Abstract
Modern neuroscience is progressively elucidating that the classic view positing distinct brain regions responsible for survival, emotion, and cognitive functions is outdated. The hypothalamus demonstrates the interdependence of these roles, as it is traditionally known for fundamental survival functions like energy and electrolyte balance, but is now recognized to also play a crucial role in emotional and cognitive processes. This review focuses on lateral hypothalamic melanin-concentrating hormone (MCH) neurons, producing the neuropeptide MCH—a relatively understudied neuronal population with integrative functions related to homeostatic regulation and motivated behaviors, with widespread inputs and outputs throughout the entire central nervous system. Here, we review early findings and recent literature outlining their role in the regulation of energy balance, sleep, learning, and memory processes.
Graphical Abstract
Graphical Abstract.
Cognition, Emotion, and Survival
“Those who study the hypothalamus have a somewhat larger commission than dealing with its role in maintaining the physical functions of the body. Man possesses a complexity of characteristics and behavior that is of an order quite different from contraction, secretion, digestion, metabolism, reproduction and adaptation, though related to all of these. That relationship is the mystery.”1
The above quote expresses the sense of awe in trying to conceptually reconcile basic physiological functions fundamental for the survival of the organism and its species, and seemingly more complex, “higher-order” cognitive, and social activities in which humans engage, such as math, politics, and the construction of complex technologies and societies.
An influential theory of the past, which has attempted to do so has been the “triune brain” theory, developed by Paul McLean and posing that the brain is composed by three distinct structures, which evolved separately and function independently. The oldest part is the “reptilian brain,” which consists of the ventral-most brain structures such as the basal ganglia and the brainstem, and is responsible for the basic, instinctual, and stereotyped behaviors and functions essential for survival. The second structure is the limbic system, involved in emotional responses and representing an evolutionary progress from reptiles to mammals. The third, most recent structure and prominent in humans is the cortex, involved in more sophisticated functions such as cognition, reasoning, planning, and rational decision-making.2–4
Today, this theory is outdated,3 as we now know that the brain did not evolve in stages, but rather that all brain structures are shared among vertebrates with differences in proportion. Additionally, brain structures do not function independently, and neither are survival, emotion, and cognition separable brain functions. Finally, the brain does not function solely by reacting to stimuli, instead, it continuously predicts and adjusts to the ever-changing internal and external factors, optimizing its responses to the environment both inside and outside the body.3
One anatomically defined region of the brain, the hypothalamus, exemplifies how survival, emotion, and cognition are interdependent in the brain. It has been known for a long time to underlie fundamental homeostatic functions, essential for the survival of the individual and the species, like energy balance, electrolyte balance, endocrine function, and reproduction. Over time, it has become increasingly clearer that it is essential also for emotional and cognitive functions (Figure 1).
Figure 1.
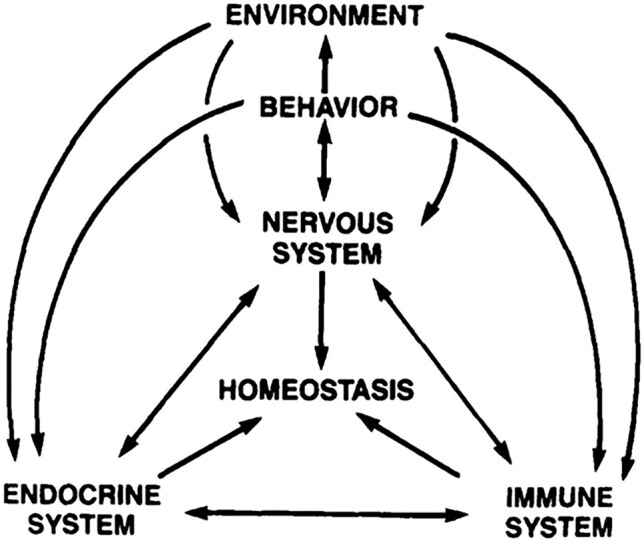
Conceptual representation of the nervous system as the central regulator of internal and external inputs and outputs (reproduced with permission from Brooks1). The hypothalamus is a structure uniquely positioned to fulfil this role.
This review focuses on how a genetically defined neuronal population in the hypothalamus, named melanin-concentrating hormone (MCH) neurons, in addition to energy homeostasis and sleep, controls cognitive and emotional processes.
The Hypothalamus
“This bit of brain, 4 grams in weight, integrates almost all higher physiological functions.”
Fred Plum and Robert Van Uitert, 1978
The hypothalamus is a heterogeneous region located at the base of the brain, which controls a variety of physiological processes essential for survival, such as feeding and energy homeostasis, thirst and osmotic homeostasis, hormone release, body temperature, sleep, locomotion, and basic social behaviors like sexual and reproductive behavior, escape and aggression.
It is an ancient and conserved structure of the forebrain,6,7 made up of several nuclei and neuronal populations, which act as internal sensors (interoceptors) of homeostatic states and orchestrate the essential behaviors aimed at counteracting needs,5 such as foraging and consuming food when in a state of energy deprivation (Figure 2). Neurons in the hypothalamus sense and respond to internal signals (hormones, nutrients, and other molecules) and initiate complex, motivated behaviors when stimulated exogenously, as shown in classical studies.5 More recently, it has been shown that hypothalamic neurons not only respond to internal stimuli on a slow timescale (minutes, hours), but are also responsive to external stimuli on a faster timescale (seconds).8 The latter are environmental cues used to predict future homeostatic needs and to orchestrate complex behaviors flexibly and depending on environmental context—as opposed to the generation of stereotyped behaviors.9,10
Figure 2.
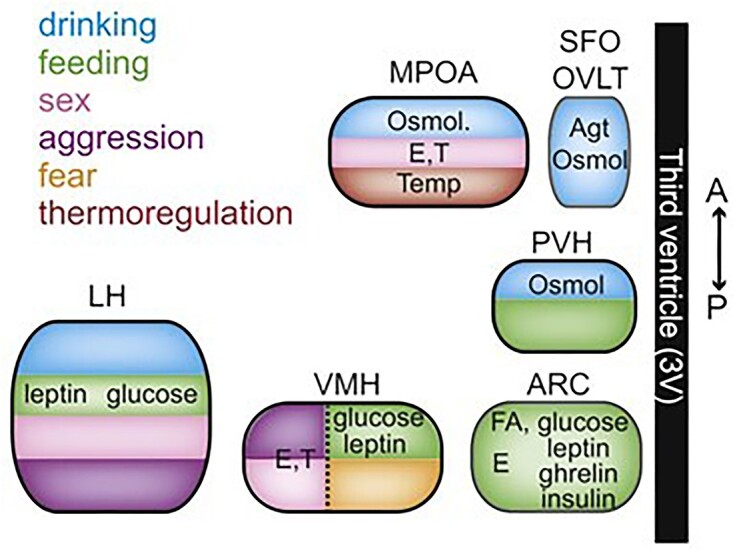
Simplified representation along the antero-posterior and medio-lateral axes of the organization and functions of hypothalamic nuclei, including homeostatic functions and motivated behaviors (reproduced with permission from Sternson5).
The hypothalamus controls the autonomic nervous system by innervating autonomic preganglionic neurons and various nuclei in the brainstem that control autonomic reflexes.10 It controls the endocrine system directly through groups of neurons that connect to the anterior and posterior pituitary gland and indirectly through autonomic innervation of endocrine glands.10
The Lateral Hypothalamic Area (LH or LHA)
Many structural and functional studies support a role of the LH in the control of feeding behavior,11 arousal,12,13 and reinforcement processes—early lesion studies showed that the LH is necessary for feeding behavior, while electrical self-stimulation revealed the LH as one of the brain areas supporting the strongest self-stimulation.14–16 The LH has extensive connections with the brainstem and hypothalamic areas that process homeostatic signals from the body, and with forebrain cognitive and hedonic systems and areas mediating stress, anxiety, arousal, and sleep.17
The LH has two main non-overlapping neuronal populations, unique to the LH and defined by their neuropeptides: orexin/hypocretin (ORX) neurons and MCH neurons. Other, less well-characterized cell types are also present in the LH, such as GAD65 neurons,18 Trh-expressing, and Sst-expressing neurons.19 Additionally, other neurotransmitter markers (glutamate and glutamate transporters, gamma-aminobutyric acid (GABA) transporters),20 neuropeptides [cocaine- and amphetamine-regulated transcript (CART), dynorphin, nesfatin], and receptors (leptin receptor) are expressed and/or co-released by LH neurons to various degrees.19,21
ORX and MCH neurons are thought to have opposing roles on arousal and feeding, as they show reciprocal activity profiles22 and are differently modulated by glucose.23,24 However, they show similarities in that both neuronal types have brain-wide inputs and outputs, not only to regions regulating feeding and arousal, but also locomotion, cognition, and reward.22,25,26
ORX neurons are identified by the expression of the neuropeptide orexin, which has an excitatory action on postsynaptic neurons.27–29 Additionally, they express and release glutamate.20 They are known to promote stable wakefulness,30,31 as their loss results in narcolepsy, a sleep disorder.32–34 They also mediate feeding,35 and control behavior under situations of high motivational relevance.36 More recently, they have also been found to be involved in locomotion37 and cognition.38
GAD65 neurons are inhibited by glucose18 and are active during sleep.39 Locally, they synapse onto neighboring MCH neurons40 and are activated by ORX neurons.41 They likely participate in GABAergic LH projections to more distant brain regions, such as the ventral tegmental area (VTA).42
MCH neurons are the focus of this review and are covered more extensively below.
The MCH System
Early Findings
The MCH peptide was first isolated from the pituitary of teleost fish, where it is secreted in circulation and causes the concentration of melanin granules in the scales, thus determining color change in response to the environment.43 Subsequently, it was found in the hypothalamus44 of rodents and humans45 (and in some peripheral tissues,46 see the section “Functions—Overview”). The mammalian MCH peptide (Figure 3) consists of 19 amino acids, encoded by the Pmch gene, which gives rise to a longer precursor peptide (165 amino acids), prepro-MCH. The Pmch gene is highly conserved between teleost fish and mammals and the MCH peptide is identical in all mammals analyzed so far.47,48 In contrast to fish, MCH in the mammalian brain is found in the LH and zona incerta (or incerto-hypothalamic area).26 In spite of slight differences in localization, in all vertebrates analyzed, MCH has been found exclusively in the hypothalamus or homologous structures.47 Alternative processing of the prepro-MCH precursor generates 2 other peptides, NGE and NEI,49 the function of which is not well known.26
Figure 3.
