375–386. doi: 10.1007/s00442-017-3956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi OJL, Bellifa M, Ziegler C, et al. 2022. Drought stress recovery of hydraulic and photochemical processes in Neotropical tree saplings. Tree Physiology 42: 114–129. doi: 10.1093/treephys/tpab092. [DOI] [PubMed] [Google Scholar]
- Mathur S, Jain L, Jajoo A.. 2018. Photosynthetic efficiency in sun and shade plants. Photosynthetica 56: 354–365. doi: 10.1007/s11099-018-0767-y. [DOI] [Google Scholar]
- Maxwell C, Griffiths H, Borland AM, Young AJ, Broadmeadow MSJ, Fordham MC.. 1995. Short-term photosynthetic responses of the C3-CAM epiphyte Guzmania monostachia var. monostachia to tropical seasonal transitions under field conditions. Functional Plant Biology 22: 771–781. doi: 10.1071/pp9950771. [DOI] [Google Scholar]
- Medina E, Olivares E, Diaz M.. 1986. Water stress and light intensity effects on growth and nocturnal acid accumulation in a terrestrial CAM bromeliad (Bromelia humilis Jacq.) under natural conditions. Oecologia 70: 441–446. doi: 10.1007/BF00379509. [DOI] [PubMed] [Google Scholar]
- Nardini A, Gortan E, Ramani M, Salleo S.. 2008. Heterogeneity of gas exchange rates over the leaf surface in tobacco: an effect of hydraulic architecture? Plant, Cell & Environment 31: 804–812. doi: 10.1111/j.1365-3040.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- Niinemets U. 2010. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecological Research 25: 693–714. doi: 10.1007/s11284-010-0712-4. [DOI] [Google Scholar]
- Niinemets U, Valladares F.. 2004. Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: optimality and constraints. Plant Biology 6: 254–268. doi: 10.1055/s-2004-817881. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Keenan TF, Hallik L.. 2015. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytologist 205: 973–993. doi: 10.1111/nph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N.. 2006. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochimica et Biophysica Acta 1757: 742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- North GB, Browne MG, Fukui K, Maharaj FDR, Phillips CA, Woodside WT.. 2016. A tale of two plasticities: leaf hydraulic conductances and related traits diverge for two tropical epiphytes from contrasting light environments. Plant, Cell & Environment 39: 1408–1419. doi: 10.1111/pce.12697. [DOI] [PubMed] [Google Scholar]
- Oliveira ECP de, Lameira OA, de Sousa FIB, Silva RJF.. 2008. Estrutura foliar de curauá em diferentes intensidades de radiação fotossinteticamente ativa. Pesquisa Agropecuária Brasileira 43: 163–169. [Google Scholar]
- Onoda Y, Westoby M, Adler PB, et al. 2011. Global patterns of leaf mechanical properties. Ecology Letters 14: 301–312. doi: 10.1111/j.1461-0248.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- Petit M, Céréghino R, Carrias J-F, et al. 2014. Are ontogenetic shifts in foliar structure and resource acquisition spatially conditioned in tank-bromeliads? Botanical Journal of the Linnean Society 175: 299–312. doi: 10.1111/boj.12171. [DOI] [Google Scholar]
- Pierce S, Winter K, Griffiths H.. 2002. The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae. Plant, Cell & Environment 25: 1181–1189. [Google Scholar]
- Pikart FC, Marabesi MA, Mioto PT, et al. 2018. The contribution of weak CAM to the photosynthetic metabolic activities of a bromeliad species under water deficit. Plant Physiology and Biochemistry 123: 297–303. doi: 10.1016/j.plaphy.2017.12.030. [DOI] [PubMed] [Google Scholar]
- Platt T, Gallegos CL, Harrison WG.. 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. Journal of Marine Research 38: 687–701. [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R.. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets U, Walter A, Fiorani F, Schurr U.. 2010. A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. Journal of Experimental Botany 61: 2043–2055. doi: 10.1093/jxb/erp358. [DOI] [PubMed] [Google Scholar]
- Popma J, Bongers F.. 1988. The effect of canopy gaps on growth and morphology of seedlings of rain forest species. Oecologia 75: 625–632. doi: 10.1007/BF00776429. [DOI] [PubMed] [Google Scholar]
- Pospíšil P. 2016. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Frontiers in Plant Science 7: 1950. doi: 10.3389/fpls.2016.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rahim MA, Fordham R.. 1991. Effect of shade on leaf and cell size and number of epidermal cells in garlic (Allium sativum). Annals of Botany 67: 167–171. doi: 10.1093/oxfordjournals.aob.a088116. [DOI] [Google Scholar]
- Rascher U, Liebig M, Lüttge U.. 2000. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant, Cell & Environment 23: 1397–1405. doi: 10.1046/j.1365-3040.2000.00650.x. [DOI] [Google Scholar]
- Read J, Stokes A.. 2006. Plant biomechanics in an ecological context. American Journal of Botany 93: 1546–1565. doi: 10.3732/ajb.93.10.1546. [DOI] [PubMed] [Google Scholar]
