Abstract
Background
Numerous groups of plants have adapted to CO2 limitations by independently evolving C4 photosynthesis. This trait relies on concerted changes in anatomy and biochemistry to concentrate CO2 within the leaf and thereby boost productivity in tropical conditions. The ecological and economic importance of C4 photosynthesis has motivated intense research, often relying on comparisons between distantly related C4 and non-C4 plants. The photosynthetic type is fixed in most species, with the notable exception of the grass Alloteropsis semialata. This species includes populations exhibiting the ancestral C3 state in southern Africa, intermediate populations in the Zambezian region and C4 populations spread around the palaeotropics.
Scope
We compile here the knowledge on the distribution and evolutionary history of the Alloteropsis genus as a whole and discuss how this has furthered our understanding of C4 evolution. We then present a chromosome-level reference genome for a C3 individual and compare the genomic architecture with that of a C4 accession of A. semialata.
Conclusions
Alloteropsis semialata is one of the best systems in which to investigate the evolution of C4 photosynthesis because the genetic and phenotypic variation provides a fertile ground for comparative and population-level studies. Preliminary comparative genomic investigations show that the C3 and C4 genomes are highly syntenic and have undergone a modest amount of gene duplication and translocation since the different photosynthetic groups diverged. The background knowledge and publicly available genomic resources make A. semialata a great model for further comparative analyses of photosynthetic diversification.
Keywords: Poaceae, photosynthesis, adaptation
INTRODUCTION
The origin of photosynthesis more than two billion years ago was a major event in Earth history, and since then the photosynthetic apparatus has been adapted continuously in response to varying environmental conditions (Raven and Geider, 2003; Tcherkez et al., 2006; Cardol et al., 2008; Raven et al., 2008; Young et al., 2012; Dusenge et al., 2019; Kumarathunge et al., 2019). In particular, atmospheric CO2 levels have repeatedly decreased, reaching very low levels ~30 million years ago (Ma) (Pagani et al., 2005). Given that CO2 represents the main substrate of photosynthesis, its depletion decreases the efficiency of carbon fixation (Ehleringer and Bjorkman, 1977; Skillman, 2008). Indeed, when CO2 is limited, Rubisco, the enzyme that fixes CO2 during photosynthesis, will fix O2 instead, exacerbating the costly process of photorespiration (Peterhansel and Maurino, 2011; Busch, 2020). Plants have developed different strategies to limit photorespiration, which becomes especially important in warm and dry environments of the low-CO2 world that prevailed after the Oligocene (Ehleringer and Monson, 1993; Ehleringer et al., 1997; Tcherkez et al., 2006). Over the last 32 million years (Christin et al., 2011), >60 lineages of plants have evolved the C4 photosynthetic pathway (Sage et al., 2011), which concentrates CO2 within the leaf before its use by Rubisco (Hatch, 1987; Sage, 2004; Sage et al., 2012). C4 plants outperform those that still use the ancestral C3 pathway in open biomes of tropical regions, and consequently, they cover vast areas of the world and account for one-quarter of terrestrial primary production (Still et al., 2003; Edwards et al., 2010; Lehmann et al., 2019). C4 plants also include major crops, such as maize, sorghum and sugarcane, and have consequently been the focus of numerous research projects (e.g. Matsuoka et al., 2001; Langdale, 2011; von Caemmerer et al., 2012; Sales et al., 2021; Zhao et al., 2022). Given the complexity of the C4 trait, its recurrent evolutionary origins are especially puzzling.
The C4 pathway requires a specialized leaf anatomy in addition to the upregulation and synchronization of numerous enzymes (Hatch, 1987; Edwards et al., 2001; Sage et al., 2012). In the C4 pathway, the initial fixation of atmospheric CO2 is performed by the enzyme phosphoenolpyruvate carboxylase (PEPC), which, unlike Rubisco, has no affinity for O2. This reaction usually happens in mesophyll cells, which are in direct contact with the atmosphere. The resulting four-carbon acids are then transformed and transported to a different cell that is isolated from the atmosphere, typically in the bundle sheaths that surround the veins, where Rubisco is localized in C4 plants. The largest lineages of C4 plants (e.g. Chloridoideae grasses) evolved this trait >30 Ma (Christin et al., 2008), and consequently, they differ broadly from C3 plants in many aspects besides those linked to the photosynthetic pathway (Heyduk et al., 2019). Most C4 research has therefore used closely related plants with contrasted photosynthetic types, which exist in various lineages of angiosperms, with examples from Amaranthaceae and Chenopodiaceae (Atriplex) (Ehleringer and Bjorkman, 1977; Kadereit et al., 2003), Asteraceae (Flaveria) (Vogan and Sage, 2011), Cleomaceae (Cleome) (Koteyeva et al., 2011), Cyperaceae (Eleocharis) (Bruhl and Perry, 1995), Molluginaceae (Mollugo) (Christin et al., 2011), Poaceae (Neurachne) (Khoshravesh et al., 2020) and more broadly across eudicots (Muhaidat et al., 2007; Muhaidat and McKown, 2013). In most cases, however, the photosynthetic types are distributed among distinct species. The only known exception is the grass Alloteropsis semialata, which includes both C4 and non-C4 individuals, providing an exciting system in which to study the drivers of C4 evolution.
The photosynthetic variation existing in A. semialata was discovered based on leaf anatomical surveys and measurements of carbon isotopes independently by Ellis (1974) and Brown (1975). The differences between photosynthetic types within this species have been repeatedly studied since then, focusing on the ecological (Ripley et al., 2007, 2010b; Ibrahim et al., 2008; Osborne et al., 2008; Bateman and Johnson, 2011; Lundgren et al., 2015), cytogenetic (Frean and Marks, 1988; Liebenberg and Fossey, 2001; Lundgren et al., 2015; Bianconi et al., 2020; Olofsson et al., 2021), physiological (Frean et al., 1980, 1983a; Lundgren et al., 2016) and biochemical variation (Ueno and Sentoku, 2006; Phansopa et al., 2020), and more recently, on evolutionary and genomic aspects of C4 photosynthesis (Ibrahim et al., 2009; Christin et al., 2012; Lundgren et al., 2015, 2019; Olofsson et al., 2016, 2021; Dunning et al., 2017, 2019a; Bianconi et al., 2018, 2020; Curran et al., 2022). In this review, we consolidate the knowledge accumulated on this study system. First, we compile and review the distribution and evolutionary history of the Alloteropsis genus and its photosynthetic types. Second, we review the photosynthetic diversity discovered within the genus and discuss its importance for our understanding of C4 evolution. Third, we present a new chromosome-level reference genome for a C3 individual of A. semialata and compare it with an existing genome for a C4 conspecific, with a special focus on synteny and gene orthology. Finally, we discuss future research directions that can build on existing knowledge and resources accumulated by different researchers over almost 50 years. We hope that this information can help to motivate future research into the photosynthetic diversity of Alloteropsis, a unique system for studying photosynthetic diversity.
EVOLUTIONARY HISTORY OF ALLOTEROPSIS
Evolutionary diversification of the Alloteropsis genus
In its latest treatment, the genus Alloteropsis included five recognized species (Clayton and Renvoize, 1982). The species Alloteropsis cimicina, A. paniculata and A. papillosa are all C4 and form a monophyletic group based on chloroplast markers (Ibrahim et al., 2009). Alloteropsis cimicina is an annual weed (Fig. 1), native across Africa, Asia and Oceania, which is invasive in the Americas (Zuloaga et al., 2003; Rocha and Miranda, 2012). Few samples of this group have been analysed with molecular data, and at present it is not clear whether A. paniculata and A. papillosa represent truly distinct species from A. cimcina or whether they are merely morphological variants. It has been suggested that A. papillosa is a possible hybrid, because the linear leaf-blades resemble A. semialata while the inflorescence and spikelet characters are reminiscent of A. cimicina (Clayton and Renvoize, 1982). The divergence of samples assigned to A. cimicina and A. paniculata was estimated as ~2.5 Ma (Olofsson et al., 2016; Bianconi et al., 2020), and these two species will be discussed jointly as ‘A. cimicina’ in this review. The A. cimicina group diverged from the other two species in the genus ~11 Ma (Fig. 2; Lundgren et al., 2015; Dunning et al., 2017).
Fig. 1.

Diversity of Alloteropsis. Pressed herbarium specimens are shown for the main groups of Alloteropsis. From top to bottom, then left to right: A. cimicina (Nyirenda, Curran, Christin ZAM2065-05; SHD), A. angusta erect (Lundgren 2015-3-3; SHD) and decumbent (Nyirenda, Curran, Bianconi, Christin ZAM1933a; SHD), A. semialata lineage I (Mapaura, Lundgren, Olofsson 4; SHD), lineage II (Nyirenda, Curran, Bianconi, Christin ZAM1936-H1; SHD), lineage III (Nyirenda, Curran, Bianconi, Christin ZAM1934; SHD) and lineage IV (Dunning, Yakandawala, Ariyarathne-06; SHD).
Fig. 2.
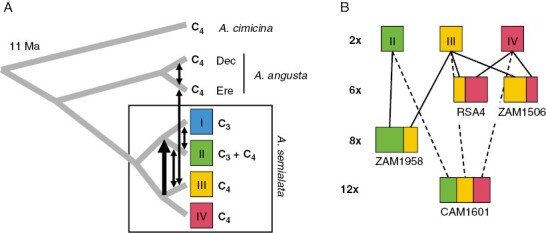
Relationships among Alloteropsis lineages. (A) The phylogenetic relationships among the main lineages of Alloteropsis are shown, based on the nuclear genome analyses by Raimondeau et al. (2022). The approximate age of the root is indicated, and the names of the group and their photosynthetic types are given on the right. Abbreviations: Dec, decumbent; Ere, erect. Arrows connecting branches indicate reported episodes of genetic exchanges (Olofsson et al., 2021; Curran et al., 2022). (B) The genetic contributions of genetic groups are represented for polyploid individuals analysed in detail (Olofsson et al., 2016, 2021; Bianconi et al., 2020). For each polyploid, the approximate proportion of their genome originating from each diploid lineage (shown at the top) is represented by a corresponding colour. Ploidy levels are indicated on the left, and the name of a representative population is given for each polyploid.
The sister species A. angusta and A. semialata have been studied in more detail, with intense species sampling increasing our confidence in the delimitation of lineages. Alloteropsis angusta was originally described as a small decumbent plant (Clayton and Renvoize, 1982), although we discovered that it also has an erect morph representing a distinct ecotype (Fig. 1; Curran et al., 2022). The decumbent and erect A. angusta ecotypes co-occur across Central and East Africa. They are divergent on the nuclear genome but share chloroplast haplotypes, indicating frequent hybridization (Fig. 2; Curran et al., 2022). All 132 samples of A. angusta analysed with carbon isotopes were probably C4, with only one anomalous non-C4 value that could need to be verified because of limited availability of material (Supplementary Data Table S1). The karyotype of the four A. angusta samples analysed was probably diploid, with a haploid genome size (1C) of ~1 Gb (Curran et al., 2022). Alloteropsis angusta diverged from A. semialata ~8 Ma (Lundgren et al., 2015; Dunning et al., 2017), although nuclear genome analyses indicate that the two species hybridize repeatedly where they come into contact (Fig. 2; Curran et al., 2022).