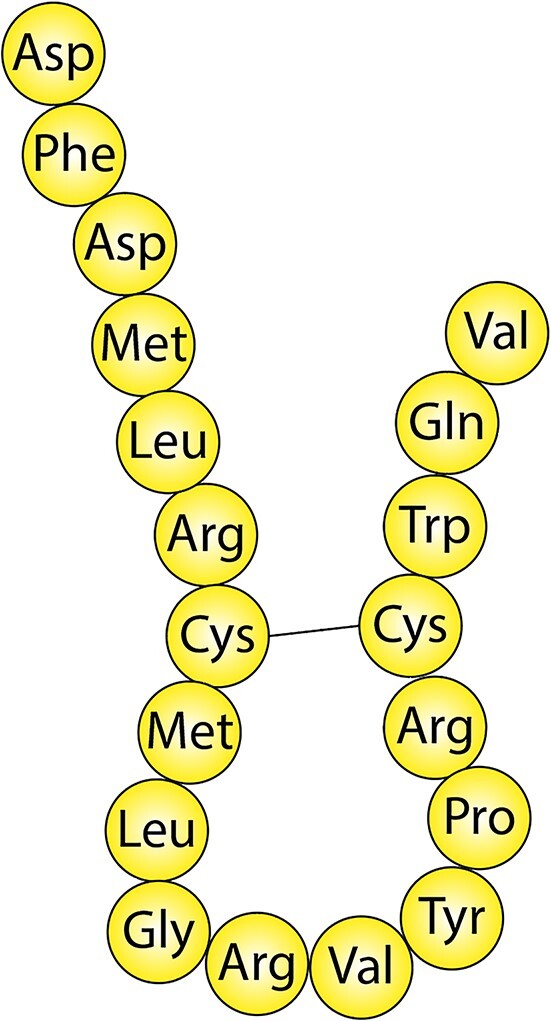
Amino-acid sequence and structure of the mammalian MCH peptide after precursor processing.
Functions—Overview
The MCH system is implicated in a variety of processes within the central nervous system (CNS). This ubiquitous peptide acts as an important neuromodulator for the organism’s homeostatic balance, acting over a large spectrum of integrative functions, especially those related to homeostatic regulation and motivated behaviors. Early evidence implicated the MCH peptide in the regulation of feeding behavior, finding its expression increased after fasting50,51 and intracerebroventricular (ICV) injection of MCH increased food consumption.51 Later, MCH peptide has also been found to be implicated in sleep52 and, more recently, learning and plasticity processes, emotion,53–55 and reproduction.56,57 The MCH system has also been found to have a role in the periphery: in the gut,54,58 in pancreatic islet function,59 in brown adipose tissue (BAT),60,61 and in the immune system,62,63 and to exert neuroendocrine actions64 in addition to non-neuroendocrine roles. MCH neurons project widely throughout the brain65,66 and receive inputs from several brain regions.22
Transmitters and Receptors Involved in the MCH System
The MCH peptide has 2 known G-protein coupled receptors: MCHR1,67–71 found in all vertebrates, and MCHR2, found in primates and carnivores but not in rodents.72,73 The two receptors are highly homologous48 and both are found predominantly expressed in the brain compared to other tissues.73–77 The binding of MCH peptide to MCHR1 can activate Gi/o coupled pathways, which decrease intracellular cAMP levels and are associated with neuronal inhibition, but also Gq coupled pathways, which lead to an increase in intracellular Ca2+ levels and are associated with neuronal activation. Instead, activation of MCHR2 causes exclusively increase in intracellular Ca2+ levels through Gq73,75 pathways. Most rodent studies on the functions of the MCH system only involve MCHR1, due to the lack of MCHR2. A mouse model engineered to express MCHR2 under the promoter of MCHR1 showed protection against diet-induced obesity by reducing feeding,78 an effect similar to MCHR1. However, additional studies are needed to fully elucidate the physiological role of MCHR2. MCH peptide itself has been reported to have mainly inhibitory effects on other hypothalamic neuron types.79
As many other neuron types,20 MCH neurons likely co-release other neurotransmitter molecules in addition to MCH peptide. This is suggested by differences in the phenotypes of animal models lacking the MCH gene (MCH-KO mice) compared to those lacking MCH neurons altogether (MCH-ablated mice), as is the case for glucose tolerance profiles.80 Evidence for the release of both classic neurotransmitters glutamate and GABA from MCH neurons and the presence of their molecular machinery in MCH neurons81,82 has been reported. A study83 found that nearly all MCH neurons express the glutamate transporter VGlut2 and that its knockout leads to a phenotype partially overlapping but different from the knockout of MCH peptide. Another study84 reported that a small subset (about 5%) of MCH terminals projecting to the locus coeruleus (LC) contains the machinery for GABA release and transmission. Finally, it has also been reported that MCH terminals projecting to the lateral septum (LS)—a site of very dense MCH innervation—release glutamate.85
MCH neurons also express the CART peptide and endocannabinoids, but this is not a specific characteristic of MCH neurons, since these molecules are expressed by several neuronal populations in the hypothalamus86 and in the rest of the brain.87,88
Inputs and Outputs of MCH Neurons
MCH neurons send extensive projections to most brain areas, notably the cortex, the olfactory areas, the hippocampal formation, the septal nuclei, the amygdala, the nucleus accumbens, the LC, the raphe and nuclei of the reticular formation, and the spinal cord.89 Intra-hypothalamic MCH projections target the arcuate nucleus, dorsomedial hypothalamus, lateral and posterior hypothalamus and tuberomammillary nuclei (TMN).89 MCH axons also contact the median eminence (ME),90 which is an interface between the CNS and the periphery and a major site of blood-brain barrier permeability. Additionally, MCH neurons have been hypothesized to interact with tanycytes,149 elongated cells in the ME and third ventricle which control the blood-hypothalamus barrier and, therefore, the entry of peripheral signals into the hypothalamus.150–152 MCH peptide has been shown to affect the beating frequency of cilia on ependymocytes91,92,148 and to regulate the permeability of the blood–brain barrier by controlling microvessel fenestration and facilitating leptin action in the arcuate nucleus.93 Additionally, MCH peptide itself is released in the cerebrospinal fluid (CSF), thus potentially reaching non-synaptically connected brain regions, which has been shown to be one of the routes for its regulation of feeding.94 MCH may act through non-synaptic communication also in the brain itself, as the expression of MCHR1 has been found associated to a subcellular structure called the primary cilium, which is involved in the detection of neurochemical messengers in the extracellular space.95 In general, MCHR1 expression in the brain largely mirrors that of MCH-containing axons.26
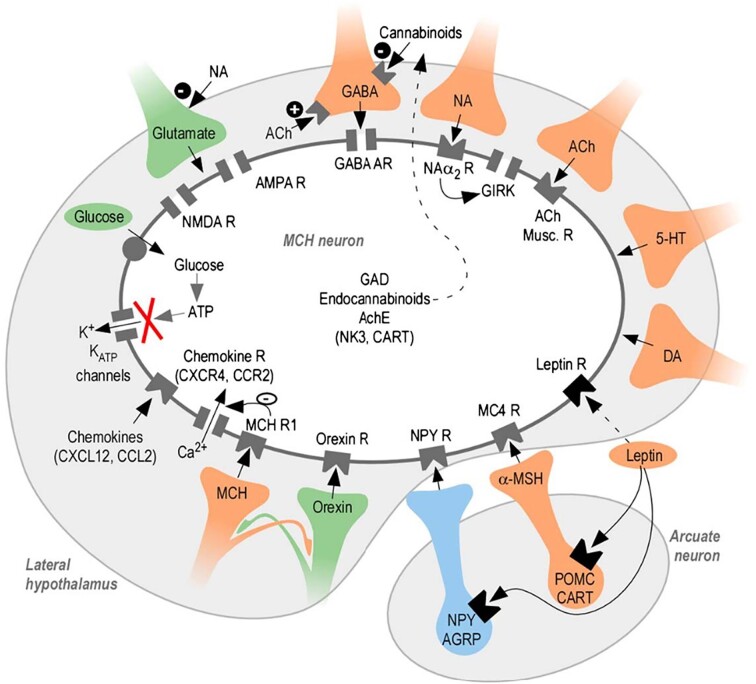
MCH neurons receive and respond to a wide variety of signals (Figure 4). They are depolarized by glutamate and hyperpolarized by GABA.96,97 They express ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) glutamate receptors, group I metabotropic glutamate receptors97,98 and GABA-A receptors.81,97,99,100 MCH neurons express MCHR1 autoreceptors, but no effect of their activation has been found.96,101
Figure 4.
Neurotransmitters and neuropeptides acting on MCH neurons and their receptors (from Guyon et al.112). MCH neurons respond to a wide variety of local and long-range signaling molecules.
In the LH, MCH neurons are intermingled with ORX neurons. Orexin neurons possess the MCHR1 and its activation by MCH peptide has an inhibitory effect.102 MCH neurons, in turn, express orexin receptors whose activation depolarizes them,97 but orexin neurons also inhibit MCH neurons through excitation of local GAD65 neurons.103
MCH neurons receive dense inputs from other hypothalamic nuclei and neuronal populations, such as oxytocin and vasopressin neurons in the paraventricular nucleus, POMC (Pro-opiomelanocortin) neurons in the arcuate nucleus, and the supraoptic nucleus.22 MCH neurons also receive projections from NPY-producing neurons of the arcuate nucleus104 and express NPY receptors,81 but their effect is not clear.96,97 MCH neurons also express the MC4R for α-MSH produced by POMC neurons in the arcuate nucleus, but this receptor may not be functional.97
They also receive inputs from cerebral nuclei (bed nucleus of the stria terminalis, nucleus accumbens, LS, and diagonal band nucleus), from the midbrain (VTA, reticular nucleus, periaqueductal gray, nucleus raphe), and from cortical areas (mostly hippocampus and amygdala nuclei).22,105 In vitro evidence shows that noradrenaline (norepinephrine) hyperpolarizes MCH neurons through α2 adrenergic receptors, and acetylcholine hyperpolarizes MCH neurons through its muscarinic receptors96,97,106 and both transmitters modulate synaptic inputs to MCH neurons.97 Serotonin also hyperpolarizes MCH neurons.97 Dopamine also depresses MCH neuron activity.107 These transmitters are involved in arousal, stress, attention, memory, motivation, and reward. MCH neurons also interact with opioids and cannabinoids in the LH108—where they have indirect excitatory actions on MCH neurons by inhibiting local GABAergic neurons109—and in the nucleus accumbens,110 and with thyrotropin-releasing hormone (TRH).111
MCH neurons also sense signaling molecules from the periphery. They respond to physiological concentrations of glucose by depolarizing24 and are affected—presumably indirectly—by feeding-related hormones such as leptin and insulin (see below).
MCH Neurons and Energy Balance
Energy homeostasis is the process through which energy stored in the body is held constant over time, and for this the amount of energy consumed and energy expended is made to match, by integrating the body’s short-term and long-term energy needs. The hypothalamus is a crucial regulator of this process, in which MCH neurons take part among others.
