In this large, multisite RCT, pretest video education shows promise for men with prostate cancer.
Abstract
PURPOSE
Germline genetic testing (GT) is recommended for men with prostate cancer (PC), but testing through traditional models is limited. The ProGen study examined a novel model aimed at providing access to GT while promoting education and informed consent.
METHODS
Men with potentially lethal PC (metastatic, localized with a Gleason score of ≥8, persistent prostate-specific antigen after local therapy), diagnosis age ≤55 years, previous malignancy, and family history suggestive of a pathogenic variant (PV) and/or at oncologist's discretion were randomly assigned 3:1 to video education (VE) or in-person genetic counseling (GC). Participants had 67 genes analyzed (Ambry), with results disclosed via telephone by a genetic counselor. Outcomes included GT consent, GT completion, PV prevalence, and survey measures of satisfaction, psychological impact, genetics knowledge, and family communication. Two-sided Fisher's exact tests were used for between-arm comparisons.
RESULTS
Over a 2-year period, 662 participants at three sites were randomly assigned and pretest VE (n = 498) or GC (n = 164) was completed by 604 participants (VE, 93.1%; GC, 88.8%), of whom 596 participants (VE, 98.9%; GC, 97.9%) consented to GT and 591 participants completed GT (VE, 99.3%; GC, 98.6%). These differences were not statistically significant although subtle differences in satisfaction and psychological impact were. Notably, 84 PVs were identified in 78 participants (13.2%), with BRCA1/2 PV comprising 32% of participants with a positive result (BRCA2 n = 21, BRCA1 n = 4).
CONCLUSION
Both VE and traditional GC yielded high GT uptake without significant differences in outcome measures of completion, GT uptake, genetics knowledge, and family communication. The increased demand for GT with limited genetics resources supports consideration of pretest VE for patients with PC.
INTRODUCTION
The identification of germline pathogenic variants (PVs) is integral to informing the treatment of prostate cancer (PC), as targeted therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is approved for patients with homologous recombination deficiency (HRD) metastatic PC (mPC).1,2 While 12% of PC cases are associated with PVs,3 systematic processes to identify germline PVs have been limited and few studies have included cancer types beyond breast, ovarian, and colorectal cancers. With an estimated 288,300 new cases of PC in 2023,4 it is imperative to identify additional service delivery models to meet the increasing demand for timely access to genetic testing (GT).
CONTEXT
Key Objective
How does pretest video education (VE) on germline genetic testing (GT) compare with traditional genetic counseling for patients with prostate cancer (PC)?
Knowledge Generated
In this randomized controlled clinical trial of 662 patients with PC, there were no statistically significant differences between arms in uptake of GT, knowledge of GT, or family communication of results. There were some differences in satisfaction and psychological impact.
Relevance
Overall, given the increased demand for GT with limited genetics resources, pretest VE is a valuable platform for facilitating GT, although further study is needed.
Despite the well-established benefits of GT for patients with cancer including informing treatment, screening, and cascade testing of family members, there has been inconsistent integration of germline genetics into oncology workflows, leading to significant gaps in care.5,6 Rates of cancer genetic counseling (GC) are unacceptably low (approximately 20%) among individuals with standard or guideline-based indications for GT within integrated health care systems,7,8 commercially insured patients,9 and minorities.10-13 Barriers to GT include long wait times and uneven access to genetics experts.14-16 Despite clear clinical guidelines recommending universal testing for an expanding number of cancers,8,17-19 GC resources are not well matched to clinical needs among patients with a high probability of carrying a germline PV.20,21 In fact, genetics professionals have cited the shortage of providers as one of the biggest challenges in the field.22
Digital health technologies may represent an opportunity to combat this problem and perform scalable genetic assessments as indications for GT continue to increase. At the time this trial was designed, while the importance of GT for patients with PC was anticipated on the basis of the high PV prevalence,3 little data on alternatives to in-person GC services existed. Randomized trials comparing traditional with alternative and streamlined delivery approaches, including those conducted by our group, have consistently shown comparable or noninferior outcomes among patients with cancer.23-28 PVs in BRCA2 are known to be implicated in the development of early-onset and aggressive PC. Furthermore, cancer-specific survival is significantly worse in those with BRCA PVs compared with those without (median survival 8.6 years v 15.7 years).29 PVs in HRD genes like BRCA2 and mismatch repair (MMR) genes, among others, have treatment implications. A novel and efficient genetics service delivery model for patients with PC will have significant clinical implications for GC, testing, and targeted treatment, with the goal of ultimately improving survival for patients with PC.
The primary aim of this study was to assess uptake of GT in patients with PC in a randomized trial of pretest video education (VE) compared with in-person GC and to measure satisfaction, distress, and knowledge by randomized arm.