- Rodrigues MA, Hamachi L, Mioto PT, Purgatto E, Mercier H.. 2016. Implications of leaf ontogeny on drought-induced gradients of CAM expression and ABA levels in rosettes of the epiphytic tank bromeliad Guzmania monostachia. Plant Physiology and Biochemistry 108: 400–411. doi: 10.1016/j.plaphy.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Rodrigues Pereira TA, da Silva LC, Azevedo AA, Francino DMT, dos Santos Coser T, Pereira JD.. 2013. Leaf morpho-anatomical variations in Billbergia elegans and Neoregelia mucugensis (Bromeliaceae) exposed to low and high solar radiation. Botany 91: 327–334. doi: 10.1139/cjb-2012-0276. [DOI] [Google Scholar]
- Rozendaal DMA, Hurtado VH, Poorter L.. 2006. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Functional Ecology 20: 207–216. doi: 10.1111/j.1365-2435.2006.01105.x. [DOI] [Google Scholar]
- Sack L, Scoffoni C.. 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytologist 198: 983–1000. doi: 10.1111/nph.12253. [DOI] [PubMed] [Google Scholar]
- Scarano FR, Duarte HM, Rôças G, et al. 2002. Acclimation or stress symptom? An integrated study of intraspecific variation in the clonal plant Aechmea bromeliifolia, a widespread CAM tank-bromeliad. Botanical Journal of the Linnean Society 140: 391–401. doi: 10.1046/j.1095-8339.2002.00112.x. [DOI] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman BC. 1942. Developmental anatomy of the shoot of Zea mays L. Annals of Botany 6: 245–282. doi: 10.1093/oxfordjournals.aob.a088407. [DOI] [Google Scholar]
- Shi Z, Li K, Zhu X, Wang F.. 2020. The worldwide leaf economic spectrum traits are closely linked with mycorrhizal traits. Fungal Ecology 43: 100877. doi: 10.1016/j.funeco.2019.100877. [DOI] [Google Scholar]
- Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Badger MR.. 2011. Photoprotection in plants: a new light on photosystem II damage. Trends in Plant Science 16: 53–60. doi: 10.1016/j.tplants.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Takahashi CA, Mercier H.. 2011. Nitrogen metabolism in leaves of a tank epiphytic bromeliad: characterization of a spatial and functional division. Journal of Plant Physiology 168: 1208–1216. doi: 10.1016/j.jplph.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Takahashi CA, Ceccantini GCT, Mercier H.. 2007. Differential capacity of nitrogen assimilation between apical and basal leaf portions of a tank epiphytic bromeliad. Brazilian Journal of Plant Physiology 19: 119–126. [Google Scholar]
- Tanaka Y, Sugano SS, Shimada T, Hara-Nishimura I.. 2013. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist 198: 757–764. doi: 10.1111/nph.12186. [DOI] [PubMed] [Google Scholar]
- Terashima I, Miyazawa S-I, Hanba YT.. 2001. Why are sun leaves thicker than shade leaves? – consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research 114: 93–105. doi: 10.1007/pl00013972. [DOI] [Google Scholar]
- Valladares F, Pearcy RW.. 1997. Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant, Cell & Environment 20: 25–36. doi: 10.1046/j.1365-3040.1997.d01-8.x. [DOI] [Google Scholar]
- Valladares F, Sanchez-Gomez D, Zavala MA.. 2006. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology 94: 1103–1116. doi: 10.1111/j.1365-2745.2006.01176.x. [DOI] [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG.. 1999. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist 143: 155–162. doi: 10.1046/j.1469-8137.1999.00427.x. [DOI] [Google Scholar]
- Winter K, Wallace BJ, Stocker GC, Roksandic Z.. 1983. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 57: 129–141. doi: 10.1007/BF00379570. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wu FZ, Bao WK, Li FL, Wu N.. 2008. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica 46: 40–48. doi: 10.1007/s11099-008-0008-x. [DOI] [Google Scholar]
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal 57: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AJ. 1991. The photoprotective role of carotenoids in higher plants. Physiologia Plantarum 83: 702–708. doi: 10.1034/j.1399-3054.1991.830426.x. [DOI] [Google Scholar]
- Zhang J, He N, Liu C, et al. 2020. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Global Change Biology 26: 2534–2543. doi: 10.1111/gcb.14973. [DOI] [PubMed] [Google Scholar]
- Zheng L-L, Zhao Q, Yu Z-Y, Zhao S-Y, Zeng D-H.. 2017. Altered leaf functional traits by nitrogen addition in a nutrient-poor pine plantation: a consequence of decreased phosphorus availability. Scientific Reports 7: 7415. doi: 10.1038/s41598-017-07170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhu H, Cao Y, et al. 2020. Effect of simulated warming on leaf functional traits of urban greening plants. BMC Plant Biology 20: 139. doi: 10.1186/s12870-020-02359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki MA, Boyce CK.. 2014. Evolution of a unique anatomical precision in angiosperm leaf venation lifts constraints on vascular plant ecology. Proceedings of the Royal Society B: Biological Sciences 281: 20132829. doi: 10.1098/rspb.2013.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