Several peripheral signals act to regulate food intake and energy expenditure: adiposity-related leptin secreted by the adipose tissue, blood-glucose regulating insulin and glucagon secreted from pancreatic cells, and meal-generated satiety signals secreted from the gut, such as CCK and ghrelin.113 These are transported into the brain through the blood–brain barrier and exert their action especially in the hypothalamus, in several nuclei and neuronal populations.
MCH neurons are considered “second order” feeding neurons as they are under the control of “first order” arcuate nucleus neurons, which receive direct signals from the periphery.108,114 Arcuate POMC neurons, expressing alpha-melanocyte stimulating hormone (α-MSH) and CART, signal satiety and inhibit food intake and energy storage. Neighboring neurons containing neuropeptide Y (NPY) and agouti-gene related peptide (AgRP) signal hunger and promote food intake and weight gain.115 NPY-AgRP are inhibited by leptin and insulin, whereas POMC neurons are stimulated by them. Both populations send projections to MCH neurons, which, in turn, negatively regulate POMC neurons.
It has emerged from various studies that MCH promotes energy conservation and accumulation by increasing food intake and reducing energy expenditure.116–118 Elevating MCH in the brain increases food intake51,119–122: acute effects of ICV MCH injection are washed out after 24 h, while chronic infusion increases food intake and body weight over time.123 Both acute and chronic ICV infusions of MCH in mice and rats lead to hyperphagia,51,119–121,124,125 which persists even in obese animals.121 Indeed, MCH levels are increased in most mouse models of obesity.126–128 Overexpression of MCH in transgenic mouse models increases body weight and leads to obesity.116 Conversely, pharmacological129–132 or genetic inhibition of MCH or blockade of MCHR180,117,133–135 leads to weight loss in both lean and obese mice, independently of food palatability.136 Additionally, MCH neuron ablation reduces age-associated weight gain.133,134
MCH neurons respond to and interact with several peripheral feeding-related signals. They are stimulated by glucose24 and regulate peripheral glucose homeostasis.137 Central infusion of MCH induces glucose intolerance.138,139 Glutamate signaling in MCH neurons is necessary for the MCH-neuron-mediated effects on glucose tolerance83 and mice with glucose-insensitive MCH neurons have altered glucose tolerance.137 Insulin, which stimulates glucose uptake and metabolism and decreases food intake and body weight when administered centrally, also modulates MCH neurons,140 as they increase the level of MCH peptide expression after insulin administration, and deletion of the insulin receptor from these neurons improves insulin sensitivity in obese mice.140 Chronic central administration of MCH promotes insulin resistance via a mechanism that is independent of weight gain.134,139 The MCH system interacts with leptin, with several studies showing evidence of a negative interaction between the two.108,141–143 Additionally, estrogen has been reported to regulate the orexigenic effect of MCH neurons, making it sexually dimorphic.144–147
MCH neurons interact with other neuronal populations involved in the control of energy balance, both in the hypothalamus and in the rest of the brain. Since LH ORX neurons have an anorexigenic action153–155 and engage in reciprocal inhibitory circuits with MCH neurons,103 these two nutrient-sensing populations are likely to generate an intra-LH circuit for antagonistic regulation of feeding and energy balance. Outside the hypothalamus, MCH neurons project, among others, to reward areas such as the VTA and nucleus accumbens (Nac).65,66,89 MCH injection and antagonism in the Nac respectively increase and decrease food intake.156,157 MCH activation is able to increase preference for palatable foods158 and rats fed a high-fat diet continue to show increased levels of MCH even after switching to a standard diet.159 Moreover, activation of MCH neurons alone is rewarding and reinforces ongoing feeding.160 These results have led to the hypothesis that MCH neurons are involved in the motivational components of feeding and food-seeking behaviors. This function likely depends on an interaction with dopamine in the Nac, as MCH-KO mice show increased dopamine release and dopamine transporters expression157,161 and GABAergic medium spiny neurons (MSNs)—the primary projection neurons of the Nac—express the MCH-R1.162
MCH neurons are involved in the regulation of energy expenditure beyond feeding alone, regulating locomotion and thermogenesis. ICV injection of MCH in mice reduces energy expenditure163 and genetic deletion of components of the MCH system results in a lean phenotype with an increased metabolic rate and increased oxygen consumption, independently of their diet.117,135,147,164 Additionally, deletion of the Pmch gene in obese leptin-deficient mice results in a reduction of body weight due to increased energy expenditure and locomotor activity.165 MCH neurons are polysynaptically connected to the BAT.60,166 In mice, ICV injection of MCH reduces thermogenesis of the BAT, reducing core body temperature125 and mice with MCH-R1 knockout have a higher core body temperature.167 MCH neurons thus promote energy saving by reducing locomotion and BAT thermogenesis. Additionally, activation of MCH signaling in the arcuate nucleus favors fat storage in the white adipose tissue and increases body weight independent of feeding.124 MCH also induces adiposity by reducing sympathetic neural activity and regulating liver metabolism.124,138
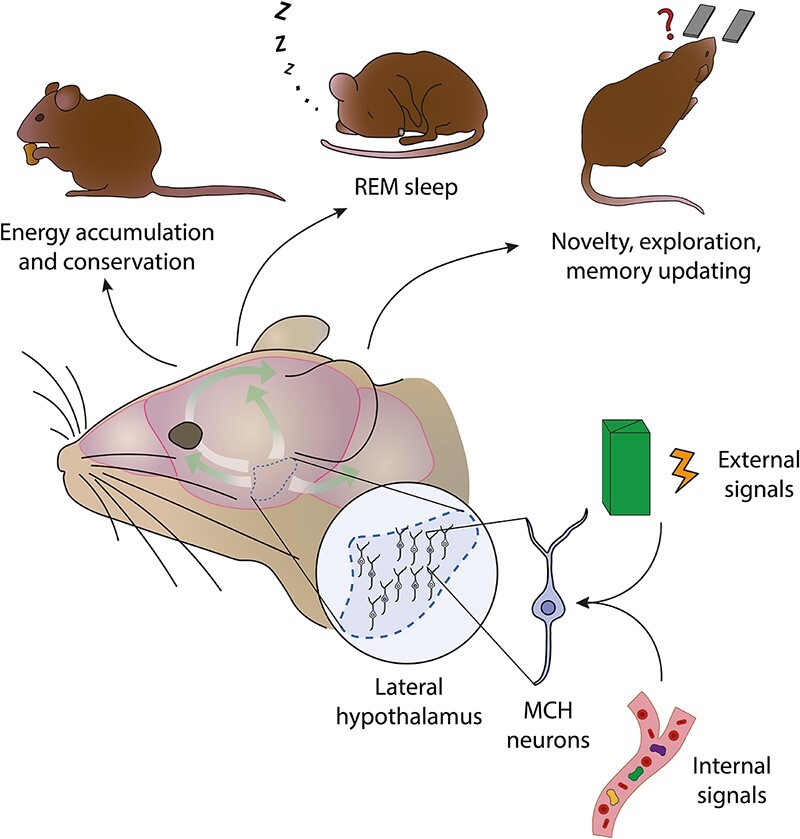
Together, these studies show that MCH neurons are involved in the control of energy homeostasis on multiple levels, by promoting feeding and, possibly, the rewarding properties of food, by promoting energy storage in the adipose tissue and by reducing energy expenditure. The latter function may be related to their role in sleep and sleep transitions.
MCH Neurons and Sleep
In addition to the regulation of energy balance, MCH neurons are involved in another homeostatic process: sleep. For some time, they have been thought to only be active during sleep,168 although now it is clear that they are also active during wakefulness.22,40,61
Sleep is a highly conserved physiological state across animals, and it serves to provide rest, memory processing, and recovery of all bodily systems. It is characterized by a decreased muscle tone and an increased threshold for responsiveness to stimuli. It has 2 main phases, non-rapid eye movements (NREM) and rapid eye movements (REM ). In NREM sleep, brain activity as measured by electroencephalogram (EEG) is highly synchronized, with high amplitude and low frequency. In REM sleep, EEG activity appears similar to activity during wakefulness, with low amplitude, high frequency, and general de-synchronization. Several systems and neuronal types in the brain have been found to regulate wake and sleep. The wake-promoting system includes several areas in the ascending reticular activating system in the pons and midbrain—among which noradrenergic neurons in the LC and dopaminergic neurons of the VTA and substantia nigra pars compacta (SNc)—and another group of neurons in the forebrain—among which histaminergic neurons in the TMN ORX neurons in the LH. The sleep-promoting system includes GABAergic neurons in the thalamic reticular nucleus, in the hypothalamic preoptic area (POA) and in the cortex. Sleep onset is thought to be due to the accumulation of various metabolites produced during wakefulness, which inactivate wake-promoting neuronal populations and activate sleep-promoting neuronal populations.169
MCH neurons appear to be sleep-promoting, as ICV injections of MCH peptide induce hypersomnia, with an increase in REM and slow-wave sleep (a stage of NREM sleep).170,171 Mouse models with deletions of components of the MCH system show increased locomotor activity and wakefulness, especially during the dark (active) phase.80,83,117,133,135,140,162,164,172,173 Additionally, MCH neurons show activation during REM sleep174,175 and after sleep rebound following sleep deprivation.170,176,177 Accordingly, in rodents MCH levels increase in the CSF during the light (sleep) phase and decrease during the dark (active) phase.178 MCH axons and the MCH-R1 are present in brain regions implicated in the control of sleep.65,71,89 For example, MCH neurons receive direct afferents from the hypothalamic suprachiasmatic nucleus (SCN), the master circadian pacemaker,178,179 and SCN neurons express MCH-R1.178,180 Activation of the MCH system in several target sites also increases the number and duration of REM sleep episodes.181–183 Optogenetic activation of MCH neurons during NREM sleep facilitates the onset of REM sleep, while activation during REM sleep extends the duration of REM sleep episodes.181,184 Interestingly, a recent study has found that MCH neurons regulate REM sleep in response to ambient temperature.185 The sleep-related functions of MCH neurons are fine-tuned by local astrocytes,186 which modulate presynaptic glutamatergic transmission onto MCH neurons in response to sleep deprivation.187
Together, these findings demonstrate an interaction between MCH neurons and sleep systems, with a role for MCH neurons especially in REM sleep. This sleep phase is important for memory consolidation,188,189 therefore it might be one of the ways through which MCH modulates learning and memory processes.