The diversity of A. semialata was studied originally in South Africa, where both C3 and C4 individuals were first discovered (Ellis, 1974). Comparisons of leaf anatomy from herbarium specimens suggested additional photosynthetic variation in A. semialata sampled from Tanzania and Zambia (Renvoize, 1987), and carbon isotopes intermediate between C3 and C4 signatures were also reported for samples from these countries (Ellis, 1981; Hattersley and Watson, 1992). Subsequent studies with a dense sampling from Central and East Africa, including live plants grown in greenhouse conditions, permitted physiological and biochemical assays, confirming that a greater photosynthetic diversity exists across Africa, and we describe here the evolutionary lineages of A. semialata and their photosynthetic types based on the most recent analyses.
Main lineages of A. semialata
Chloroplast markers suggested that the species originated in Central-East Africa, then acquired important chloroplast diversity as it migrated outside of this centre of origin (Lundgren et al., 2015; Bianconi et al., 2020). Seven main lineages of chloroplast haplotypes correspond to a reduced set of four distinct nuclear lineages, with the photosynthetic types appearing to be mostly consistent within each of these four groups (Fig. 2; Olofsson et al., 2016; Bianconi et al., 2020). Despite evidence of recurrent hybridization among them, the four nuclear lineages are recovered in both population genetics and phylogenetic analyses (Olofsson et al., 2016, 2021; Bianconi et al., 2020) and are therefore useful to summarize the diversification of the species. Their most recent common ancestor is estimated at ~2.5 Ma (Bianconi et al. 2020; Raimondeau et al., 2022), and each has followed a different evolutionary trajectory since then.
The nuclear lineage I, which encompasses chloroplast group A, results from a southern migration from its centre of origin and is now distributed across South Africa, Mozambique and Zimbabwe (Fig. 3; Lundgren et al., 2015; Bianconi et al. 2020). All individuals analysed so far are diploids (Lundgren et al., 2015; Bianconi et al., 2020). Based on carbon isotopes, no lineage I individuals use the derived C4 photosynthetic type (Fig. 3; Supplementary Data Table S2), and the live plants analysed in greenhouse conditions showed characteristics of C3 plants, including high CO2 compensation points, low PEPC activity in the leaves, low leaf transcript abundance of genes encoding C4 enzymes and low levels of C4 enzymes themselves (Fig. 4; Frean et al., 1980; Barrett et al., 1983; Ueno and Sentoku, 2006; Ripley et al., 2007; Lundgren et al., 2016, 2019; Dunning et al., 2019a). The leaf anatomy of plants from this group is, moreover, indicative of a C3 photosynthetic type (Fig. 4; Ellis, 1974; Frean et al., 1983a, b; Lundgren et al., 2019). In two individuals from this group, the inner bundle sheath (also known as the mestome sheath) was shown to contain glycine decarboxylase (Ueno and Sentoku, 2006; Lundgren et al., 2019), an enzyme used in recycling of the products of O2 fixation by Rubisco. This might suggest that the plant performed a weak photorespiratory pump, as seen in other species described as ‘type I intermediates’ (Edwards and Ku, 1987; Sage et al., 2012). However, the abundance of glycine decarboxylase in other tissues (Ueno and Sentoku, 2006; Lundgren et al., 2019) and the C3-like physiology of the plants analysed (Frean et al., 1980; Barrett et al., 1983; Ripley et al., 2007; Lundgren et al., 2016) suggest that such a pump is weak, at best. Nuclear lineage I can thus be considered as encompassing mostly, if not only, C3 individuals.
Fig. 3.
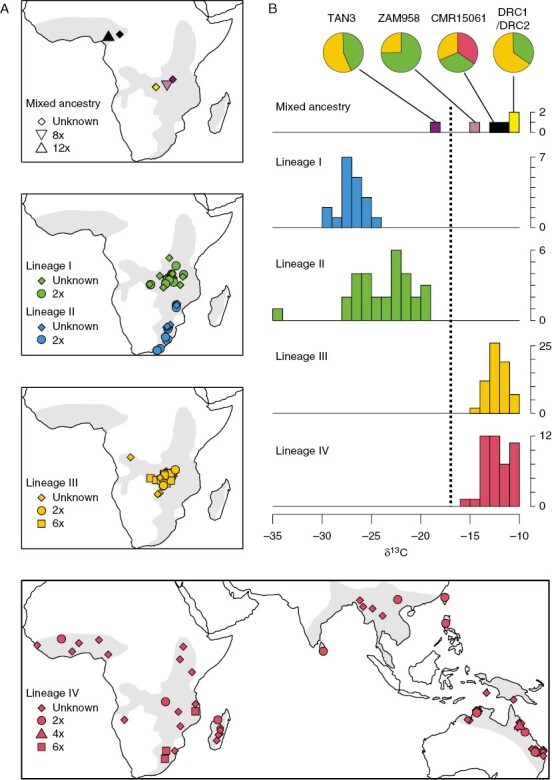
Geographical distribution and photosynthetic diversity of Alloteropsis semialata. (A) For each of the four main lineages of A. semialata (lineage I in blue, lineage II in green, lineage III in orange and lineage IV in red), the distribution of known samples is shown, with shapes indicating their ploidy level (for data, see Supplementary Data Table S2). Individuals of mixed ancestry unassigned to these lineages are shown separately. The grey area on each map shows the putative range of the species, based on known individuals plus reported samples on GBIF (https://www.gbif.org/). (B) The distribution of carbon isotope ratios (δ13C) is shown with histograms for each group. In the case of individuals with mixed ancestries, the relative parental contributions of the four lineages are shown with pie charts.
Fig. 4.
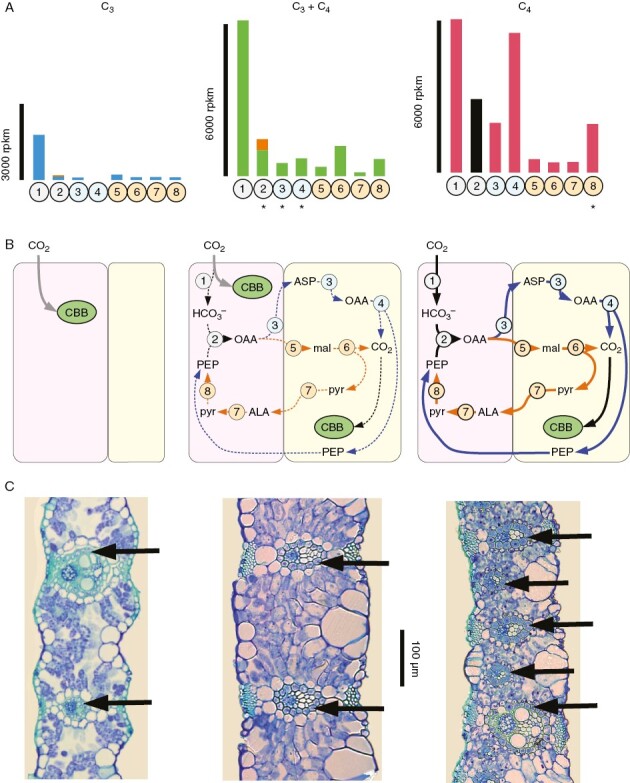
Photosynthetic types within Alloteropsis semialata. The figure shows: (A) the C4 gene expression patterns; (B) putative carbon acquisition pathways; and (C) leaf anatomy for a C3 individual of A. semialata (RSA5, left column), a C3 + C4 individual (TAN1, middle column) and a C4 individual (AUS1, right column). (A) The expression levels of genes encoding enzymes involved in the C4 pathway [in reads per million mappable reads per kilobase (rpkm)] were retrieved from Dunning et al. (2017); 1 = carbonic anhydrase (gene βca-2P3); 2 = phosphoenolpyruvate carboxylase [sum of genes ppc-1P3 and ppc-1P6 (shown in orange), including the laterally acquired ppc-1P3_LGT-A present in AUS1 shown in black]; 3 = aspartate aminotransferase (gene aspat-3P4); 4 = phosphoenolpyruvate carboxykinase (laterally acquired gene pck-1P1_LGT-C); 5 = NADP-malate dehydrogenase (gene nadpmdh-3P4); 6 = NADP-malic enzyme (gene nadpme-1P4); 7 = alanine aminotransferase (alaat-1P5); and 8 = pryuvate, phosphate dikinase (ppdk-1P2). Asterisks below gene numbers indicate those that were consistently upregulated in the C3 + C4 versus C3 or C4 versus C3 + C4 comparisons (see Dunning et al., 2019a). (B) The putative C4 cycles show the metabolites across the mesophyll (in pink) and bundle sheath (in yellow) cells, with enzymes represented by circles and numbered as in (A), the PCK pathway in blue and the NADP-ME pathway in orange. Abbreviations: ALA, alanine; ASP, aspartate; CBB, Calvin–Benson–Bassham cycle; mal, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; pyr, pyruvate. (C) The leaf cross-sections were retrieved from the paper by Bianconi et al. (2022). The black arrows point to the inner bundle sheath of each vein.
The nuclear lineage II is associated mostly with chloroplast groups B and C and migrated slowly around the centre of origin of the species (Lundgren et al., 2015; Bianconi et al., 2020). It is tightly associated with the Central Zambezian miombo woodlands, where it occurs in both open and wooded grasslands (Olofsson et al., 2021), and has been identified in Burundi, Democratic Republic of Congo, Malawi, Tanzania and Zambia (Fig. 3). All individuals analysed so far are diploid (Bianconi et al., 2020; Olofsson et al., 2021). None of the individuals from this lineage is C4, based on carbon isotopes (Lundgren et al., 2015; Olofsson et al., 2021), although the lineage contains isotopic intermediates that probably grew using both C3 and C4 pathways (Fig. 3; Lundgren et al., 2016; Olofsson et al., 2021). Moreover, its individuals have CO2 compensation points, leaf anatomy, PEPC abundance and C4 gene transcript abundance in the leaves that are intermediate between C3 and C4 types (Fig. 4; Lundgren et al., 2016, 2019; Dunning et al., 2019a). All the evidence suggests that individuals from lineage II assimilate some of their carbon via the C3 cycle and some via the C4 cycle, and consequently, they were termed ‘C3+C4’ (Dunning et al., 2017). This type corresponds to ‘type II intermediates’ reported in a number of other lineages of plants (Monson et al., 1986; Sage et al., 2012, 2018; Schlüter and Weber, 2016; Lyu et al., 2022). Moreover, the variation in carbon isotopes, CO2 compensation points and leaf anatomy within lineage II (Fig. 3; Lundgren et al., 2016, 2019; Olofsson et al., 2021) suggest that the strength of the C4 cycle varies among populations and could potentially even include individuals without any C4 cycle.
The nuclear lineage III is associated mostly with the chloroplast lineages F and G, which are sister to all other chloroplast lineages of A. semialata (Lundgren et al., 2015; Olofsson et al., 2016). However, its nuclear genome is consistently sister to that of lineage IV (Olofsson et al., 2016; Bianconi et al., 2020). It is hypothesized that this mismatch is a result of genomic swamping, with unidirectional pollen flow from lineage III into a distantly related maternal lineage resulting in the apparent substitution of the nuclear genome in a pattern that mirrors chloroplast capture (Bianconi et al., 2020). Lineage III is restricted mostly to the Central Zambezian miombo woodlands, where it overlaps with lineage II (Fig. 3), and the two can be found in mixed populations (Olofsson et al., 2021). Its individuals include both diploids and hexaploids (Olofsson et al., 2021). All individuals from lineage III analysed so far have carbon isotopes typical of C4 plants (Fig. 3; Lundgren et al., 2015; Olofsson et al., 2021). The populations analysed showed high leaf transcript abundance of genes encoding C4 enzymes (Dunning et al., 2019a). The assessed populations from Zambia and Tanzania have very low CO2 compensation points and leaves where chloroplasts are concentrated in the inner bundle sheath, confirming that they are C4 (Lundgren et al., 2019).