METHODS
Participants
From January 2017 through December 2019, patients were recruited for a randomized controlled trial evaluating in-person, pretest VE or GC from three sites: Dana-Farber Cancer Institute (DFCI, Boston, MA), University of Texas Southwestern Medical Center (Dallas, TX), and Karmanos Cancer Institute (Detroit, MI). Eligible patients were English-speaking and age ≥18 years, with one of the following criteria: mPC (hormone-sensitive, de novo, or castration-resistant [mCRPC]); localized PC with a Gleason score of ≥8; rising prostate-specific antigen (PSA) level after prostatectomy or radiation; persistent PSA after prostatectomy; PC at age ≤55 years; any PC with a personal history of other malignancy, or biopsy with high-grade prostatic intraepithelial neoplasia, or small acinar proliferation; and/or cancer family history (CFH) potentially indicating a germline PV (eg, premenopausal breast cancer, cancers of the ovary, pancreas, and colorectum, in ≥1 first- or second-degree relatives). Patients were excluded if they had previous cancer GC or GT, active hematologic malignancy, or localized PC previously treated and in remission for ≥2 years without CFH.
Procedures
Institutional review board approval was obtained at all study sites. The VE was developed by a team of cancer genetic counselors (CGCs), geneticists, and medical oncologists who wrote the script for narration (ninth-grade reading level), created a storyboard, and provided accompanying visuals (graphic representations of DNA, pedigree, consent form, test report, and illustrative imagery). Production was iterative as feedback was incorporated from the lay community. The final video was a professionally produced, 8-minute summary of the key educational components of a cancer GC visit including the choice in GT, the role of genes in cancer, multigene panel testing, psychosocial implications of testing, genetic discrimination, inheritance patterns, types of results, cascade testing, screening, prevention, and treatment implications.30,31
Eligible patients were identified through their medical oncologist or through the cancer genetics practice at each site. Of the 784 eligible patients approached by study coordinators, 662 (84%) consented to study participation. Data were not collected on nonconsenting patients. Consented patients were randomly assigned 3:1 to pretest VE or in-person GC (Fig 1). Random assignment occurred through a central process managed by the Office of Data Quality at DFCI, with stratification by hormone-sensitive/castrate-resistant PC. Per standard clinical practice, CFH was collected via a secure link to an electronic family history questionnaire. The CFH was reviewed and updated at the time of their visit for participants randomly assigned to the GC arm and at the time of result disclosure by a CGC for participants in the VE arm.
FIG 1.
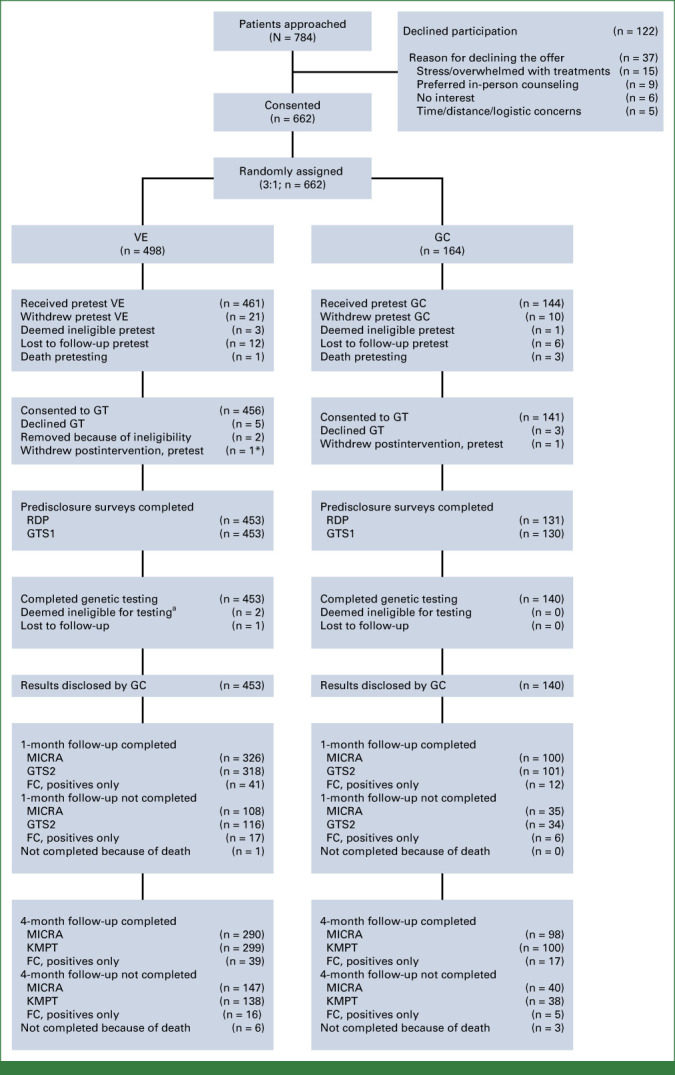
CONSORT diagram. FC, family communication; GC, genetic counseling; GT, genetic test; GTS1/2, Genetic Testing Satisfaction Survey at time point 1 and 2; KMPT, knowledge of multigene panel testing (KnowGene); MICRA, Multidimensional Impact of Cancer Risk Assessment; RDP, result disclosure; VE, video education.