MCH Neurons and Learning
Living in a changing and partially unknown environment requires behavioral adaptation. Upon specific events, new behaviors must be learned, consolidated, stored, and then recalled in response to specific stimuli. Therefore, learning and memory processes are crucial for the survival of organisms. Learning is the process of acquiring new knowledge about the world, and memory is the process of retaining and reshaping that knowledge over time.190
Behavioral adaptation requires the integration of internal and external signals, therefore learning in the brain needs to be adapted to changing external variables, such as rewards,191 punishments, context, and internal variables such as nutritional state,192 attention, and motivation. Likewise, memory storage is not a linear and fixed sequence of events but is the dynamic outcome of several interacting processes—from initial acquisition, to consolidation, retrieval, and integration with other memories—and it is modulated by those variables. The biological basis for learning and memory storage in the brain is synaptic plasticity, the ability of neurons to change the strength of their synapses with use.190 Thus, memory storage does not need to rely on specialized “storage neurons,” but rather the capacity for storing memory is built into the architecture of neuronal circuits and is made possible by a plethora of cellular mechanisms that allow synapses to strengthen and weaken in the short and long term.190
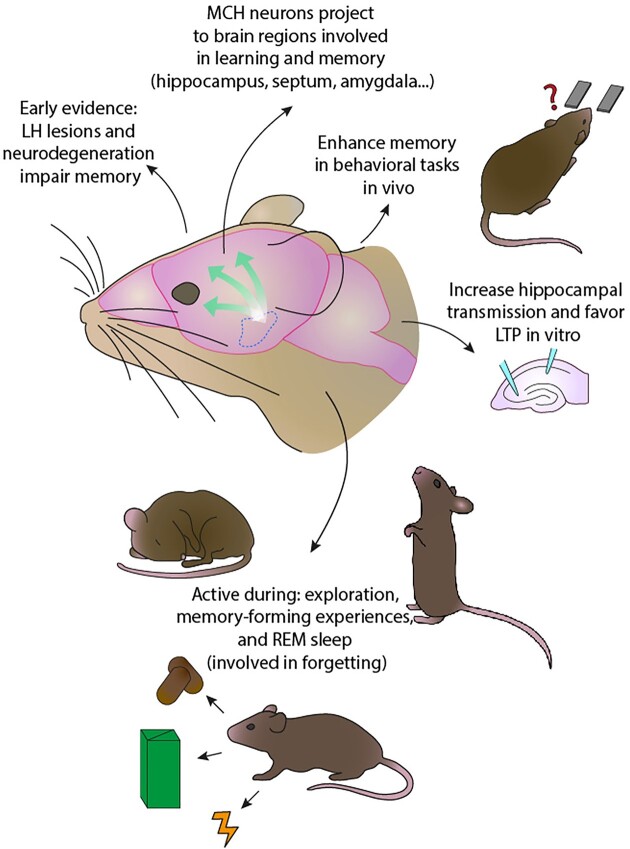
Accumulating evidence points to a role of MCH neurons in the modulation of learning and memory (Figure 5), alongside other hypothalamic populations, such as orexin and AgRP neurons.38,193–195 While the evolutionary reasons for the involvement of a brain region devoted to homeostasis and survival, as the hypothalamus, in learning and memory processes may not appear obvious at first, recent evidence linking metabolism and memory may help. In the Drosophila fruit fly, it has been found that under conditions of starvation, the formation of long-term memory is suppressed and the activity of neurons normally underlying it is absent.192 Exogenously stimulating these neurons restores long-term memory but reduces survival, providing evidence that the brain coordinates memory processes, which have a high energy cost, with metabolic needs to favor survival.192 Additionally, fruit flies are able to form “metabolic memories,” which help balance food choice with caloric intake and the hypothalamus-like pars intercerebralis of the Drosophila brain is necessary for this kind of learning.196 High-fat diet, which impairs this process in the fruit fly, also alters the expression of memory-related genes in the hypothalamus of mice, suggesting that this structure may be involved in metabolism-related learning also in mammals and that this learning may be disrupted in obesity.196
Figure 5.
Summary of evidence supporting a role of MCH neurons in cognitive function, as suggested by early and recent studies.
Early evidence for the involvement of the hypothalamus in learning and memory dates back to the 60s and 70s. Rats with lesions to the LH were unable to form a new associative memory, but retained an association formed before the lesion.197 Electrical stimulation of the LH after a learning task was found to facilitate long-term memory,198 while LH lesion impaired the formation of flavor-to-nutrient (appetitive) and flavor-to-toxin (aversive) post-ingestive associations.199 On the clinical side, “Alzheimer’s neurofibrillary changes” were found in the hypothalamus and brainstem of “senile dementia,” ie, Alzheimer’s disease (AD), patients, in addition to the hippocampus.200 Other, non-neurodegenerative, lesions in the hypothalamus are also associated with memory impairment201 (for more information on MCH neurons and neurodegenerative disorders, see below).
MCH neurons project to brain regions involved in learning and memory, such as the hippocampal formation, subiculum, cerebral cortex, basolateral amygdala, and shell of the nucleus accumbens (Nac).65,66,89,202 They were shown to be required for learning to select nutrient-containing foods158 and responding to food cues203 but also for non-food-related memory formation, such as object recognition,40 and activation of MCH neurons seems to enhance learning and memory processes.204–208 MCH peptide was found to improve memory performance when infused in the hippocampus and amygdala in rats209 and to revert the amnesic effects of a nitric-oxide-synthase inhibitor, a known disruptor of hippocampal plasticity.206 In vitro, MCH peptide applied to brain slices induces a dose-dependent and long-lasting increase in hippocampal synaptic transmission.207 In hippocampal slices from rats injected with MCH and trained in a memory task, the frequency threshold for the induction of long-term potentiation (LTP) was found to be reduced and expression of NMDA-receptor subunits important for plasticity was increased.208,210 Additionally, MCH peptide was found to decrease hippocampal LTP thresholds by increasing synaptic transmission.208,211 On the other hand, mice with a knock-out of the MCHR1 show impairments in various behavioral learning tasks,204,212 reduced expression of both AMPA213 and NMDA204 glutamate receptors in the hippocampus and impaired LTP and long-term depression.212,213
MCH neurons project to the hippocampus, specifically to GABAergic basket cells, and they also innervate cholinergic neurons of the medial septal nucleus, which, in turn, project to the hippocampus.214 Thus, MCH neurons communicate with the hippocampus both directly and indirectly, and these circuits have been hypothesized to participate in the organization of exploratory behavior during foraging, as the hippocampus is well known to be crucial for spatial exploration and memory. Additionally, they have been found to increase the signal-to-noise ratio of the dorsolateral septum (dLS),215 an output structure of the hippocampus. This would allow hippocampal cognitive maps to be transformed into behavioral actions, and, indeed, enhancing MCH signaling in the dLS facilitates hippocampal-dependent memory formation.215
MCH neurons themselves may also play a direct role in exploratory behavior, as they have been found to be active especially when animals explore novel objects.22,175 MCH activity during encounters with objects is higher if the object is novel and decreases over time as the object becomes familiar.40 Optogenetic silencing of object associated MCH activity during initial exploration prevents the recognition of the object on subsequent exposure, showing the necessity of MCH activity for the acquisition of memory about the object.40 MCH activity is under inhibitory control of local hypothalamic GAD65 neurons, whose silencing instead improves future object recognition.40 These results suggest a role of MCH signaling as a “novelty signal” favoring memory acquisition.
MCH neurons have also been found to be endogenously active during learning-driving aversive experience and this activation is necessary for a correct extinction of fear as, in its absence, mice display overactive, relapsing fear behavior.216 A hallmark of pathological fear in human PTSD is the presence of fear responses in safe situations erroneously perceived as dangerous, due to inflexible coupling of cues which are no longer predictive of danger to fearful behavioral responses.217–219 Exposure therapy is often not enough to provide a full recovery from these fear responses220–222 and these results closely mirror this phenomenon with silencing of MCH neurons during the initial aversive experience. Therefore, this can be used as an animal model for dysfunctional safety learning without disruption of the initial and useful fear learning. In this study,216 as in the study investigating MCH function during novel object exploration,40 the activity of MCH neurons during the early stages of a sensory experience determines whether the memory of that experience is correctly expressed behaviorally later on. Together, they point to an important role of MCH neurons in memory updating.
More recently, MCH activity has been found to co-occur with and to specifically drive events of self-paced exploratory rearing in mice,223 an innate behavior during which animals stand on their hind legs to sample the environment.224 This finding opens the possibility that MCH neurons participate in the active seeking of novel information, in addition to processing it for storage in memory and future retrieval. Furthermore, LC noradrenergic (LC-NA) neurons—classically involved in stress responses225–227—have been found to inhibit MCH neurons and thus impair exploratory rearing,223 thus providing a mechanistic substrate for the reduction of exploration under stressful conditions.228
Whether wake-active and REM-sleep-active MCH neurons constitute two functionally separate subpopulations is still not fully elucidated, as one study reported a 70% overlap,175 while another reported that only a minority of recorded MCH neurons were active during both wakefulness and REM sleep229 (these differences could be due, for example, to recording MCH neurons in slightly different anatomical locations). Although most studies on MCH neuron function in learning and memory processes suggest that they facilitate memory formation (including hippocampus-dependent forms of memory), a recent study has reported that inhibition of MCH neurons active during REM-sleep facilitates the retention of hippocampus-dependent memory,229 therefore proposing that REM-active MCH neurons favor hippocampal forgetting (or disrupt hippocampal consolidation188) during sleep.229 An explanation for these apparently conflicting results could be that MCH neurons are indeed functionally separated into wake-active and sleep-active, and the former facilitate memory formation while the latter facilitate instead memory erasure. Alternatively, it is possible that MCH neurons themselves do not drive plasticity processes towards a specific direction, but rather function as generic “eligibility trace” for plasticity, facilitating either potentiation or depotentiation depending on other incoming inputs, promoting memory updating in general.230 In this case, the exact timing and context of MCH manipulations (activation/inactivation) during behavioral experiments would be crucial in determining the outcome on learning and memory processes.
MCH System and Cognitive Decline: Alzheimer’s and Parkinson’s Diseases
AD patients show neurofibrillary degeneration in the LH231 and MCH neurons show aggregates in AD, which, together with loss of ORX neurons, may underlie the sleep disturbances associated with this pathology.232 In scopolamine-induced memory impaired mice and in AD mouse models, nasal cavity administration of MCH peptide improved memory impairments and reduced amyloid beta in AD mice.233
Some Current Questions
Although causal evidence is accumulating that MCH neurons control cognition, many key questions remain, some of which we highlight here.
First, which actions of MCH neurons are mediated by the MCH neuropeptide vs GABA/glutamate that they also co-release? In some cases, this has been probed by antagonists, and the data suggest that key actions of MCH neurons indeed rely on MCH peptide. This could be probed further by targeted, and preferably conditional/inducible, knockout of MCH receptors in specific neurons.
Second, are “sleep” and “wake” MCH neurons the same or different subsets of neurons? This is a point of current debate: Some studies indicated that a major subset of MCH cells active during sleep are also active during exploration,175 but others find that sleep and wake MCH cells are separate.229
Third, is cognitive modulation by MCH neurons related to (or even explained by) their role in arousal/sleep? In other words, is sleep-promotion the primary role of MCH cells, and do their other impacts arise secondarily to that? If this is the case, then awake activity dynamics of MCH cells should be inversely correlated with arousal dynamics. This remains to be directly tested.