The nuclear lineage IV is associated mostly with chloroplast groups D and E (Lundgren et al., 2015). It migrated rapidly away from the centre of origin of the species to Southern Africa, Western Africa, Madagascar, Asia and Australia (Fig. 3; Lundgren et al., 2015; Bianconi et al., 2020), where it is now considered a keystone species (Bateman and Johnson, 2011). In South Africa, it overlaps with the C3 lineage I, and the two occasionally form mixed populations (Frean et al., 1980). It is present in a variety of biomes, and its broader niche is likely to be associated with its rapid dispersal (Lundgren et al., 2015), although whether each individual possesses a broad niche or whether the group is able to adapt rapidly to local conditions is unknown. This lineage IV contains mostly diploids in Asia, Australia and large parts of Africa, but also hexaploids in Southern Africa (Lundgren et al., 2015; Bianconi et al., 2020) and a single tetraploid reported from Australia (Olofsson et al., 2021). All individuals from this lineage have carbon isotopes indicative of C4 photosynthesis (Fig. 3; Lundgren et al., 2015; Bianconi et al., 2020). They show high PEPC abundance in the leaves, high transcript abundance of genes for C4 photosynthesis, an abundance of inner bundle sheath cells filled with chloroplasts and high leaf abundance of C4 enzymes that follow the expected cellular compartmentalization (Fig. 4; Frean et al., 1983a, b; Ueno and Sentoku, 2006; Lundgren et al., 2016, 2019; Dunning et al., 2019a). These plants, however, have Rubisco in the mesophyll cells, which is unexpected for C4 plants and led to them being referred to as ‘C4-like’ (Ueno and Sentoku, 2006). Given that the physiology and carbon isotopes are typical of C4 plants (Fig. 3) and it is not known whether their mesophyll Rubisco is active, they must be considered as performing C4 photosynthesis.
Species status of A. semialata
Differences in photosynthetic types and ploidy levels observed in South Africa led to a suspicion that C3 and C4A. semialata populations were different species (Frean and Marks, 1988; Liebenberg and Fossey, 2001), and two subspecies were defined in South Africa (Gibbs Russell, 1983). The morphological characters used to recognize these two subspecies are not applied easily in other regions of Africa (Gibbs Russell, 1983), and the variation in photosynthetic type and other anatomical traits goes beyond that described in South Africa, meaning that many individuals would fall outside these existing subspecies (Curran et al., 2022). More generally, the four nuclear lineages of A. semialata described here hybridize naturally in the wild (Fig. 2; Olofsson et al., 2016, 2021; Bianconi et al., 2020) and produce healthy F1 hybrids in the greenhouse (Bianconi et al., 2020, 2022). To date, we have not been able to obtain seeds for F2 individuals despite F1 hybrids producing flowers. Some hybrid incompatibility might exist, although experimental tests are lacking. Given the morphological variation existing within each lineage and the recurrence of genetic exchanges among them, they should all be considered as part of the same species complex. In the absence of morphological characterization of a large number of individuals of known genetic ancestry, we advocate for the identification of nuclear lineages based on genetic data. This is especially important because some parts of the species ranges that present genetically divergent lineages remain poorly sampled, especially in Angola and the Democratic Republic of Congo.
Recurrent polyploidization in A. semialata
Although the four nuclear lineages are clearly distinct in diploids, some polyploids are placed phylogenetically outside these lineages, with discrepancies among nuclear markers, incongruence between nuclear and organelle genomes, and even disagreement among organelles (Olofsson et al., 2016, 2019, 2021; Bianconi et al., 2020). This includes dodecaploids (12×) from Cameroon (Bianconi et al., 2020), hexaploids (6×)/octoploids (8×) from Zambia (Olofsson et al., 2021) and plants of unknown ploidy in Tanzania and the Democratic Republic of Congo (Fig. 3; Olofsson et al., 2016; Bianconi et al., 2020). Among these individuals, the known polyploids present genomic contributions from multiple nuclear lineages: II + III in Zambia (Fig. 2; Olofsson et al., 2021) and II + III + IV in Cameroon (Fig. 2; Bianconi et al., 2020); and the hexaploids from lineages III and IV each present contributions from the other lineage (Fig. 2; Olofsson et al., 2016; Bianconi et al., 2020). The affinities of other polyploids reported in the past, including tetra-, octo- and dodecaploids in addition to hexaploids (Ellis, 1981; Frean and Marks, 1988; Liebenberg and Fossey, 2001), remain unknown in the absence of genetic analyses. The available evidence already indicates that polyploidy allows the mixing of distinct lineages, in some cases corresponding to different photosynthetic types. Based on their position in the nuclear and organelle phylogenies, polyploids emerged recurrently within A. semialata (Olofsson et al., 2019; Bianconi et al., 2020). Polyploidy seems to have a huge effect in some cases, with the polyploids being much more abundant than diploids in regions of Africa (Fig. 3). The exact consequences of the recurrent polyploidization on the niche and the phenotype, however, remain to be investigated in detail. At present, the extent of gene flow among polyploids and between diploids and polyploids is not fully understood, although polyploidy has been proposed as a mechanism preventing reproductive interference among photosynthetic types in mixed populations (Olofsson et al., 2021).
Reproductive and life-history characteristics of A. semialata
Alloteropsis semialata is a perennial species, and although its lifespan has never been evaluated, we have kept some plants for 10 years in climatically controlled greenhouse conditions (12 h daylight, 25/20 °C day/night temperature) without any signs of senescence. The species forms rosettes, from which long stems emerge carrying the inflorescence (Fig. 1). The base of the leaf is thickened, forming bulb-like structures, and new bulbs and tillers emerge continuously from rhizomes. In some accessions, the bulbs are compressed together and form a single compact structure that cannot be separated without disrupting the bulb tissue. In others, the stolons place the bulbs further apart, and these then form separate individuals that can be repotted to produce clones. The extent of such vegetative growth has not been quantified in the wild, but we have identified wild individuals that have formed tens of bulbs, each bearing a stem and inflorescence. This suggests that clonal propagation is frequent in the wild, and it is certainly constant in the greenhouse.
In comparison, sexual reproduction is uneven in the greenhouse. Some accessions seem to flower more frequently than others, and the seed set is also fairly variable. Based on our own experience, polyploids are able to self-fertilize, as can some of the C3 + C4 (lineage II), which occasionally even develop cleistogamous inflorescences among the rosette leaves. In contrast, all seedlings obtained from diploid individuals from lineages I, III and IV that were genotyped resulted from crosses between distinct accessions, suggesting a predominantly outcrossing system (Bianconi et al., 2022). The different lineages of A. semialata can interbreed freely to produce F1 hybrids (Bianconi et al., 2022). Inflorescences with pollen were observed in most F1 hybrids, but to date we have not been able to obtain seeds for F2 offspring. Evidence of introgression suggests that such crosses do occur episodically in the wild (Olofsson et al., 2016, 2019; Bianconi et al., 2020).
INSIGHTS INTO C4 EVOLUTION
Three transitions to a full C4 physiology
The Alloteropsis genus belongs to the Paniceae tribe of grasses, which contains multiple independent origins of C4 photosynthesis (GPWG, 2012). Its closest relatives identified so far are C3 (Entolasia sp., Amphicarpum sp. and Panicum pygmaeum) (GPWG, 2012), and the genus is likely to have emerged from a C3 lineage within the Boivinellinae (Ibrahim et al., 2009). All Alloteropsis accessions are C4 except the non-C4A. semialata, which are nested within the genus (Fig. 2). It was therefore hypothesized that the non-C4A. semialata might represent a reversal from a C4 ancestral state (Ibrahim et al., 2009). Further investigation of the history of individual anatomical and biochemical C4 components, however, argues strongly against this scenario (Dunning et al., 2017). Alloteropsis cimicina and the pair A. semialata/A. angusta co-opted different tissues for the C4 pathway. The former uses the outer bundle sheath to segregate Rubisco and the Calvin–Benson–Bassham cycle, with a large proportion of bundle sheath tissue achieved via a dramatic expansion of these cells (Christin et al., 2013). In stark contrast, both A. semialata and A. angusta use the inner bundle sheath to segregate Rubisco and achieved a large proportion of bundle sheath tissue via the addition of minor veins, without drastic enlargement of bundle sheath cells (Fig. 4; Ellis, 1974; Brown, 1977; Frean et al., 1983a; Renvoize, 1987; Christin et al., 2013; Lundgren et al., 2019). The details of the C4 pathway in A. cimicina have not been established with biochemical assays, but transcriptomic analyses indicate a high transcript abundance of genes encoding the NADP-malic enzyme (NADP-ME) and other enzymes associated with the NADP-ME type (Dunning et al., 2017, 2019a). Early analyses described some South African A. semialata as using the NADP-ME type, with potential changes depending on the temperature (Frean et al., 1983b), although this has not been confirmed subsequently. All other samples were characterized as being of the phosphoenolpyruvate carboxykinase decarboxylase (PCK) type (Prendergast et al., 1987; Ueno and Sentoku, 2006). Subsequent transcriptomic analyses suggested that, at 25 °C, all assessed C4 accessions of either A. semialata or A. angusta use mainly the PCK C4 type, with various amounts of NADP-ME activity (Fig. 4; Dunning et al., 2017, 2019a). In addition to the differences associated with the C4 type discussed above, enzymes used for the C4 pathway in both A. cimicina and A. angusta/A. semialata are not necessarily encoded for by the same gene, with different paralogues encoding the C4 copy of aspartate aminotransferase in the two groups (Dunning et al., 2017). The numerous anatomical, biochemical and genetic mismatches unambiguously support at least two independent C4 origins within the Alloteropsis genus.
The history of photosynthetic diversification among the A. angusta/A. semialata group is more difficult to decipher because the two species use homologous anatomical and biochemical C4 components. However, genes for the C4 enzymes show evidence of adaptation under positive selection on the branches individually leading to each of the two species, but not in their common ancestor (Dunning et al., 2017). Biochemical adaptation therefore appears to have happened twice in the group, although it cannot be ruled out that the common ancestor of the two species possessed some C4 components and that only the subsequent gene adaptation occurred after their split. A full C4 pathway therefore evolved three times in this small genus, and this was helped by exchanges of C4 genes within the genus (Dunning et al., 2017).
Alloteropsis is not the only group in which recurrent C4 origins appeared: C3, C3–C4, C4-like and C4 species co-exist within the genus Flaveria, with two independent origins of C3–C4 intermediacy and C4-like (McKown et al., 2005); in Molluginaceae, C3–C4 intermediacy evolved at least twice, and two fully developed C4 originated twice within the same species, Mollugo cerviana (Christin et al., 2011); and new photosynthetic pathways have evolved repeatedly within the subtribe Neurachninae (Christin et al., 2012). Whether the most recent common ancestor of A. semialata was C3 + C4 remains an open question. Such a scenario would imply that the C3A. semialata have lost their previous C3 + C4 characters, because no traces of genes previously used for a weak C4 cycle have been detected in these populations. The exact history of components sustaining a C3 + C4 state, such as the proliferation of chloroplasts containing active Rubisco in the bundle sheath and overexpression of some C4 enzymes, will need to be revisited once the mutations underlying these traits are identified. Indeed, if such components existed in the ancestors of the C3A. semialata, sequences that differ from the ancestral state would be expected following mutations to revert to a C3 physiology.