Finally, are the “classic” functions of MCH in feeding and energy balance separate from their “new” functions in cognitive control? This is not necessarily so, since much of cognitive control presumably evolved to facilitate survival, and energy optimization (eating, metabolism) is a critical element of survival. Therefore, it is possible that the energy and cognition roles of MCH neurons are tied together to facilitate integrated control of cognition and energy balance. There are some recent indications about how some elements of this might work,94,160,203,234,235 but an integrated model accounting for all findings remains to be produced.
Contributor Information
Cristina Concetti, Neurobehavioural Dynamics Laboratory, ETH Zürich, Schorenstrasse 16, Schwerzenbach 8603, Switzerland.
Daria Peleg-Raibstein, Neurobehavioural Dynamics Laboratory, ETH Zürich, Schorenstrasse 16, Schwerzenbach 8603, Switzerland.
Denis Burdakov, Neurobehavioural Dynamics Laboratory, ETH Zürich, Schorenstrasse 16, Schwerzenbach 8603, Switzerland.
Conflict of Interest
The authors have no conflict of interest to declare.
Data Availability
All relevant data are already included in this review article.
References
- 1. Brooks CM. The history of thought concerning the hypothalamus and its functions. Brain Res Bull. 1988;20(6):657–667. [DOI] [PubMed] [Google Scholar]
- 2. Roxo MR, Franceschini PR, Zubaran C, Kleber FD, Sander JW.. The limbic system conception and its historical evolution. Sci World J. 2011;11:2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steffen PR, Hedges D, Matheson R.. The brain is adaptive not triune: how the brain responds to threat, challenge, and change. Front Psychiatry. 2022;13:802606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacLean PD. The triune brain in evolution: role in paleocerebral functions. https://books.google.ch/books?id=4PmLFmNdHL0C&;printsec=frontcover&redir_esc=y#v=onepage&q&f=false. 1990. [DOI] [PubMed]
- 5. Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77(5):810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tessmar-Raible K, Raible F, Christodoulou Fet al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129(7):1389–1400. [DOI] [PubMed] [Google Scholar]
- 7. Venkatesh B, Si-Hoe SL, Murphy D, Brenner S.. Transgenic rats reveal functional conservation of regulatory controls between the Fugu isotocin and rat oxytocin genes. Proc Natl Acad Sci USA. 1997;94(23):12462–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bracey EF, Burdakov D.. Fast sensory representations in the lateral hypothalamus and their roles in brain function. Physiol Behav. 2020;222:112952. [DOI] [PubMed] [Google Scholar]
- 9. Burdakov D, Peleg-Raibstein D.. Hypothalamic heuristics for survival. Trends Endocrinol Metabol. 2019;30(10):689–691. [DOI] [PubMed] [Google Scholar]
- 10. Saper CB, Lowell BB.. The hypothalamus. Curr Biol. 2014;24(23):R1111–R1116. [DOI] [PubMed] [Google Scholar]
- 11. Burdakov D, Karnani MM, Gonzalez A.. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav. 2013;121:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saper CB, Scammell TE, Lu J.. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. [DOI] [PubMed] [Google Scholar]
- 13. Herrera CG, Ponomarenko A, Korotkova T, Burdakov D, Adamantidis A.. Sleep and metabolism: the multitasking ability of lateral hypothalamic inhibitory circuitries. Front Neuroendocrin. 2017;44:27–34. [DOI] [PubMed] [Google Scholar]
- 14. Stuber GD, Wise RA.. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elmquist JK, Elias CF, Saper CB.. From lesions to leptin hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. [DOI] [PubMed] [Google Scholar]
- 16. Huguet G, Aldavert-Vera L, Kádár E, Peña De Ortiz S, Morgado-Bernal I, Segura-Torres P.. Intracranial self-stimulation to the lateral hypothalamus, a memory improving treatment, results in hippocampal changes in gene expression. Neuroscience. 2009;162(2):359–374. [DOI] [PubMed] [Google Scholar]
- 17. Reppucci CJ, Petrovich GD.. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct. 2016;221(6):2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karnani MM, Szabó G, Erdélyi F, Burdakov D.. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J Physiology. 2013;591(4):933–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mickelsen LE, Bolisetty M, Chimileski BRet al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat Neurosci. 2019;22(4):642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schöne C, Burdakov D.. Glutamate and GABA as rapid effectors of hypothalamic “peptidergic” neurons. Front Behav Neurosci. 2012;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonnavion P, Mickelsen LE, Fujita A, De Lecea L, Jackson AC.. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J Physiol. 2016;594(22):6443–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. González JA, Iordanidou P, Strom M, Adamantidis A, Burdakov D.. Awake dynamics and brain-wide direct inputs of hypothalamic MCH and orexin networks. Nat Commun. 2016;7(1):11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kosse C, Gonzalez A, Burdakov D.. Predictive models of glucose control: roles for glucose-sensing neurones. Acta Physiologica. 2015;213(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burdakov D, Gerasimenko O, Verkhratsky A.. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25(9):2429–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peyron C, Tighe DK, Van Den Pol ANet al. Neurons containing hypocretin (Orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bittencourt JC. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endocrinol. 2011;172(2):185–197. [DOI] [PubMed] [Google Scholar]
- 27. Eriksson KS, Sergeeva O, Brown RE, Haas HL.. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21(23):9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burdakov D, Liss B, Ashcroft FM.. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium—calcium exchanger. J Neurosci. 2003;23(12):4951–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kukkonen JP, Leonard CS.. Orexin receptor signalling cascades. British J Pharmacol. 2014;171(2):314–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inutsuka A, Yamanaka A. The regulation of sleep and wakefulness by the hypothalamic neuropeptide orexin/hypocretin. Nagoya J Med Sci. 2013;75:29–36. PMID: 23544265 [PMC free article] [PubMed] [Google Scholar]
- 31. Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24(1):429–458. [DOI] [PubMed] [Google Scholar]
- 32. Chemelli RM, Willie JT, Sinton CMet al. Narcolepsy in orexin knockout mice molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. [DOI] [PubMed] [Google Scholar]
- 33. Lin L, Faraco J, Li Ret al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (Orexin) receptor 2 gene. Cell. 1999;98(3):365–376. [DOI] [PubMed] [Google Scholar]
- 34. Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E.. Hypocretin (orexin) deficiency in human narcolepsy. Lancet North Am Ed. 2000;355(9197):39–40. [DOI] [PubMed] [Google Scholar]
- 35. Sakurai T, Amemiya A, Ishii Met al. Orexins and orexin receptors: a Family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 36. Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G.. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karnani MM, Schöne C, Bracey EFet al. Role of spontaneous and sensory orexin network dynamics in rapid locomotion initiation. Prog Neurobiol. 2020;187:101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aitta-Aho T, Pappa E, Burdakov D, Apergis-Schoute J.. Cellular activation of hypothalamic hypocretin/orexin neurons facilitates short-term spatial memory in mice. Neurobiol Learn Mem. 2016;136:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hassani OK, Henny P, Lee MG, Jones BE.. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32(3):448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kosse C, Burdakov D.. Natural hypothalamic circuit dynamics underlying object memorization. Nat Commun. 2019;10(1):2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kosse C, Schöne C, Bracey E, Burdakov D.. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc Natl Acad Sci USA. 2017;114(17):4525–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharpe MJ, Marchant NJ, Whitaker LRet al. Lateral hypothalamic GABAergic neurons encode reward predictions that are relayed to the ventral tegmental area to regulate learning. Curr Biol. 2017;27(14):2089–2100.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI.. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305(5932):321–323. [DOI] [PubMed] [Google Scholar]
- 44. Vaughan JM, Fischer WH, Hoeger C, Rivier J, Vale W. Characterization of melanin-concentrating hormone from rat hypothalamus*. Endocrinology. 1989;125(3):1660–1665. [DOI] [PubMed] [Google Scholar]
- 45. Mouri T, Takahashi K, Kawauchi Het al. Melanin-concentrating hormone in the human brain. Peptides. 1993;14(3):643–646. [DOI] [PubMed] [Google Scholar]
- 46. Hervieu G, Nahon J-L.. Pro-melanin concentrating hormone messenger ribonucleic acid and peptides expression in peripheral tissues of the rat. Neuroendocrinology. 1995;61(4):348–364. [DOI] [PubMed] [Google Scholar]
- 47. Diniz GB, Bittencourt JC.. The melanin-concentrating hormone (MCH) system: a tale of two peptides. Front Neurosci. 2019;13:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pissios P, Maratos-Flier E.. Melanin-concentrating hormone: from fish skin to skinny mammals. Trends Endocrinol Metabol. 2003;14(5):243–248. [DOI] [PubMed] [Google Scholar]
- 49. Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125(4):2056–2065. [DOI] [PubMed] [Google Scholar]
- 50. Presse F, Sorokovsky I, Max J-P, Nicolaidis S, Nahon J-L.. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71(3):735–745. [DOI] [PubMed] [Google Scholar]
- 51. Qu D, Ludwig DS, Gammeltoft Set al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. [DOI] [PubMed] [Google Scholar]
- 52. Ferreira JGP, Bittencourt JC, Adamantidis A.. Melanin-concentrating hormone and sleep. Curr Opin Neurobiol. 2017;44:152–158. [DOI] [PubMed] [Google Scholar]
- 53. Rana T, Behl T, Sehgal Aet al. Exploring the role of neuropeptides in depression and anxiety. Prog Neuro-Psychopharmacol Biol Psychiatry. 2021;114:110478. [DOI] [PubMed] [Google Scholar]
- 54. He X, Li Y, Zhang Net al. Melanin-concentrating hormone promotes anxiety and intestinal dysfunction via basolateral amygdala in mice. Front Pharmacol. 2022;13:906057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Antal-Zimanyi I, Khawaja X.. The role of melanin-concentrating hormone in energy homeostasis and mood disorders. J Mol Neurosci. 2009;39(1-2):86–98. [DOI] [PubMed] [Google Scholar]
- 56. Naufahu J, Cunliffe AD, Murray JF.. The roles of melanin-concentrating hormone in energy balance and reproductive function: are they connected?. Reproduction. 2013;146(5):R141–R150. [DOI] [PubMed] [Google Scholar]
- 57. Williamson-Hughes PS, Grove KL, Smith MS.. Melanin concentrating hormone (MCH): a novel neural pathway for regulation of GnRH neurons. Brain Res. 2005;1041(2):117–124. [DOI] [PubMed] [Google Scholar]