Multiple origins of C 4 components within A. semialata
The group consisting of lineages III and IV of A. semialata is wholly C4, and it is likely that its most recent common ancestor was also C4, leading to the inference of a single origin in this species. However, individual C4 components unambiguously originated multiple times within the group. The best examples are given by C4 genes acquired from distantly related species, because these are easy to identify across samples (Christin et al., 2012; Dunning et al., 2017). The main enzyme of the C4 pathway, PEPC, is encoded in grasses by seven different gene lineages that emerged through repeated single-gene or whole-genome duplication events (Christin et al., 2014). Of these, two are upregulated in the C3 + C4 and some C4 individuals of A. semialata (Figs 4 and 5; Dunning et al., 2017), and these co-opted genes show evidence of biochemical adaptation for the C4 context (Phansopa et al., 2020). Other C4 accessions of A. semialata (and A. angusta) overexpress only one of these two genes, and it is not always the same copy (Fig. 5; Dunning et al., 2017), meaning that the C4-specific PEPC from different C4 accessions effectively have different origins even if they originated in the common ancestor from the group (Fig. 5). A completely distinct gene of PEPC is used by the Australian A. semialata, and this gene was acquired laterally, from the Andropogoneae Themeda triandra (Christin et al., 2012; Dunning et al., 2019b), with important biochemical adaptations that pre-date the transfer (Phansopa et al., 2020). This laterally acquired copy replaced the ancestral native copies, which became pseudogenes (Fig. 5). A similar process happened in Africa with two other PEPC genes acquired laterally from other groups of grasses (Cenchrinae and Melinidinae; Christin et al., 2012), each present in a subset of individuals, creating a mosaic of PEPC origins within the species (Olofsson et al., 2016). These PEPC examples show that C4 components can originate independently within a single C4 lineage. The C4A. semialata also present important C4 anatomical variation (Lundgren et al., 2019) and variation in the expression of C4-related genes (Dunning et al., 2019a), offering an excellent system in which to unravel the secondary adaptations that happened among sub-lineages after the initial transition to C4 photosynthesis.
Fig. 5.
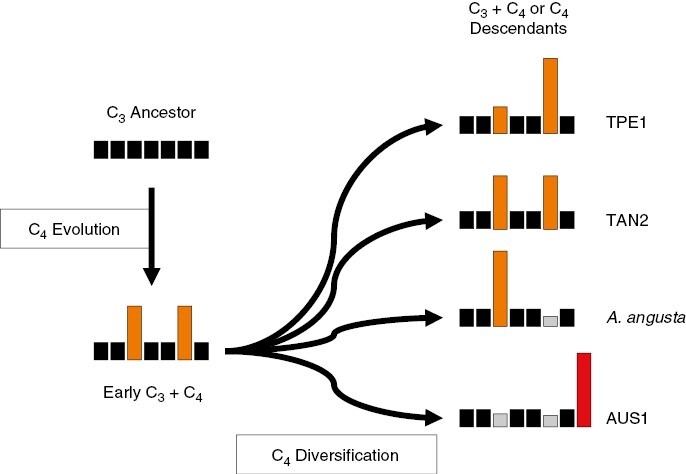
History of phosphoenolpyruvate carboxylase (PEPC) co-option in Alloteropsis. Each bar approximates the expression level of one of the seven genes encoding PEPC existing in grasses, in black for functional non-C4 genes, in orange for genes co-opted in C4 photosynthesis, in red for laterally acquired C4 genes and in grey for non-functional pseudogenes. The name of an individual illustrating each pattern is indicated on the right (Dunning et al., 2017; Phansopa et al., 2020).
Studies of A. semialata suggest that an initial C4trait can emerge via a few changes
The C4 pathway observed in older C4 lineages is often complex, relying on numerous anatomical and biochemical specialities (Hatch, 1987; Sage, 2004; Sage et al., 2012). Anatomically, a higher proportion of vascular bundle sheath tissue, as a result of a greater number of veins and larger size of the bundle sheath cells, strongly increases C4 evolvability in grasses (Christin et al., 2013). Biochemical changes are numerous and very diverse depending on the photosynthetic subtype (Burgess and Hibberd, 2015). Such features are likely to encompass those that were needed to evolve the C4 trait (primary adaptations), but also those that evolved later (secondary adaptations) and those that evolved for unrelated reasons (Heyduk et al., 2019). In fact, most comparative transcriptomic studies among C4 and C3 relatives identify several hundreds of differentially expressed genes, many of which would probably represent secondary adaptations (Bräutigam et al., 2011, 2014; Lyu et al., 2021).
Given that they share a recent common ancestor, comparisons of the different groups of A. semialata offer an opportunity to distinguish the primary adaptations that were acquired during the initial transition to C4 photosynthesis from those that evolved later. In such comparative endeavours, it is especially important to sample the diversity within each group to identify those features restricted to a subsample of C4 plants, which represent secondary adaptations. Leaf anatomy comparisons revealed that the only character that differs consistently between C4 and non-C4A. semialata is the proliferation of minor veins (Fig. 4), which is therefore sufficient to support the transition between C3 + C4 and C4 in this species (Lundgren et al., 2019). As in A. semialata, vein density seems to be an important factor for C4 evolvability in Neurachne (Khoshravesh et al., 2020), in contrast to C3 and C4 relatives within the Chenopodiaceae, which present similar vein density (Voznesenskaya et al., 2013; Freitag and Kadereit, 2014). Certain A. semialata C4 individuals differ in other leaf anatomy characters (e.g. bundle sheath cell enlargement) from the non-C4A. semialata (Lundgren et al., 2019), but these are likely to represent secondary adaptations. In terms of leaf transcriptomes, only three genes encoding known C4 enzymes (aspartate aminotransferase, phosphoenolpyruvate carboxykinase and phosphoenolpyruvate carboxylase) were consistently upregulated in the C3 + C4 compared with the C4A. semialata, and only pyruvate orthophosphate dikinase was upregulated in the C4 compared with the C3 + C4A. semialata (Fig. 4; Dunning et al., 2019a). Other C4-related genes were upregulated only in a subset of C4 populations (e.g. malate dehydrogenase), suggesting that they represent secondary adaptations that were not involved in the initial transition to C4 photosynthesis.
Not all aspects of the C4 trait have been studied in A. semialata. For example, the intracellular anatomical variation has been investigated in very few individuals, and the enzyme kinetics and regulation are largely unknown (but for PEPC, see Phansopa et al., 2020). There are, therefore, likely to be more differences between the groups of A. semialata than those reported here. Nevertheless, the evidence available to date suggests that the transition to complete reliance on the C4 pathway involves relatively few primary adaptations and can emerge via the upregulation of only four genes (aspat, pck, ppc and ppdk) accompanied by a single key alteration in leaf development involving the proliferation of minor veins. Many other features traditionally associated with C4 photosynthesis instead represent secondary adaptations, which improved existing C4 traits and might allow this species to perform extremely well in a diversity of ecological niches. These secondary adaptations can, moreover, evolve in distinct populations and be combined later, following genetic exchanges among distinct lineages and adaptive introgression (Olofsson et al., 2016). The presence of distinct populations occupying different environments and accumulating new mutations might thus accelerate the evolutionary diversification of photosynthesis in some groups (Olofsson et al., 2016; Dunning et al., 2017).
Hybridization and the origin of C 3 + C 4 A. semialata
C3 + C4 plants are found in a number of angiosperm lineages (Sage et al., 2012, 2018; Lundgren and Christin, 2017), and it has been hypothesized that they might result from natural crosses between C3 and C4 plants (Kadereit et al., 2017). For example, within Salsoleae, the ‘type I intermediate’ species Salsola divaricata was proposed to be of hybrid origin (Tefarikis et al., 2022). In Flaveria, F1 hybrids obtained by artificially crossing C3, C3–C4 and C4 species showed a varying degree of inheritance of C4 phenotypes, suggesting that a hybrid origin of natural C3–C4 intermediates was unlikely (Kadereit et al., 2017). Furthermore, coordinated changes in gene expression, protein sequences and physiological and anatomical traits along the C4 evolutionary pathway defended the hypothesis of C3–C4 species being evolutionarily intermediate steps (Lyu et al., 2021). In contrast, a recent study using transcriptomes from 17 Flaveria species showed recurrent hybridization among them, which might have a certain impact into the development of C4 photosynthesis in the lineage (Morales-Briones and Kadereit, 2022).
In A. semialata, hybrids between C3 and C4 indeed resemble naturally occurring C3 + C4A. semialata in their physiology, although their C3 + C4 state relies on different components (Bianconi et al., 2022). In particular, hybrids between C3 and C4 parents in semi-controlled greenhouse conditions possess some of the minor veins that characterize the C4A. semialata (Bianconi et al., 2022), as do a few naturally occurring C3 + C4 × C4 hybrids identified in the wild (Supplementary Data Fig. S1). Such minor veins are generally absent from the natural C3 + C4 populations (Lundgren et al., 2019). Although these differences indicate that the origin of C3 + C4 is not likely to stem simply from an ancient hybridization between C3 and C4 parents, gene flow between the different A. semialata lineages might have played a role in diversification of the photosynthetic trait. A multigene coalescent species tree analysis highlights that the placement of the C3 + C4 clade is not fully resolved, with approximately equal proportions of gene trees placing this clade as sister to the C3 or C4 lineages (Bianconi et al., 2020). This pattern points to an episode of hybridization, which is likely to be relatively ancient because the genes encoding the C4 enzymes in the C3 + C4 intermediates that are sister to the C4 clade (e.g. aspartate aminotransferase) generally lack the traces of adaptive amino acid replacement seen in the C4 populations (Dunning et al., 2017). These gene tree analyses indicate that enzyme adaptation happened after the hybridization event. Gene flow continues to connect the C4 and C3 + C4 lineages periodically, as evidenced by the more recent transfer of a gene encoding PCK between lineages (Dunning et al., 2017).
Shared history, photosynthetic diversification and secondary adaptations all influence the ecophysiological behaviour of A. semialata
C4 photosynthesis brings a suite of benefits for CO2 fixation in hot, dry and high-light environments, including improved light-, water- and nitrogen-use efficiencies (Pearcy and Ehleringer, 1984; Sage and Pearcy, 1987; Long, 1999). However, it has long been recognized that the photosynthetic pathway is only one of a suite of ecophysiological characters needed to succeed in any particular environment. These might be inherited from non-C4 ancestors or evolve as environmental adaptations during C4 diversification (Osmond et al., 1982; Pearcy and Ehleringer, 1984). Alloteropsis semialata has therefore been used as a study system to investigate how the photosynthetic pathway interacts with ancestral and novel functional traits to determine ecological behaviour in relationship to temperature, water deficits, fire and atmospheric CO2 concentration (Supplementary Data Table S3). Two populations of A. semialata, a C3 diploid belonging to nuclear lineage I and a C4 hexaploid from nuclear lineage IV, have been used for detailed ecophysiological studies. These plants grow in mixed populations close to their range limits in South Africa, but their ecological behaviour differs significantly.
In A. semialata, the rate of leaf photosynthesis in light-saturated conditions, the quantum efficiency in light-limited conditions and the rate of leaf growth were all greater in the C4 than the C3 type at high temperatures (Osborne et al., 2008), as expected from theory and past work in other species (Monson and Jaeger, 1991; Kephart et al., 1992). However, the temperature threshold for a C4 advantage occurred at a lower temperature (15–17 °C) than is typically observed in comparisons of older C4 species with C3 counterparts (e.g. Ehleringer and Bjorkman, 1977). Furthermore, the benefit of C3 photosynthesis expected at lower temperatures was compromised by exposure to chilling in the range 10–15 °C, which causes photodamage to photosystem II in both C3 and C4 types (Osborne et al., 2008). This shared failure in C3 and C4 lineages to tolerate chilling is likely to reflect their shared recent tropical history (Osborne et al., 2008). In contrast, tolerance of freezing differs markedly between these C3 and C4 populations because the C3 lineage has evolved a physiological response that protects leaves from freezing damage after a period of cold acclimatization (Osborne et al., 2008). A common garden experiment in South Africa showed that differential freezing tolerance led to complete canopy senescence in field conditions during winter in the C4 type, but allowed the C3 type to retain functioning leaves (Ibrahim et al., 2008). Differential leaf survival was significant because daytime leaf temperatures on cloudless winter days exceeded 25 °C, at which C4 photosynthesis would provide a significant performance advantage (Ibrahim et al., 2008). In this species, adaptations (or a lack of adaptations) to avert chilling and freezing damage are therefore more important determinants of ecophysiological behaviour in cool conditions than the differential limitation of C3 and C4 photosynthesis by low temperatures.
C3 and C4 South African populations of A. semialata are also differentially impacted by water availability. C4 leaves have a higher water-use efficiency of photosynthesis than the C3 type in well-watered conditions (Ibrahim et al., 2008). However, drought conditions offset this benefit by causing a greater non-stomatal (i.e. metabolic) limitation of photosynthesis in the C4 than the C3 type (Ripley et al., 2007). In a common garden, this difference in ecophysiological behaviour negated the C4 photosynthetic advantage over the C3 during drought events (Ibrahim et al., 2008). The general susceptibility of C4 photosynthesis to drought limitation observed in multiple species is thought to be non-stomatal and metabolic in origin because photosynthetic inhibition is independent of ambient CO2 concentration (Ghannoum et al., 2003; Ghannoum, 2009). In other wild grass species, the metabolic limitation to CO2 assimilation doubled the time taken in C4 compared with C3 types to recover photosynthetic potential after rewatering (Ripley et al., 2010a). Evidence from A. semialata showed that the depression in CO2 assimilation is not caused by the restriction of alternative electron sinks when photochemistry is limited by water deficits (Ripley et al., 2007), but the mechanism remains unknown. In the case of drought responses, the differences in ecophysiological behaviour between C3 and C4 populations of A. semialata therefore relate directly to photosynthetic diversification.
Life history and biomass allocation also differ between South African C3 and C4A. semialata. C4 plants allocate a smaller proportion of biomass to leaves and roots than the C3 type, and a greater proportion to bulbs and flowers (Ripley et al., 2008). This difference in allocation strategy might reflect the higher nitrogen-use efficiency of the C4 than the C3 type, which means that biomass productivity is greater and might be allocated more flexibly to storage and reproduction for a given amount of nitrogen. Alternatively, it might represent a secondary adaptation to disturbance by fire in the C4 type, which tends to occupy more fire-prone habitats (Ripley et al., 2008, 2010b). A controlled burning experiment supported the hypothesis that the studied C4 population was better fire adapted than the C3 type. After an experimental fire during the winter dry season, spring growth was little impacted in the C4 type, owing to the remobilization of belowground resources and faster aboveground productivity, but was significantly impaired in the C3 type (Ripley et al., 2010b). These growth patterns have important ecological implications that must only be associated with C4 photosynthesis indirectly.
Finally, experiments in controlled environmental conditions have evaluated the photosynthetic and growth responses of C3 and C4A. semialata to historical CO2 concentrations corresponding to glacial and interglacial levels (Ripley et al., 2013). C4 photosynthesis provides the greatest benefits for CO2 fixation at the lowest, glacial CO2 level, with increases in photosynthetic capacity in the C3 type compensating for CO2 limitation at the interglacial level (Ripley et al., 2013). This photosynthetic acclimatization in the C3 leaves was associated with significant increases in nitrogen concentration. However, the total pool of nitrogen in C3 plants was unchanged by CO2 treatments, such that the acclimatization response was associated with lower nitrogen-use efficiency and biomass. In contrast, tissue nitrogen concentrations within C4A. semialata were little impacted by CO2 (Ripley et al., 2013). The work indicated that leaf acclimatization to CO2 is mediated by whole plant resource use.
Work using A. semialata, therefore, showed that ecophysiological behaviour arose from the interactions of shared history with photosynthetic diversification and new secondary adaptations. Several aspects of physiological function are clearly related to diversification of the photosynthetic pathway within this species, especially the drought and CO2 responses. However, the work has emphasized the importance of adaptations (or lack of adaptations) in non-photosynthetic characters for ecophysiological behaviour, even for environmental factors closely associated with C4 performance, such as temperature. Comparison of closely related C3 and C4 populations of A. semialata have shown that such secondary adaptations might arise rapidly during photosynthetic pathway diversification.
ADDITIONAL GENOMIC RESEARCH USING C3 AND C4A. SEMIALATA
Resources for comparative genomics will become essential for further dissection of the evolution of C4 photosynthesis, as the precise genetic changes responsible for the evolution of this complex phenotype are characterized. As part of this review, we present additional genomic resources for A. semialata, which now includes de novo reference genomes for each of the four nuclear lineages, two of which (one C3 and one C4) are assembled at the chromosome scale. We conduct some preliminary analyses that highlight the types of questions that can be asked with these data (i.e. the role of structural rearrangements and gene duplication in the emergence of C4 photosynthesis). We hope that the background information gathered here, and the publication of additional resources, will encourage other researchers to use A. semialata as a model for C4 evolution.
Reference genomes for each of the four nuclear lineages in A. semialata
Like the rest of the Paniceae tribe, the genome of A. semialata is composed of nine distinct chromosomes (Frean and Marks, 1988). The haploid genome size (1C) is ~1 Gb in diploid individuals and slightly lower in polyploid individuals (Olofsson et al., 2016, 2021), suggesting that genome downsizing occurs after polyploidization. We previously assembled a chromosome-level reference genome for one diploid accession from lineage IV originating from Australia (AUS1; Dunning et al., 2019b). We also generated assemblies of varying contiguity for one representative from each of the other three lineages, all diploid accessions (Raimondeau et al., 2022). Here, we present Omni-C sequence data for one of the C3 individuals from lineage I for which a draft genome assembly was already available (individual RSA5-3; Raimondeau et al., 2022). We use these data for HiRise scaffolding, obtaining a chromosome-level genome assembly for this C3 RSA5-3 individual, which we then compare with the C4 AUS1 (for full details of the methods and results, see Supplementary Data Methods S1 and Results). In addition to the reference genomes, there are publicly available whole-genome resequencing data for almost 100 individuals, RAD data for several hundred others representing the entirety of the A. semialata diversity sampled to date, and transcriptome data (available from GenBank Sequence Read Archive under BioProject PRJNA401220, PRJNA481434, PRJNA560360, PRJNA649872, PRJNA666779, PRJNA752516, PRJNA715711 and PRJNA824797).
The role of structural variants in the emergence of C4 photosynthesis
Structural rearrangements (e.g. inversions) reduce recombination in that region of the genome and can therefore loci beneficial loci together (Rieseberg, 2001; Hipp et al., 2010; Huang and Rieseberg, 2020). These structural rearrangements can drive adaptive shifts (Hoffman and Rieseberg, 2008), and they could potentially play a role in the emergence of C4 photosynthesis. To identify structural rearrangements, the first step is to establish the syntenic regions between genomes. Both assemblies consist of nine chromosomes and differ in their overall size (RSA5-3 = 621 Mb and AUS1 = 747 Mb). This difference largely reflects the estimated 1C genome sizes (Table 1) and is explained, in part, by the expansion of ‘copy and paste’ transposable elements, especially Ty3-retrotransposons (Supplementary Data Table S4). The physical locations of orthologous genes on the nine chromosomes shows that synteny is well conserved between the C3 and C4 genomes, with most matches placed along the diagonal (Fig. 6). Indeed, only 1480 one-to-one orthologues were not in syntenic positions (Supplementary Data Figs S2 and S3; Tables S5 and S6), indicating that only ~10% of genes have changed physical location since the divergence of the two genomes. The synteny plots include several duplicated segments, for instance between chromosome 1 and both chromosomes 4 and 7 (Fig. 6), which probably represent remnants of an ancient Poales whole-duplication event that is also visible in other grasses (Paterson et al., 2004, 2009). The plots also show several more recent rearrangements, including inversions (e.g. on chromosomes 2, 3 and 6; Fig. 6) and translocations (e.g. on chromosomes 5 and 9; Fig. 6). Further data are needed to confirm that these represent true chromosomal changes and not assembly artefacts, but comparisons with the Setaria italica genome indicate that three of them are specific to RSA5-3 (i.e. they differ both between RSA5-3 and AUS1 and between RSA5-3 and S. italica), while two are specific to AUS1 (Fig. 6). A further five inversions exist between both A. semialata and S. italica (Fig. 6).
Table 1.
Comparison of chromosome-level reference genomes of Alloteropsis semialata
| Parameter | ASEM_RSA5 | ASEM_AUS1 |
|---|---|---|
| Lineage | I, C3 | IV, C4 |
| Origin | South Africa (−32.70, 27.53) | Australia (−19.62, 146.96) |
| 1C genome size (Mb) | 870 | 1100 |
| Assembled genome size (Mb) | 621 | 747 |
| Number of scaffolds | 1323 | 687 |
| Size across nine chromosomes (Mb) | 582 | 782 |
| Annotated genes | 71 593 | 75 709 |
| Annotated genes with Panicoideae homologues | 30 060 | 29 126 |
Fig. 6.
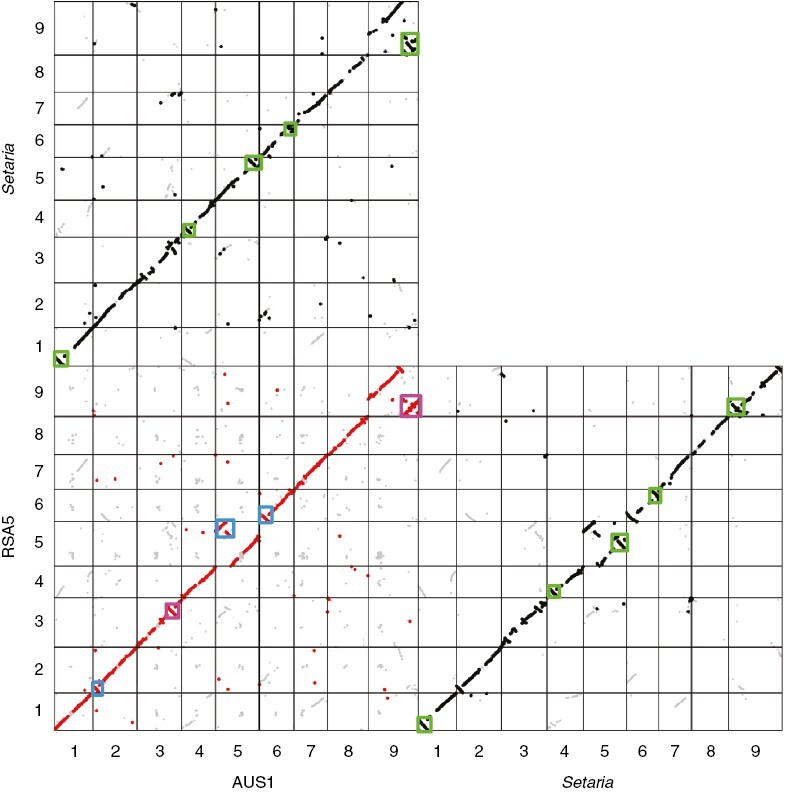
Synteny comparisons based on coding DNA. Pairs of synteny blocks (i.e. genes in the same position in both comparisons) are shown along the nine chromosomes of Alloteropsis semialata (genomes RSA5-3 and AUS1) and Setaria italica v2.2. High-quality matches (at least eight reciprocal best matches in a nine-match window) are shown in black or red, with the others in grey. Rectangles highlight major rearrangement, in blue for those specific to RSA5-3, in magenta for those specific to AUS1 and in green for those that differ between S. italica and both A. semialata assemblies.