- 58. Kokkotou E, Moss AC, Torres Det al. Melanin-concentrating hormone as a mediator of intestinal inflammation. Proc Natl Acad Sci USA. 2008;105(30):10613–10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pissios P, Ozcan U, Kokkotou Eet al. Melanin concentrating hormone is a novel regulator of islet function and growth. Diabetes. 2007;56(2):311–319. [DOI] [PubMed] [Google Scholar]
- 60. Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, Mckinley MJ.. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110(3):515–526. [DOI] [PubMed] [Google Scholar]
- 61. Izawa S, Yoneshiro T, Kondoh Ket al. Melanin-concentrating hormone-producing neurons in the hypothalamus regulate brown adipose tissue and thus contribute to energy expenditure. J Physiol. 2021;600(4):815–827. [DOI] [PubMed] [Google Scholar]
- 62. Verlaet M, Adamantidis A, Coumans Bet al. Human immune cells express ppMCH mRNA and functional MCHR1 receptor. FEBS Lett. 2002;527(1-3):205–210. [DOI] [PubMed] [Google Scholar]
- 63. Lakaye B, Coumans B, Harray S, Grisar T.. Melanin-concentrating hormone and immune function. Peptides. 2009;30(11):2076–2080. [DOI] [PubMed] [Google Scholar]
- 64. Kennedy AR, Todd JF, Stanley SAet al. Melanin-concentrating hormone (MCH) suppresses thyroid stimulating hormone (TSH) release, in Vivo and in Vitro, via the hypothalamus and the pituitary. Endocrinology. 2001;142(7):3265–3268. [DOI] [PubMed] [Google Scholar]
- 65. Hervieu GJ, Cluderay JE, Harrison Det al. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12(4):1194–1216. [DOI] [PubMed] [Google Scholar]
- 66. Saito Y, Cheng M, Leslie FM, Civelli O.. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435(1):26–40. [DOI] [PubMed] [Google Scholar]
- 67. Bächner D, Kreienkamp H-J, Weise C, Buck F, Richter D.. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1). FEBS Lett. 1999;457(3):522–524. [DOI] [PubMed] [Google Scholar]
- 68. Chambers J, Ames RS, Bergsma Det al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400(6741):261–265. [DOI] [PubMed] [Google Scholar]
- 69. Lembo PMC, Grazzini E, Cao Jet al. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1(5):267–271. [DOI] [PubMed] [Google Scholar]
- 70. Shimomura Y, Mori M, Sugo Tet al. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261(3):622–626. [DOI] [PubMed] [Google Scholar]
- 71. Saito Y, Nothacker H-P, Wang Z, Lin SHS, Leslie F, Civelli O.. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400(6741):265–269. [DOI] [PubMed] [Google Scholar]
- 72. An S, Cutler G, Zhao JJet al. Identification and characterization of a melanin-concentrating hormone receptor. Proc Natl Acad Sci USA. 2001;98(13):7576–7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hill J, Duckworth M, Murdock Pet al. Molecular cloning and functional characterization of MCH2, a novel Human MCH receptor. J Biol Chem. 2001;276(23):20125–20129. [DOI] [PubMed] [Google Scholar]
- 74. Mori M, Harada M, Terao Yet al. Cloning of a novel G protein-coupled receptor, SLT, a subtype of the melanin-concentrating hormone receptor. Biochem Biophys Res Commun. 2001;283(5):1013–1018. [DOI] [PubMed] [Google Scholar]
- 75. Rodriguez M, Beauverger P, Naime Iet al. Cloning and molecular characterization of the novel human melanin-concentrating hormone receptor MCH2. Mol Pharmacol. 2001;60:632–639. PMID: 11562423 [PubMed] [Google Scholar]
- 76. Sailer AW, Sano H, Zeng Zet al. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Natl Acad Sci USA. 2001;98(13):7564–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang S, Behan J, O’neill Ket al. Identification and pharmacological characterization of a novel human melanin-concentrating hormone receptor, MCH-R2. J Biol Chem. 2001;276(37):34664–34670. [DOI] [PubMed] [Google Scholar]
- 78. Chee MJS, Pissios P, Prasad D, Maratos-Flier E.. Expression of melanin-concentrating hormone receptor 2 protects against diet-induced obesity in male mice. Endocrinology. 2014;155(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gao X-B. Electrophysiological effects of MCH on neurons in the hypothalamus. Peptides. 2009;30(11):2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Whiddon BB, Palmiter RD.. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci. 2013;33(5):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Harthoorn LF, Sañé A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25(8):1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92(1-2):263–271. [DOI] [PubMed] [Google Scholar]
- 83. Schneeberger M, Tan K, Nectow ARet al. Functional analysis reveals differential effects of glutamate and MCH neuropeptide in MCH neurons. Mol Metab. 2018;13:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Del Cid-Pellitero E, Jones BE.. Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience. 2012;223:269–276. [DOI] [PubMed] [Google Scholar]
- 85. Chee MJS, Arrigoni E, Maratos-Flier E.. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35(8):3644–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848(1-2):101–113. [DOI] [PubMed] [Google Scholar]
- 87. Subhedar NK, Nakhate KT, Upadhya MA, Kokare DM.. CART in the brain of vertebrates: circuits, functions and evolution. Peptides. 2014;54:108–130. [DOI] [PubMed] [Google Scholar]
- 88. Mechoulam R, Parker LA.. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64(1):21–47. [DOI] [PubMed] [Google Scholar]
- 89. Bittencourt JC, Presse F, Arias Cet al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319(2):218–245. [DOI] [PubMed] [Google Scholar]
- 90. Berk ML, Finkelstein JA.. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull. 1982;8(5):511–526. [DOI] [PubMed] [Google Scholar]
- 91. Conductier G, Brau F, Viola Aet al. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat Neurosci. 2013;16(7):845–847. [DOI] [PubMed] [Google Scholar]
- 92. Conductier G, Martin AO, Risold P-Yet al. Control of ventricular ciliary beating by the melanin concentrating hormone-expressing neurons of the lateral hypothalamus: a Functional imaging survey. Front Endocrinol. 2013;4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jiang H, Gallet S, Klemm Pet al. MCH neurons regulate permeability of the median eminence barrier. Neuron. 2020;107(2):306–319.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Noble EE, Hahn JD, Konanur VRet al. Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell Metab. 2018;28(1):55–68.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Diniz GB, Battagello DS, Klein MOet al. Ciliary melanin-concentrating hormone receptor 1 (MCHR1) is widely distributed in the murine CNS in a sex-independent manner. J Neurosci Res. 2020;98(10):2045–2071. [DOI] [PubMed] [Google Scholar]
- 96. Gao X-B, Ghosh PK, Van Den Pol AN. Neurons synthesizing melanin-concentrating hormone identified by selective reporter gene expression after transfection In vitro: transmitter responses. J Neurophysiol. 2003;90(6):3978–3985. [DOI] [PubMed] [Google Scholar]
- 97. Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. PMID: 15157424. [DOI] [PubMed] [Google Scholar]
- 98. Huang H, Van Den Pol AN. Rapid direct excitation and long-lasting enhancement of NMDA response by group I metabotropic glutamate receptor activation of hypothalamic melanin-concentrating hormone neurons. J Neurosci. 2007;27(43):11560–11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bäckberg M, Ultenius C, Fritschy J‐M, Meister B.. Cellular localization of GABAA receptor α subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16(7):589–604. [DOI] [PubMed] [Google Scholar]
- 100. Moragues N, Ciofi P, Lafon P, Tramu G, Garret M.. GABAA receptor ε subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967(1-2):285–289. [DOI] [PubMed] [Google Scholar]
- 101. Gao X‐B, Van Den Pol AN.. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533(1):237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rao Y, Lu M, Ge Fet al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28(37):9101–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Apergis-Schoute J, Iordanidou P, Faure Cet al. Optogenetic evidence for inhibitory signaling from Orexin to MCH neurons via local microcircuits. J Neurosci. 2015;35(14):5435–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Broberger C, De Lecea L, Sutcliffe JG, Hökfelt T.. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402(4):460–474. [PubMed] [Google Scholar]
- 105. Niu J-G, Yokota S, Tsumori T, Oka T, Yasui Y.. Projections from the anterior basomedial and anterior cortical amygdaloid nuclei to melanin-concentrating hormone-containing neurons in the lateral hypothalamus of the rat. Brain Res. 2012;1479:31–43. [DOI] [PubMed] [Google Scholar]
- 106. Bayer L, Eggermann E, Serafin Met al. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130(4):807–811. [DOI] [PubMed] [Google Scholar]
- 107. Conductier G, Nahon J-L, Guyon A.. Dopamine depresses melanin concentrating hormone neuronal activity through multiple effects on α2-noradrenergic, D1 and D2-like dopaminergic receptors. Neuroscience. 2011;178:89–100. [DOI] [PubMed] [Google Scholar]
- 108. Al-Massadi O, Dieguez C, Schneeberger Met al. Multifaceted actions of melanin-concentrating hormone on mammalian energy homeostasis. Nat Rev Endocrinol. 2021;17(12):745–755. [DOI] [PubMed] [Google Scholar]
- 109. Huang H, Acuna-Goycolea C, Li Y, Cheng HM, Obrietan K, Van Den Pol AN.. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci. 2007;27(18):4870–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lopez CA, Guesdon B, Baraboi E-D, Roffarello BM, Hétu M, Richard D.. Involvement of the opioid system in the orexigenic and hedonic effects of melanin-concentrating hormone. Am J Physiol Regulat Integr Comp Physiol. 2011;301(4):R1105–R1111. [DOI] [PubMed] [Google Scholar]
- 111. Zhang X, Van Den Pol AN. Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: implications for TRH-mediated anorexic and arousal actions. J Neurosci. 2012;32(9):3032–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Guyon A, Conductier G, Rovere C, Enfissi A, Nahon J-L.. Melanin-concentrating hormone producing neurons: activities and modulations. Peptides. 2009;30(11):2031–2039. [DOI] [PubMed] [Google Scholar]
- 113. Woods SC, Seeley RJ, Porte D, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280(5368):1378–1383. [DOI] [PubMed] [Google Scholar]
- 114. Myers MG, Olson DP.. Central nervous system control of metabolism. Nature. 2012;491(7424):357–363. [DOI] [PubMed] [Google Scholar]
- 115. Vohra MS, Benchoula K, Serpell CJ, Hwa WE.. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur J Pharmacol. 2022;915:174611. [DOI] [PubMed] [Google Scholar]
- 116. Ludwig DS, Tritos NA, Mastaitis JWet al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107(3):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E.. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396(6712):670–674. [DOI] [PubMed] [Google Scholar]
- 118. Mul JD, Yi CX, van den Berg SAet al. Pmch expression during early development is critical for normal energy homeostasis. Am J Physiol Endocrinol Metab. 2010;298:E477–E488. [DOI] [PubMed] [Google Scholar]
- 119. Rossi M, Beak SA, Choi S-Jet al. Investigation of the feeding effects of melanin concentrating hormone on food intake—action independent of galanin and the melanocortin receptors. Brain Res. 1999;846(2):164–170. [DOI] [PubMed] [Google Scholar]