To evaluate the role of structural rearrangements in C4 evolution, we selected the most relevant genes involved in C4 photosynthesis in A. semialata, based on gene expression levels in leaves, and located them on their physical position in both assemblies (Moreno-Villena et al., 2018; Dunning et al., 2019a). All C4 genes are present in both genomes, except tpt-1P1, which is absent in the C3 accession, and most of them show a conserved chromosome location and copy number (Fig. 7). Focusing on the four genes that were consistently upregulated in C3 + C4 and C4 versus C3 (Fig. 4), we observed that they are located in genomic regions that rearranged between AUS1 and RSA5-3. The gene aspat-3P4 is located on chromosome 5 in both individuals but seems to be affected by a large inversion or translocation (Fig. 7). The gene ppdk-1P2 is surprisingly duplicated in RSA5-3, the C3 individual (Fig. 7). The genes pck-1P1 and ppc-1P3 were laterally transferred into the C4 accession AUS1 from other C4 grasses (Christin et al., 2012) and are, therefore, located on chromosomes 7 and 8 in the AUS1 genome but absent in RSA5-3 (Fig. 7). However, native copies persist in both genomes. For ppc-1P3, the native copy is located on chromosome 4 in both accessions, and RSA5-3 has an additional duplication on chromosome 6 (Fig. 7). For pck-1P1, both native copies are located on chromosome 9 but translocated to different chromosome arms (Fig. 7). These large variants usually have a significant effect in gene regulation and gene function (Alonge et al., 2020; Hämälä et al., 2021; Yuan et al., 2021), but further work is required to determine whether these structural rearrangements have played a role in the evolution of C4 photosynthesis in A. semialata.
Fig. 7.
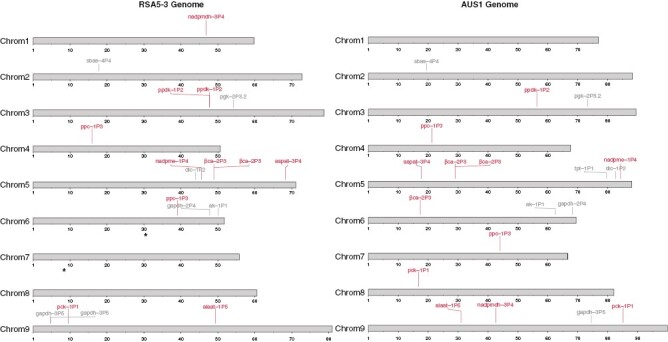
Genomic distribution of C4 genes. The two Alloteropsis semialata assemblies are represented by chromosomes, and expressed C4 genes (Dunning et al., 2019a) are located according to their genomic positions. The genes differentially expressed among C3, C3 + C4 and C4 groups are shown in pink, with other C4-related genes in grey. The two laterally transferred copies are marked with an asterisk. Gene names are abbreviated according to Moreno-Villena et al. (2018). Abbreviations: ak, adenylate kinase protein; alaat, alanine aminotransferase; aspat, aspartate aminotransferase; βca, β-carbonic anhydrase; dic, dicarboxylate carrier; gapdh, glyceraldehyde-3-phosphate dehydrogenase; nadpmdh, NADP-malate dehydrogenase; nadpme, NADP-malic enzyme; pck, phosphoenolpyravute carboxykinase; pgk, phosphoglycerate kinase; ppc, phosphoenolpyruvate carboxylase; ppdk, pyruvate, phosphate dikinase; sbas, sodium bile acid symporter family; tpt, triosephosphate-phosphate translocator.
Gene duplication plays no lasting role in C4 evolution
The evolution of C4 involves the increased expression of numerous enzymes, and gene duplication can play an important role in gene expression through a dosage effect. Indeed, it has previously been shown that there is a dosage effect on the expression of two C4 genes (pck and pepc) in A. semialata (Bianconi et al., 2018). However, differences in gene copy number appear to be transient, being rendered obsolete by the fixation of regulatory mutations increasing expression levels (Bianconi et al., 2018). When comparing the C3 and C4 reference genomes, we see that gene duplication is common, with the number of genes increasing by gene duplication being 24% in RSA5-3 and 21% in AUS1 since the split of the two accessions. However, only a few C4 genes are duplicated, and these are in the C3 individual. This confirms previous work that although it might be important for the initial emergence of C4 photosynthesis, these copy number variants are not maintained over longer evolutionary periods (Bianconi et al., 2018). However, it is important to note that some recent duplicates creating gene copy number variation might be collapsed in reference genomes and therefore hidden in the absence of read depth analyses (Bianconi et al., 2018).
Furthermore, neofunctionalization of retained duplicated genes can contribute to the evolution of novel traits. Most known C4 genes do not seem to have followed this pathway (Gowik and Westhoff 2011; Williams et al., 2012; van den Bergh et al., 2014); however, comparisons of seven monocot genomes led to 21 orthologous genes that were duplicated and retained in parallel in two distinct C4 origins (Emms and Kelly, 2015), suggesting that these might be newly identified genes that contributed to the evolution or optimization of C4 photosynthesis through duplication and neofunctionalization.
CONCLUSIONS
The genus Alloteropsis in general, and the species A. semialata in particular, constitute outstanding systems for retracing the events that led to C4 evolution and the ecological and physiological consequences of this gradual transition. In comparison to what is known in other species, A. semialata has maintained an unparalleled diversity of photosynthetic traits, and across the wider genus there is extensive variation in C4 anatomy, biochemistry and physiology. This phenotypic variation is accompanied by important genetic diversity, both among and within groups. This diversity provides the perfect ground for comparative analyses, enabling the identification of the traits that differ consistently among groups. Such an approach has been applied to C4 ecology, physiology, anatomy and transcriptomics, and this system would also be very suitable for quantitative genetics (Simpson et al., 2022). In addition, this diversity provides ample information to reconstruct the genomic history of the group and the genes within them using phylogenetic approaches. The genome provided here for a C3 individual, together with the previous one we published for a C4 individual (Dunning et al., 2019b), will enable future research into the genetic architecture of C4 evolution. Properties that are beneficial for comparative analyses, such as slow growth that allows the maintenance of accessions and the retention of diversity among accessions, however, represent limitations for experimental work. To our knowledge, no transformation has been attempted with any Alloteropsis species. In addition, A. semialata infrequently sets flowers and seeds in the greenhouse, hampering controlled crosses. If these limitations were to be overcome, the group would represent one of the best systems in which to study the genomic factors that control expression of the C4 trait in grasses.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Methods S1. Figure S1: leaf cross-section of a naturally occurring C3 + C4 × C4 hybrid identified in the wild, showing the intermittent presence of minor veins. Figure S2: genomic distribution of one-to-one orthologues. Figure S3: origins of genes in the two genomes of Alloteropsis semialata. Table S1: carbon isotope ratios and coordinates of Alloteropsis angusta samples. Table S2: sampled populations of Alloteropsis semialata. Table S3: mean environmental parameters for C3, C3 + C4 and C4Alloteropsis semialata populations extracted from Lundgren and Christin (2017). Table S4: statistics of transposable element annotation for AUS1 and RSA5-3 genome assemblies. Table S5: one-to-one orthologues between the two genomes of Alloteropsis semialata. Table S6: position and syntenic relationship among pairs of genes belonging to the same orthogroup.
ACKNOWLEDGEMENTS
We thank Vanja Milenkovic for her help in generating carbon isotope ratios for samples of Alloteropsis angusta.
Contributor Information
Lara Pereira, Ecology and Evolutionary Biology, School of Biosciences, University of Sheffield, Western Bank, Sheffield S10 2TN,UK.
Matheus E Bianconi, Ecology and Evolutionary Biology, School of Biosciences, University of Sheffield, Western Bank, Sheffield S10 2TN,UK.
Colin P Osborne, Plants, Photosynthesis and Soil, School of Biosciences, University of Sheffield, Western Bank, Sheffield S10 2TN, UK.
Pascal-Antoine Christin, Ecology and Evolutionary Biology, School of Biosciences, University of Sheffield, Western Bank, Sheffield S10 2TN,UK.
Luke T Dunning, Ecology and Evolutionary Biology, School of Biosciences, University of Sheffield, Western Bank, Sheffield S10 2TN,UK.
FUNDING
L.P. is supported by a NERC Standard Grant (NE/V000012/1), P.-A.C. was funded by the Royal Society (grants RGF\EA\181050 and URF\R\180022), and L.T.D. is supported by a Natural Environment Research Council Independent Research Fellowship (NE/T011025/1).
DATA AVAILABILITY
Raw sequencing data for AUS1 and RSA5-3 are available from the NCBI Sequence Read Archive under BioProject PRJNA481434 and PRJNA824797. Genome assemblies, annotations, extracted coding sequence and proteins for AUS1 and RSA5-3 generated as part of this review are available from Dryad (doi:10.5061/dryad.c866t1gb1).