- 120. Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon J, Levens N.. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague–Dawley rats. Int J Obes. 2002;26(10):1289–1295. [DOI] [PubMed] [Google Scholar]
- 121. Gomori A, Ishihara A, Ito Met al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E583–E588. [DOI] [PubMed] [Google Scholar]
- 122. Whitlock BK, Daniel JA, Mcmahon CDet al. Intracerebroventricular melanin-concentrating hormone stimulates food intake in sheep. Domest Anim Endocrinol. 2005;28(2):224–232. [DOI] [PubMed] [Google Scholar]
- 123. Rossi M, Choi SJ, O'shea D, Miyoshi T, Ghatei MA, Bloom SR.. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138(1):351–355. [DOI] [PubMed] [Google Scholar]
- 124. Imbernon M, Beiroa D, Vázquez MJet al. Central melanin-concentrating hormone influences liver and adipose metabolism via specific hypothalamic nuclei and efferent autonomic/JNK1 pathways. Gastroenterology. 2013;144(3):636–649.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ito M, Gomori A, Ishihara Aet al. Characterization of MCH-mediated obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E940–E945. [DOI] [PubMed] [Google Scholar]
- 126. Stricker-Krongrad A, Dimitrov T, Beck B.. Central and peripheral dysregulation of melanin-concentrating hormone in obese Zucker rats. Mol Brain Res. 2001;92(1-2):43–48. [DOI] [PubMed] [Google Scholar]
- 127. Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T.. Differential regulation of melanin-concentrating hormone and orexin genes in the Agouti-related protein/melanocortin-4 receptor system. Biochem Biophys Res Commun. 2000;268(1):88–91. [DOI] [PubMed] [Google Scholar]
- 128. Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV.. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting in ob/ob and db/db mice, but is stimulated by Leptin. Diabetes. 1998;47(2):294–297. [DOI] [PubMed] [Google Scholar]
- 129. Ito M, Ishihara A, Gomori Aet al. Melanin-concentrating hormone 1-receptor antagonist suppresses body weight gain correlated with high receptor occupancy levels in diet-induced obesity mice. Eur J Pharmacol. 2009;624(1-3):77–83. [DOI] [PubMed] [Google Scholar]
- 130. Mashiko S, Ishihara A, Gomori Aet al. Antiobesity effect of a melanin-concentrating hormone 1 receptor antagonist in diet-induced obese mice. Endocrinology. 2005;146(7):3080–3086. [DOI] [PubMed] [Google Scholar]
- 131. Shearman LP, Camacho RE, Sloan Stribling Det al. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475(1-3):37–47. [DOI] [PubMed] [Google Scholar]
- 132. Ploj K, Benthem L, Kakol‐Palm Det al. Effects of a novel potent melanin-concentrating hormone receptor 1 antagonist, AZD1979, on body weight homeostasis in mice and dogs: MCH 1 receptor antagonism and body weight homeostasis. British J Pharmacology. 2016;173(18):2739–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Alon T, Friedman JM.. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci. 2006;26(2):389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jeon JY, Bradley RL, Kokkotou EGet al. MCH−/− mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes. 2006;55(2):428–434. [DOI] [PubMed] [Google Scholar]
- 135. Marsh DJ, Weingarth DT, Novi DEet al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99(5):3240–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Noble EE, Wang Z, Liu CMet al. Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat Commun. 2019;10(1):4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kong D, Vong L, Parton LEet al. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral Glucose homeostasis. Cell Metab. 2010;12(5):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Al-Massadi O, Quiñones M, Clasadonte Jet al. MCH regulates SIRT1/FoxO1 and reduces POMC neuronal activity to induce hyperphagia, adiposity, and glucose intolerance. Diabetes. 2019;68(12):2210–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pereira-Da-Silva Má, De Souza CláT, Gasparetti AL, Saad MáJA, Velloso LíA.. Melanin-concentrating hormone induces insulin resistance through a mechanism independent of body weight gain. J Endocrinol. 2005;186(1):193–201. [DOI] [PubMed] [Google Scholar]
- 140. Hausen AC, Ruud J, Jiang Het al. Insulin-dependent activation of MCH neurons impairs locomotor activity and Insulin sensitivity in obesity. Cell Rep. 2016;17(10):2512–2521. [DOI] [PubMed] [Google Scholar]
- 141. Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139(11):4739–4742. [DOI] [PubMed] [Google Scholar]
- 142. Huang Q, Viale A, Picard F, Nahon J-L, Richard D.. Effects of leptin on melanin-concentrating hormone expression in the brain of lean and obese lepob/lepob mice. Neuroendocrinology. 1999;69(3):145–153. [DOI] [PubMed] [Google Scholar]
- 143. Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E.. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142(2):680–686. [DOI] [PubMed] [Google Scholar]
- 144. Mogi K, Funabashi T, Mitsushima D, Hagiwara H, Kimura F.. Sex difference in the response of melanin-concentrating hormone neurons in the lateral hypothalamic area to glucose, as revealed by the expression of phosphorylated cyclic adenosine 3′,5′-monophosphate response element-binding protein. Endocrinology. 2005;146(8):3325–3333. [DOI] [PubMed] [Google Scholar]
- 145. Santollo J, Eckel LA.. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol Behav. 2008;93(4-5):842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Messina M, Boersma G, Overton J, Eckel L.. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88(4-5):523–528. [DOI] [PubMed] [Google Scholar]
- 147. Chen Y, Hu C, Hsu C-Ket al. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143(7):2469–2477. [DOI] [PubMed] [Google Scholar]
- 148. Faubel R, Westendorf C, Bodenschatz E, Eichele G.. Cilia-based flow network in the brain ventricles. Science. 2016;353(6295):176–178. [DOI] [PubMed] [Google Scholar]
- 149. Nampoothiri S, Nogueiras R, Schwaninger M, Prevot V.. Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis. Nat Metab. 2022;4(7):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. J Neuroendocrinol. 2014;26(11):753–760. [DOI] [PubMed] [Google Scholar]
- 151. Bolborea M, Dale N.. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36(2):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Elizondo‐Vega R, Cortes‐Campos C, Barahona MJ, Oyarce KA, Carril CA, García‐Robles MA.. The role of tanycytes in hypothalamic glucosensing. J Cell Mol Med. 2015;19(7):1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tabuchi S, Tsunematsu T, Black SWet al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hara J, Beuckmann CT, Nambu Tet al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. [DOI] [PubMed] [Google Scholar]
- 155. González JA, Jensen LT, Iordanidou P, Strom M, Fugger L, Burdakov D.. Inhibitory interplay between orexin neurons and eating. Curr Biol. 2016;26(18):2486–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Georgescu D, Sears RM, Hommel JDet al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25(11):2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mul JD, La Fleur SE, Toonen PWet al. Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat. PLoS One. 2011;6(5):e19600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Domingos AI, Sordillo A, Dietrich MOet al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2:e01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Morganstern I, Chang G-Q, Karatayev O, Leibowitz SF.. Increased orexin and melanin-concentrating hormone expression in the perifornical lateral hypothalamus of rats prone to overconsuming a fat-rich diet. Pharmacol Biochem Behav. 2010;96(4):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dilsiz P, Aklan I, Sayar Atasoy Net al. MCH neuron activity is sufficient for reward and reinforces feeding. Neuroendocrinology. 2020;110(3-4):258–270. [DOI] [PubMed] [Google Scholar]
- 161. Pissios P, Frank L, Kennedy ARet al. Dysregulation of the mesolimbic dopamine system and reward in MCH−/− mice. Biol Psychiatry. 2008;64(3):184–191. [DOI] [PubMed] [Google Scholar]
- 162. Chee MJ, Hebert AJ, Briançon N, Flaherty SE, Pissios P, Maratos-Flier E.. Conditional deletion of melanin-concentrating hormone receptor 1 from GABAergic neurons increases locomotor activity. Mol Metab. 2019;29:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Guesdon B, Paradis É, Samson P, Richard D.. Effects of intracerebroventricular and intra-accumbens melanin-concentrating hormone agonism on food intake and energy expenditure. Am J Physiol Regulat Integr Comp Physiol. 2009;296(3):R469–R475. [DOI] [PubMed] [Google Scholar]
- 164. Kokkotou E, Jeon JY, Wang Xet al. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regulat Integr Comp Physiol. 2005;289(1):R117–R124. [DOI] [PubMed] [Google Scholar]
- 165. Segal-Lieberman G, Bradley RL, Kokkotou Eet al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA. 2003;100(17):10085–10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC.. Lateral hypothalamic ‘command neurons’ with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci. 2007;25(8):2404–2412. [DOI] [PubMed] [Google Scholar]
- 167. Åstrand A, Bohlooly-Y M, Larsdotter Set al. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol Regulat Integr Comp Physiol. 2004;287(4):R749–R758. [DOI] [PubMed] [Google Scholar]
- 168. Jego S, Adamantidis A. MCH neurons: vigilant workers in the night. Sleep. 2013;36(12):1783–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Datta S. Cellular and chemical neuroscience of mammalian sleep. Sleep Med. 2010;11(5):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Verret L, Goutagny R, Fort Pet al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Torterolo P, Sampogna S, Chase MH.. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009;1268:76–87. [DOI] [PubMed] [Google Scholar]
- 172. Zhou D, Shen Z, Strack AM, Marsh DJ, Shearman LP.. Enhanced running wheel activity of both Mch1r- and Pmch-deficient mice. Regul Pept. 2005;124(1-3):53–63. [DOI] [PubMed] [Google Scholar]
- 173. Willie JT, Sinton CM, Maratos-Flier E, Yanagisawa M.. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156(4):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hassani OK, Lee MG, Jones BE.. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep–wake cycle. Proc Natl Acad Sci USA. 2009;106(7):2418–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Blanco-Centurion C, Luo S, Spergel DJet al. Dynamic network activation of hypothalamic MCH neurons in REM sleep and exploratory behavior. J Neurosci. 2019;39(25):4986–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Modirrousta M, Mainville L, Jones BE.. Orexin and MCH neurons express c-fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21(10):2807–2816. [DOI] [PubMed] [Google Scholar]
- 177. Kitka T, Adori C, Katai Zet al. Association between the activation of MCH and orexin immunorective neurons and REM sleep architecture during REM rebound after a three day long REM deprivation. Neurochem Int. 2011;59(5):686–694. [DOI] [PubMed] [Google Scholar]
- 178. Pelluru D, Konadhode R, Shiromani PJ.. MCH neurons are the primary sleep-promoting group. Sleep. 2013;36(12):1779–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Abrahamson EE, Leak RK, Moore RY.. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12(2):435–440. [DOI] [PubMed] [Google Scholar]
- 180. Chee MJS, Pissios P, Maratos-Flier E.. Neurochemical characterization of neurons expressing melanin-concentrating hormone receptor 1 in the mouse hypothalamus. J Comp Neurol. 2013;521(10):2208–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jego S, Glasgow SD, Herrera CGet al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16(11):1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Monti JM, Lagos P, Jantos H, Torterolo P.. Increased REM sleep after intra-locus coeruleus nucleus microinjection of melanin-concentrating hormone (MCH) in the rat. Prog Neuro Psychopharmacol Biol Psychiatry. 2015;56:185–188. [DOI] [PubMed] [Google Scholar]
- 183. Kroeger D, Bandaru SS, Madara JC, Vetrivelan R.. Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience. 2019;406:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tsunematsu T, Ueno T, Tabuchi Set al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34(20):6896–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Komagata N, Latifi B, Rusterholz T, Bassetti CLA, Adamantidis A, Schmidt MH.. Dynamic REM sleep modulation by ambient temperature and the critical role of the melanin-concentrating hormone system. Curr Biol. 2019;29(12):1976–1987.e4. [DOI] [PubMed] [Google Scholar]
- 186. Pelluru D, Konadhode RR, Bhat NR, Shiromani PJ.. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6 J mice. Eur J Neurosci. 2016;43(10):1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Briggs C, Hirasawa M, Semba K.. Sleep deprivation distinctly alters glutamate transporter 1 apposition and excitatory transmission to Orexin and MCH neurons. J Neurosci. 2018;38(10):2505–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. RobertStickgoldPhDb EJW, Stickgold R. Memory, sleep, and dreaming: experiencing consolidation. Sleep Med Clin. 2011;6:97–108. PMID: 21516215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Feld GB, Born J.. Sculpting memory during sleep: concurrent consolidation and forgetting. Curr Opin Neurobiol. 2017;44:20–27. [DOI] [PubMed] [Google Scholar]
- 190. Kandel ER, Dudai Y, Mayford MR.. The Molecular and systems biology of memory. Cell. 2014;157(1):163–186. [DOI] [PubMed] [Google Scholar]
- 191. Niv Y. Reinforcement learning in the brain. J Math Psychol. 2009;53(3):139–154. [Google Scholar]
- 192. Plaçais P-Y, Preat T.. To favor survival under food shortage, the brain disables costly memory. Science. 2013;339(6118):440–442. [DOI] [PubMed] [Google Scholar]
- 193. Petrovich GD. Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front Syst Neurosci. 2018;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Zimmer MR, Schmitz AE, Dietrich MO.. Activation of Agrp neurons modulates memory-related cognitive processes in mice. Pharmacol Res. 2019;141:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Beck B, Pourié G.. Ghrelin, neuropeptide Y, and other feeding-regulatory peptides active in the hippocampus: role in learning and memory. Nutr Rev. 2013;71(8):541–561. [DOI] [PubMed] [Google Scholar]
- 196. Zhang Y, Liu G, Yan J, Zhang Y, Li B, Cai D.. Metabolic learning and memory formation by the brain influence systemic metabolic homeostasis. Nat Commun. 2015;6(1):6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Schwartz M, Teitelbaum P.. Dissociation between learning and remembering in rats with lesions to the lateral hypothalamus. J Comp Physiol Psychol. 1974;87(3):384–398. [DOI] [PubMed] [Google Scholar]
- 198. Destrade C, Jaffard R.. Post-trial hippocampal and lateral hypothalamic electrical stimulation facilitation on long-term memory of appetitive and avoidance learning tasks. Behav Biol. 1978;22(3):354–374. [DOI] [PubMed] [Google Scholar]
- 199. Touzani K, Sclafani A.. Lateral hypothalamic lesions impair flavour-nutrient and flavour-toxin trace learning in rats. Eur J Neurosci. 2002;16(12):2425–2433. [DOI] [PubMed] [Google Scholar]
- 200. Ishii T. Distribution of Alzheimer’s neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta Neuropathol. 1966;6(2):181–187. [DOI] [PubMed] [Google Scholar]
- 201. Ptak R, Birtoli B, Imboden Het al. Hypothalamic amnesia with spontaneous confabulations: A clinicopathologic study. Neurology. 2001;56:1597–1600. PMID: 11402128 [DOI] [PubMed] [Google Scholar]
- 202. Borowsky B, Durkin MM, Ogozalek Ket al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8(8):825–830. [DOI] [PubMed] [Google Scholar]
- 203. Subramanian KS, Lauer LT, Hayes AMRet al. Hypothalamic melanin-concentrating hormone neurons integrate food-motivated appetitive and consummatory processes. Nature Communications. 2023;14(1):1755. doi: 10.1038/s41467-023-37344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Adamantidis A, Thomas E, Foidart Aet al. Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur J Neurosci. 2005;21(10):2837–2844. [DOI] [PubMed] [Google Scholar]
- 205. Monzon ME, De Souza MM, Izquierdo LA, Izquierdo I, Barros DM, De Barioglio SR.. Melanin-concentrating hormone (MCH) modifies memory retention in rats. Peptides. 1999;20(12):1517–1519. [DOI] [PubMed] [Google Scholar]
- 206. Varas M, Pérez M, Monzón María, De Barioglio SR. Melanin-concentrating hormone, hippocampal nitric oxide levels and memory retention. Peptides. 2002;23(12):2213–2221. [DOI] [PubMed] [Google Scholar]
- 207. Varas M, Pérez M, Ramírez O, De Barioglio SR. Melanin concentrating hormone increase hippocampal synaptic transmission in the rat. Peptides. 2002;23(1):151–155. [DOI] [PubMed] [Google Scholar]
- 208. Varas MM, Pérez MF, Ramírez OA, De Barioglio SR. Increased susceptibility to LTP generation and changes in NMDA-NR1 and -NR2B subunits mRNA expression in rat hippocampus after MCH administration. Peptides. 2003;24(9):1403–1411. [DOI] [PubMed] [Google Scholar]
- 209. Monzon ME, de Souza MM, Izquierdo LAet al. Melanin-concentrating hormone (MCH) modifies memory retention in rats. Peptides. 2009;30:2066–2070. [DOI] [PubMed] [Google Scholar]
- 210. Sherwood A, Wosiski‐Kuhn M, Nguyen Tet al. The role of melanin-concentrating hormone in conditioned reward learning. Eur J Neurosci. 2012;36(8):3126–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Harris JJ, Concetti C, Peleg-Raibstein D, Burdakov D.. A role for MCH neuron firing in hippocampal plasticity and learning. bioRxiv. 2022. doi: 10.1101/2022.12.01.518339. [DOI]
- 212. Le Barillier L, Léger L, Luppi P‐H, Fort P, Malleret G, Salin P‐A.. Genetic deletion of melanin-concentrating hormone neurons impairs hippocampal short-term synaptic plasticity and hippocampal-dependent forms of short-term memory. Hippocampus. 2015;25(11):1361–1373. [DOI] [PubMed] [Google Scholar]
- 213. Pachoud B, Adamantidis A, Ravassard Pet al. Major impairments of glutamatergic transmission and long-term synaptic plasticity in the hippocampus of mice lacking the melanin-concentrating hormone receptor-1. J Neurophysiol. 2010;104(3):1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lima FFB, Sita LV, Oliveira ARet al. Hypothalamic melanin-concentrating hormone projections to the septo-hippocampal complex in the rat. J Chem Neuroanat. 2013;47:1–14. [DOI] [PubMed] [Google Scholar]
- 215. Liu J-J, Tsien RW, Pang ZP.. Hypothalamic melanin-concentrating hormone regulates hippocampus-dorsolateral septum activity. Nat Neurosci. 2022;25(1):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Concetti C, Bracey EF, Peleg-Raibstein D, Burdakov D.. Control of fear extinction by hypothalamic melanin-concentrating hormone–expressing neurons. Proc Natl Acad Sci USA. 2020;117(36):22514–22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Pitman RK, Sanders KM, Zusman RMet al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51(2):189–192. [DOI] [PubMed] [Google Scholar]
- 218. Milton AL. Fear not: recent advances in understanding the neural basis of fear memories and implications for treatment development. F1000Research. 2019;8:F1000 Faculty Rev–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z.. Animal model for PTSD: from clinical concept to translational research. Neuropharmacology. 2012;62(2):715–724. [DOI] [PubMed] [Google Scholar]
- 220. Bryant RA, Moulds ML, Nixon RVD.. Cognitive behaviour therapy of acute stress disorder: a four-year follow-up. Behav Res Ther. 2003;41(4):489–494. [DOI] [PubMed] [Google Scholar]
- 221. Foa EB, Kozak MJ.. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99(1):20–35. [PubMed] [Google Scholar]
- 222. Foa EB, Mclean CP.. The efficacy of exposure therapy for anxiety-related disorders and its underlying mechanisms: the case of OCD and PTSD. Annu Rev Clin Psychol. 2016;12(1):1–28. [DOI] [PubMed] [Google Scholar]
- 223. Concetti C, Viskaitis P, Grujic Net al. Exploratory rearing is governed by hypothalamic MCH cells according to the locus coeruleus. bioRxiv. 2023. doi: 10.1101/2023.07.19.549648. [DOI] [PMC free article] [PubMed]
- 224. Lever C, Burton S, O’keefe J.. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17(1-2):111–134. [DOI] [PubMed] [Google Scholar]
- 225. Benarroch EE. Locus coeruleus. Cell Tissue Res. 2018;373(1):221–232. [DOI] [PubMed] [Google Scholar]
- 226. Morris LS, Mccall JG, Charney DS, Murrough JW.. The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci Adv. 2020;4:2398212820930321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Poe GR, Foote S, Eschenko Oet al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. 2020;21(11):644–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Sturman O, Germain P-L, Bohacek J.. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Ann NY Acad Sci. 2018;21:1–10. [DOI] [PubMed] [Google Scholar]
- 229. Izawa S, Chowdhury S, Miyazaki Tet al. REM sleep–active MCH neurons are involved in forgetting hippocampus-dependent memories. Science. 2019;365(6459):1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Burdakov D, Peleg-Raibstein D.. The hypothalamus as a primary coordinator of memory updating. Physiol Behav. 2020;223:112988. [DOI] [PubMed] [Google Scholar]
- 231. Saper CB, German DC.. Hypothalamic pathology in Alzheimer’s disease. Neurosci Lett. 1987;74(3):364–370. [DOI] [PubMed] [Google Scholar]
- 232. Mladinov M, Oh JY, Petersen Cet al. A post-mortem study of melanin-concentrating hormone (MCH) neurons in Alzheimer’s disease and progressive supranuclear palsy: the complex degeneration pattern of the lateral hypothalamic area. Alzheimer’s Dementia. 2021;17(S6): e054313. [Google Scholar]
- 233. Oh ST, Liu QF, Jeong HJet al. Nasal Cavity administration of melanin-concentrating hormone improves memory impairment in memory-impaired and Alzheimer's Disease mouse models. Mol Neurobiol. 2019;56(12):8076–8086. [DOI] [PubMed] [Google Scholar]
- 234. Lord MN, Subramanian K, Kanoski SE, Noble EE.. Melanin-concentrating hormone and food intake control: sites of action, peptide interactions, and appetition. Peptides. 2021;137:170476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Terrill SJ, Subramanian KS, Lan Ret al. Nucleus accumbens melanin-concentrating hormone signaling promotes feeding in a sex-specific manner. Neuropharmacology. 2020;178:108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are already included in this review article.