LITERATURE CITED
- Alonge M, Wang X, Benoit, et al. 2020. Major impacts of widespread structural variation on gene expression and crop improvement in tomato. Cell 182: 145–161.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu M-JA, Tang Q, Wang Y, Essemine J, et al. 2022. Evolution of gene regulatory network of C4 photosynthesis in the genus Flaveria reveals the evolutionary status of C3-C4 intermediate species. Plant Communications 4: 100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DR, Frean ML, Cresswell CF.. 1983. C3 and C4 photosynthetic and anatomical forms of Alloteropsis semialata (R. Br.) Hitchcock: I. Variability in photosynthetic characteristics, water utilization efficiency and leaf anatomy. Annals of Botany 51: 801–809. doi: 10.1093/oxfordjournals.aob.a086531. [DOI] [Google Scholar]
- Bateman BL, Johnson CN.. 2011. The influences of climate, habitat and fire on the distribution of cockatoo grass (Alloteropsis semialata) (Poaceae) in the Wet Tropics of northern Australia. Australian Journal of Botany 59: 315–323. doi: 10.1071/bt10266. [DOI] [Google Scholar]
- van den Bergh E, Külahoglu C, Bräutigam A, et al. 2014. Gene and genome duplications and the origin of C4 photosynthesis: birth of a trait in the Cleomaceae. Current Plant Biology 1: 2–9. [Google Scholar]
- Bianconi ME, Dunning LT, Moreno-Villena JJ, Osborne CP, Christin P-A.. 2018. Gene duplication and dosage effects during the early emergence of C4 photosynthesis in the grass genus Alloteropsis. Journal of Experimental Botany 69: 1967–1980. doi: 10.1093/jxb/ery029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi ME, Dunning LT, Curran EV, et al. 2020. Contrasted histories of organelle and nuclear genomes underlying physiological diversification in a grass species. Proceedings of the Royal Society B: Biological Sciences 287: 20201960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi ME, Sotelo G, Curran EV, et al. 2022. Upregulation of C4 characteristics does not consistently improve photosynthetic performance in intraspecific hybrids of a grass. Plant, Cell & Environment 45: 1398–1411. doi: 10.1111/pce.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Kajala K, Wullenweber J, et al. 2011. An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiology 155: 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Schliesky S, Külahoglu C, Osborne CP, Weber APM.. 2014. Towards an integrative model of C4 photosynthetic subtypes: insights from comparative transcriptome analysis of NAD-ME, NADP-ME, and PEP-CK C4 species. Journal of Experimental Botany 65: 3579–3593. doi: 10.1093/jxb/eru100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WV. 1975. Variations in anatomy, associations, and origins of Kranz tissue. American Journal of Botany 62: 395–402. doi: 10.1002/j.1537-2197.1975.tb14062.x. [DOI] [Google Scholar]
- Brown WV. 1977. The Kranz syndrome and its subtypes in grass systematics. Memoirs of the Torrey Botanical Club 10: 1–97. [Google Scholar]
- Bruhl JJ, Perry S.. 1995. Photosynthetic pathway-related ultrastructure of C3, C4 and C3-like C3-C4 intermediate sedges (Cyperaceae), with special reference to Eleocharis. Functional Plant Biology 22: 521–530. doi: 10.1071/pp9950521. [DOI] [Google Scholar]
- Burgess SJ, Hibberd JM.. 2015. Insights into C4 metabolism from comparative deep sequencing. Current Opinion in Plant Biology 25: 138–144. doi: 10.1016/j.pbi.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Busch FA. 2020. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. The Plant Journal 101: 919–939. doi: 10.1111/tpj.14674. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP, Furbank RT.. 2012. The development of C4 rice: current progress and future challenges. Science 336: 1671–1672. [DOI] [PubMed] [Google Scholar]
- Cardol P, Bailleul B, Rappaport F, et al. 2008. An original adaptation of photosynthesis in the marine green alga Ostreococcus. Proceedings of the National Academy of Sciences of the United States of America 105: 7881–7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF.. 2011. Complex evolutionary transitions and the significance of C3–C4 intermediate forms of photosynthesis in Molluginaceae. Evolution; International Journal of Organic Evolution 65: 643–660. doi: 10.1111/j.1558-5646.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- Christin P-A, Edwards EJ, Besnard G, et al. 2012. Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer. Current Biology 22: 445–449. doi: 10.1016/j.cub.2012.01.054. [DOI] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, et al. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences of the United States of America 110: 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Arakaki M, Osborne CP, et al. 2014. Shared origins of a key enzyme during the evolution of C4 and CAM metabolism. Journal of Experimental Botany 65: 3609–3621. doi: 10.1093/jxb/eru087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Besnard G, Samaritani E, et al. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biology 18: 37–43. [DOI] [PubMed] [Google Scholar]
- Clayton WD, Renvoize SA.. 1982. Flora of Tropical East Africa. Gramineae (Part 3). Rotterdam: AA Balkema. [Google Scholar]
- Curran EV, Scott MS, Olofsson JK, et al. 2022. Hybridization boosts dispersal of two contrasted ecotypes in a grass species. Proceedings of the Royal Society B: Biological Sciences 289: 20212491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning LT, Lundgren MR, Moreno‐Villena JJ, et al. 2017. Introgression and repeated co‐option facilitated the recurrent emergence of C4 photosynthesis among close relatives. Evolution; International Journal of Organic Evolution 71: 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning LT, Moreno-Villena JJ, Lundgren MR, et al. 2019a. Key changes in gene expression identified for different stages of C4 evolution in Alloteropsis semialata. Journal of Experimental Botany 70: 3255–3268. doi: 10.1093/jxb/erz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning LT, Olofsson JK, Parisod C, et al. 2019b. Lateral transfers of large DNA fragments spread functional genes among grasses. Proceedings of the National Academy of Sciences of the United States of America 116: 4416–4425. doi: 10.1073/pnas.1810031116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenge ME, Duarte AG, Way DA.. 2019. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist 221: 32–49. [DOI] [PubMed] [Google Scholar]
- Edwards GE, Furbank RT, Hatch MD, Osmond CB.. 2001. What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiology 125: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB. 1987. Biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman NK, eds. The biochemistry of plants, Vol. 10. New York: Academic Press, 275–325. [Google Scholar]
- Edwards EJ, Osborne CP, Strömberg CA, et al.; C4 Grasses Consortium. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328: 587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Björkman O.. 1977. Quantum yields for CO2 uptake in C3 and C4 plants: dependence on temperature, CO2, and O2 concentration. Plant Physiology 59: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK.. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics 24: 411–439. [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR.. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- Ellis RP. 1974. Anomalous vascular bundle sheath structure in Alloteropsis semialata leaf blades. Bothalia: African Biodiversity and Conservation 11: 273–275. doi: 10.4102/abc.v11i3.1460. [DOI] [Google Scholar]
- Ellis RP. 1981. Relevance of comparative leaf anatomy in taxonomic and functional research on the South African Poaceae. DSc Thesis, University of Pretoria, South Africa. [Google Scholar]
- Emms DM, Kelly S.. 2015. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biology 16: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frean ML, Marks E.. 1988. Chromosome numbers of C3 and C4 variants within the species Alloteropsis semialata (R. Br.) Hitchc. (Poaceae). Botanical Journal of the Linnean Society 97: 255–259. [Google Scholar]
- Frean M, Barrett D, Cresswell CF.. 1980. Variability in leaf surface features and water efficiency utilisation in C3 and C4 forms of Alloteropsis semialata (R. Br.) hitchc. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa 15: 99–103. doi: 10.1080/00725560.1980.9648893. [DOI] [Google Scholar]
- Frean ML, Ariovich D, Cresswell CF.. 1983a. C3 and C4 photosynthetic and anatomical forms of Alloteropsis semialata (R. Br.) Hitchcock: 2. A comparative investigation of leaf ultrastructure and distribution of chlorenchyma in the two forms. Annals of Botany 51: 811–821. doi: 10.1093/oxfordjournals.aob.a086532. [DOI] [Google Scholar]
- Frean ML, Barrett DR, Ariovich D, Wolfson M, Cresswell CF.. 1983b. Intraspecifíc variability in Alloteropsis semialata (R. Br.) Hitchc. Bothalia: African Biodiversity and Conservation 14: 901–913. doi: 10.4102/abc.v14i3/4.1260. [DOI] [Google Scholar]
- Freitag H, Kadereit G.. 2014. C3 and C4 leaf anatomy types in Camphorosmeae (Camphorosmoideae, Chenopodiaceae). Plant Systematics and Evolution 300: 665–687. [Google Scholar]
- Ghannoum O. 2009. C4 photosynthesis and water stress. Annals of Botany 103: 635–644. doi: 10.1093/aob/mcn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW.. 2003. Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytologist 159: 599–608. doi: 10.1046/j.1469-8137.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- Gibbs Russell GE. 1983. The taxonomic position of C3 and C4Alloteropsis semialata (Poaceae) in southern Africa. Bothalia: African Biodiversity & Conservation 14: 205–213. [Google Scholar]
- Gowik U, Westhoff P.. 2011. The path from C-3 to C-4 photosynthesis. Plant Physiology 155: 56–63. doi: 10.1104/pp.110.165308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II. 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist 193: 304–312. [DOI] [PubMed] [Google Scholar]
- Hämälä T, Wafula EK, Guiltinan MJ, Ralph PE, dePamphilis CW, Tiffin P.. 2021. Genomic structural variants constrain and facilitate adaptation in natural populations of Theobroma cacao, the chocolate tree. Proceedings of the National Academy of Sciences of the United States of America 118: e2102914118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta (BBA) - Reviews on Bioenergetics 895: 81–106. [Google Scholar]
- Hattersley PW, Watson L.. 1992. Diversification of photosynthesis. In: Chapman, GP, eds. Grass Evolution and Domestication. Cambridge: Cambridge University Press, 38–116. [Google Scholar]
- Heyduk K, Moreno-Villena JJ, Gilman IS, Christin PA, Edwards EJ.. 2019. The genetics of convergent evolution: insights from plant photosynthesis. Nature Reviews Genetics 20: 485–493. doi: 10.1038/s41576-019-0107-5. [DOI] [PubMed] [Google Scholar]
- Hipp AL, Rothrock PE, Whitkus R, Weber JA.. 2010. Chromosomes tell half of the story: the correlation between karyotype rearrangements and genetic diversity in sedges, a group with holocentric chromosomes. Molecular Ecology 19: 3124–3138. doi: 10.1111/j.1365-294X.2010.04741.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Rieseberg LH.. 2008. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annual Review of Ecology, Evolution, and Systematics 39: 21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Rieseberg LH.. 2020. Frequency, origins, and evolutionary role of chromosomal inversions in plants. Frontiers in Plant Science 11: 296. doi: 10.3389/fpls.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim DG, Gilbert ME, Ripley BS, Osborne CP.. 2008. Seasonal differences in photosynthesis between the C3 and C4 subspecies of Alloteropsis semialata are offset by frost and drought. Plant, Cell & Environment 31: 1038–1050. doi: 10.1111/j.1365-3040.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim DG, Burke T, Ripley BS, Osborne CP.. 2009. A molecular phylogeny of the genus Alloteropsis (Panicoideae, Poaceae) suggests an evolutionary reversion from C4 to C3 photosynthesis. Annals of Botany 103: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit G, Borsch T, Weising K, Freitag H.. 2003. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. International Journal of Plant Sciences 164: 959–986. doi: 10.1086/378649. [DOI] [Google Scholar]
- Kadereit G, Bohley K, Lauterbach M, Tefarikis DT, Kadereit JW.. 2017. C3–C4 intermediates may be of hybrid origin – a reminder. New Phytologist 215: 70–76. doi: 10.1111/nph.14567. [DOI] [PubMed] [Google Scholar]
- Kephart KD, Buxton DR, Taylor SE.. 1992. Growth of C3 and C4 perennial grasses under reduced irradiance. Crop Science 32: 1033–1038. doi: 10.2135/cropsci1992.0011183x003200040040x. [DOI] [Google Scholar]
- Khoshravesh R, Stata M, Busch FA, et al. 2020. The evolutionary origin of C4 photosynthesis in the grass subtribe Neurachninae. Plant Physiology 182: 566–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Roalson EH, Edwards GE.. 2011. Diversity in forms of C4 in the genus Cleome (Cleomaceae). Annals of Botany 107: 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarathunge DP, Medlyn BE, Drake, JE, et al. 2019. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytologist 222: 768–784. [DOI] [PubMed] [Google Scholar]
- Langdale JA. 2011. C4 cycles: past, present, and future research on C4 photosynthesis. The Plant Cell 23: 3879–3892. doi: 10.1105/tpc.111.092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann CE, Griffith DM, Simpson KJ, et al. 2019. Functional diversification enabled grassy biomes to fill global climate space. BioRxiv. doi:10.1101/583625. [Google Scholar]
- Liebenberg EJL, Fossey A.. 2001. Comparative cytogenetic investigation of the two subspecies of the grass Alloteropsis semialata (Poaceae). Botanical Journal of the Linnean Society 137: 243–248. [Google Scholar]
- Long SP. 1999. Environmental responses. In: Sage RF, Monson RK, eds. C4 Plant Biology. San Diego: Academic Press, 215–249. [Google Scholar]
- Lundgren MR, Christin PA.. 2017. Despite phylogenetic effects, C3–C4 lineages bridge the ecological gap to C4 photosynthesis. Journal of Experimental Botany 68: 241–254. doi: 10.1093/jxb/erw451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren MR, Besnard G, Ripley BS, et al. 2015. Photosynthetic innovation broadens the niche within a single species. Ecology Letters 18: 1021–1029. doi: 10.1111/ele.12484. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Christin PA, Escobar EG, et al. 2016. Evolutionary implications of C3–C4 intermediates in the grass Alloteropsis semialata. Plant, Cell & Environment 39: 1874–1885. doi: 10.1111/pce.12665. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Dunning LT, Olofsson JK, et al. 2019. C4 anatomy can evolve via a single developmental change. Ecology Letters 22: 302–312. doi: 10.1111/ele.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu M-JA, Gowik U, Kelly S, et al. 2021. The coordination of major events in C4 photosynthesis evolution in the genus Flaveria. Scientific Reports 11: 15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M.. 2001. Molecular engineering of C4 photosynthesis. Annual Review of Plant Biology 52: 297–314. [DOI] [PubMed] [Google Scholar]
- McKown AD, Moncalvo J-M, Dengler NG.. 2005. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany 92: 1911–1928. [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH.. 1991. Photosynthetic characteristics of C3-C4 intermediate Flaveria floridana (Asteraceae) in natural habitats: evidence of advantages to C3-C4 photosynthesis at high leaf temperatures. American Journal of Botany 78: 795–800. doi: 10.1002/j.1537-2197.1991.tb14481.x. [DOI] [Google Scholar]
- Monson RK, Moore BD, Ku MSB, Edwards GE.. 1986. Co-function of C3- and C4-photosynthetic pathways in C3, C4 and C3–C4 intermediate Flaveria species. Planta 168: 493–502. doi: 10.1007/BF00392268. [DOI] [PubMed] [Google Scholar]
- Morales-Briones DF, Kadereit G. 2022. Exploring the possible role of hybridization in the 1 evolution of photosynthetic pathways in Flaveria (Asteraceae), the prime model of C4 photosynthesis evolution. bioRxiv. doi:10.1101/2022.01.31.478436. [Google Scholar]
- Moreno-Villena JJ, Dunning LT, Osborne CP, Christin PA.. 2018. Highly expressed genes are preferentially co-opted for C4 photosynthesis. Molecular Biology and Evolution 35: 94–106. doi: 10.1093/molbev/msx269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat R, McKown AD.. 2013. Significant involvement of PEP-CK in carbon assimilation of C4 eudicots. Annals of Botany 111: 577–589. doi: 10.1093/aob/mct017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat R, Sage RF, Dengler NG.. 2007. Diversity of Kranz anatomy and biochemistry in C4 eudicots. American Journal of Botany 94: 362–381. doi: 10.3732/ajb.94.3.362. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Bianconi M, Besnard G, et al. 2016. Genome biogeography reveals the intraspecific spread of adaptive mutations for a complex trait. Molecular Ecology 25: 6107–6123. doi: 10.1111/mec.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Dunning LT, Lundgren MR, et al. 2019. Population-specific selection on standing variation generated by lateral gene transfers in a grass. Current Biology 29: 3921–3927.e5. doi: 10.1016/j.cub.2019.09.023. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Curran EV, Nyirenda F, et al. 2021. Low dispersal and ploidy differences in a grass maintain photosynthetic diversity despite gene flow and habitat overlap. Molecular Ecology 30: 2116–2130. doi: 10.1111/mec.15871. [DOI] [PubMed] [Google Scholar]
- Osborne CP, Wythe EJ, Ibrahim DG, Gilbert ME, Ripley BS.. 2008. Low temperature effects on leaf physiology and survivorship in the C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany 59: 1743–1754. doi: 10.1093/jxb/ern062. [DOI] [PubMed] [Google Scholar]
- Osmond, CB, Winter, K, Ziegler, H.. 1982. Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Physiological Plant Ecology II. Encyclopedia of Plant Physiology, Vol. 12 B. Berlin: Springer, 479–547. [Google Scholar]
- Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S.. 2005. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309: 600–603. doi: 10.1126/science.1110063. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Chapman B.. 2004. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proceedings of the National Academy of Sciences of the United States of America 101: 9903–9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, et al. 2009. The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Ehleringer J.. 1984. Comparative ecophysiology of C3 and C4 plants. Plant, Cell & Environment 7: 1–13. [Google Scholar]
- Peterhansel C, Maurino VG.. 2011. Photorespiration redesigned. Plant Physiology 155: 49–55. doi: 10.1104/pp.110.165019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phansopa C, Dunning LT, Reid JD, Christin PA.. 2020. Lateral gene transfer acts as an evolutionary shortcut to efficient C4 biochemistry. Molecular Biology and Evolution 37: 3094–3104. doi: 10.1093/molbev/msaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast HDV, Hattersley PW, Stone NE.. 1987. New structural/biochemical associations in leaf blades of C4 grasses (Poaceae). Functional Plant Biology 14: 403–420. doi: 10.1071/pp9870403. [DOI] [Google Scholar]
- Raimondeau P, Bianconi ME, Pereira L, Parisod C, Christin PA, Dunning LT. 2022. Variation in the rate lateral gene transfers accumulate in a grass lineage. bioRxiv, 2022-10. [DOI] [PubMed] [Google Scholar]
- Raven JA, Geider RJ.. 2003. Adaptation, acclimation and regulation in algal photosynthesis. In: Larkum AWD, Douglas SE, Raven JA, eds. Photosynthesis in Algae. Advances in Photosynthesis and Respiration, Vol. 14. Dordrecht: Springer, 385–412. [Google Scholar]
- Raven JA, Cockell, CS, De La Rocha, CL.. 2008. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvoize SA. 1987. A survey of leaf-blade anatomy in grasses XI. Paniceae. Kew Bulletin 42: 739–768. doi: 10.2307/4110087. [DOI] [Google Scholar]
- Rieseberg LH. 2001. Chromosomal rearrangements and speciation. Trends in Ecology & Evolution 16: 351–358. doi: 10.1016/s0169-5347(01)02187-5. [DOI] [PubMed] [Google Scholar]
- Ripley BS, Gilbert ME, Ibrahim DG, Osborne CP.. 2007. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany 58: 1351–1363. doi: 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- Ripley BS, Abraham TI, Osborne CP.. 2008. Consequences of C4 photosynthesis for the partitioning of growth: a test using C3 and C4 subspecies of Alloteropsis semialata under nitrogen-limitation. Journal of Experimental Botany 59: 1705–1714. doi: 10.1093/jxb/erm210. [DOI] [PubMed] [Google Scholar]
- Ripley B, Frole K, Gilbert M.. 2010a. Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) panicoid grasses. Annals of Botany 105: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley B, Donald G, Osborne CP, Abraham T, Martin T.. 2010b. Experimental investigation of fire ecology in the C3 and C4 subspecies of Alloteropsis semialata. Journal of Ecology 98: 1196–1203. doi: 10.1111/j.1365-2745.2010.01700.x. [DOI] [Google Scholar]
- Ripley BS, Cunniff J, Osborne CP.. 2013. Photosynthetic acclimation and resource use by the C3 and C4 subspecies of Alloteropsis semialata in low CO2 atmospheres. Global Change Biology 19: 900–910. doi: 10.1111/gcb.12091. [DOI] [PubMed] [Google Scholar]
- Rocha AES, Miranda IS.. 2012. Nova ocorrência de Poaceae para a América do Sul: Alloteropsis (Panicoideae/Poaceae). Acta Amazonica 42: 457–460. [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist 161: 341–370. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62: 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW.. 1987. The nitrogen use efficiency of C3 and C4 plants: I. Leaf nitrogen, growth, and biomass partitioning in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 84: 954–958. doi: 10.1104/pp.84.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F.. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63: 19–47. doi: 10.1146/annurev-arplant-042811-105511. [DOI] [PubMed] [Google Scholar]
- Sage RF, Monson RK, Ehleringer JR, Adachi S, Pearcy RW.. 2018. Some like it hot: the physiological ecology of C4 plant evolution. Oecologia 187: 941–966. [DOI] [PubMed] [Google Scholar]
- Sales CR, Wang Y, Evers JB, Kromdijk J.. 2021. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. Journal of Experimental Botany 72: 5942–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter U, Weber AP.. 2016. The road to C4 photosynthesis: evolution of a complex trait via intermediary states. Plant and Cell Physiology 57: 881–889. doi: 10.1093/pcp/pcw009. [DOI] [PubMed] [Google Scholar]
- Simpson CJ, Reeves G, Tripathi A, Singh P, Hibberd JM.. 2022. Using breeding and quantitative genetics to understand the C4 pathway. Journal of Experimental Botany 73: 3072–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillman JB. 2008. Quantum yield variation across the three pathways of photosynthesis: not yet out of the dark. Journal of Experimental Botany 59: 1647–1661. doi: 10.1093/jxb/ern029. [DOI] [PubMed] [Google Scholar]
- Still CJ, Berry JA, Collatz GJ, DeFries RS.. 2003. Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochemical Cycles 17: 6-1–6-14. doi: 10.1029/2001gb001807. [DOI] [Google Scholar]
- Tcherkez GG, Farquhar GD, Andrews TJ.. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences of the United States of America 103: 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefarikis DT, Morales-Briones DF, Yang Y, Edwards G, Kadereit G.. 2022. On the hybrid origin of the C2Salsola divaricata agg. (Amaranthaceae) from C3 and C4 parental lineages. New Phytologist 234: 1876–1890. doi: 10.1111/nph.18098. [DOI] [PubMed] [Google Scholar]
- Ueno O, Sentoku N.. 2006. Comparison of leaf structure and photosynthetic characteristics of C3 and C4Alloteropsis semialata subspecies. Plant, Cell & Environment 29: 257–268. doi: 10.1111/j.1365-3040.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- Vogan PJ, Sage RF.. 2011. Water‐use efficiency and nitrogen‐use efficiency of C3‐C4 intermediate species of Flaveria Juss. (Asteraceae). Plant, Cell & Environment 34: 1415–1430. doi: 10.1111/j.1365-3040.2011.02340.x. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Akhani H, Roalson EH, Edwards GE.. 2013. Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate, and C4 photosynthesis. Journal of Experimental Botany 64: 3583–3604. doi: 10.1093/jxb/ert191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Aubry S, Hibberd JM. 2012. Molecular evolution of genes recruited into C4 photosynthesis. Trends in Plant Science 17: 213–220. [DOI] [PubMed] [Google Scholar]
- Young JN, Rickaby REM, Kapralov MV, Filatov DA.. 2012. Adaptive signals in algal Rubisco reveal a history of ancient atmospheric carbon dioxide. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 483–492. doi: 10.1098/rstb.2011.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Bayer PE, Batley J, Edwards D.. 2021. Current status of structural variation studies in plants. Plant Biotechnology Journal 19: 2153–2163. doi: 10.1111/pbi.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wang Y, Lyu MJA, Zhu XG.. 2022. Two major metabolic factors for an efficient NADP-malic enzyme type C4 photosynthesis. Plant Physiology 189: 84–98. doi: 10.1093/plphys/kiac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga FO, Morrone O, Davidse O, et al. 2003. Alloteropsis. In: Soreng RJ, Pennington SJ, eds. Catalogue of New World Grasses (Poaceae): III. Subfamilies Panicoideae, Aristidoideae, Arundinoideae and Danthonioideae v.46. Washington: Smithsonian Institution, 16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data for AUS1 and RSA5-3 are available from the NCBI Sequence Read Archive under BioProject PRJNA481434 and PRJNA824797. Genome assemblies, annotations, extracted coding sequence and proteins for AUS1 and RSA5-3 generated as part of this review are available from Dryad (doi:10.5061/dryad.c866t1gb1).