achronic lymphocytic leukemia
Intervention
The GC arm consisted of a traditional in-person pretest visit with a CGC (standard of care), in which the participant typically spent 30-45 minutes discussing their CFH, the potential impact of identifying inherited cancer risk for themselves and their family, and the benefits, risks, and limitations of GT. On the VE arm, a research coordinator (RC) played the VE for the participant on an iPad in a designated clinic space (consultation or examination room). Participants were offered the opportunity to consent to GT by the CGC or RC (depending on arm) at the end of their visit. Participants in the VE arm could request access to a CGC at any time.
Participants were consented to GT of 67 genes (Appendix, online only) through Ambry Genetics, and interpretation of sequence variations was performed according to the American College of Medical Genetics and Genomics guidelines.32 Both PVs and likely PVs were denoted as PVs for analysis in this study.
Insurance was billed for the GT, and out-of-pocket costs were waived to avoid biasing study end points. Participants on both arms, irrespective of the test results, received their result via telephone disclosure by a CGC (standard of care). Those with a PV were encouraged to follow-up in clinic with a CGC and cancer genetics physician.
Measures and Statistical Analyses
The study was designed with 3:1 random assignment of 660 participants to account for an estimated 10% attrition, cost savings, greater power to detect between arm differences, and smaller confidence intervals. Although a noninferiority design for the primary end point was considered, it was not selected because of feasibility and to identify superiority of either arm if present. Demographic information, PC characteristics, CFH, and outcomes, including consent to and completion of GT, were tabulated. Forward stepwise logistic regression was performed with two separate outcome variables: all PV results and BRCA1/2 only PV results, with relevant clinical and demographic variables as covariates.
Survey measures for secondary outcomes collected at or shortly after the time of intervention included Result Disclosure Survey and an adapted version of the previously validated Genetic Testing Satisfaction Survey (GTSS).33 All analyses of surveys administered after the disclosure of GT results were performed separately for those with and without PVs. At 1 month after result disclosure, the GTSS was repeated (GTSS2) and the Multidimensional Impact of Cancer Risk Assessment (MICRA)34,35 and Family Communication Survey (FCS) were administered. The FCS was administered to participants with a PV only. The KnowGene scale, a validated measure of knowledge of cancer multigene panel testing, was delivered 4 months after the result disclosure36 (Appendix).
RESULTS
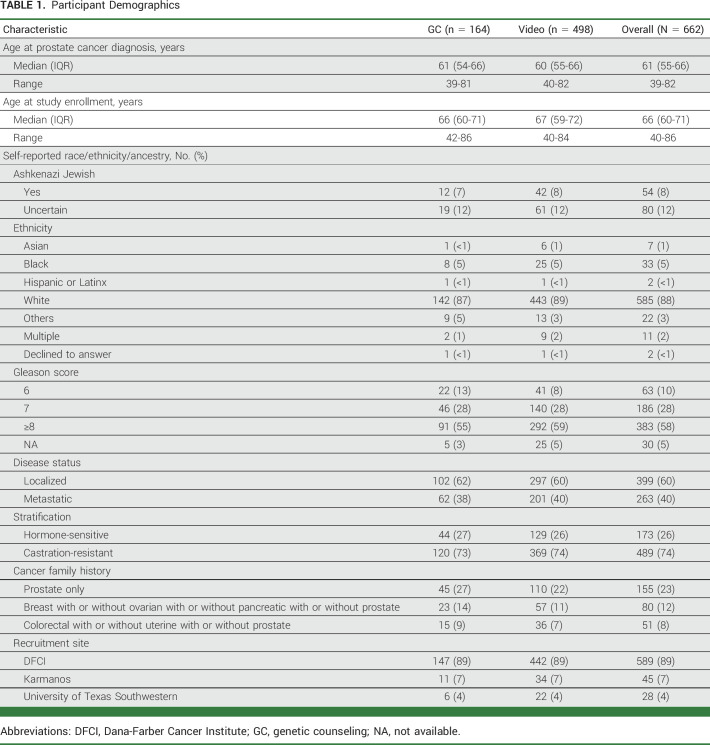
From January 2017 through December 2019, 662 patients consented to the study. The median age at study entry was 66 years with a range of 40-86 years, and at PC diagnosis, it was 61 years with a range of 39-82 years. Most participants were White (88%), 40% had mPC, and 74% had castrate-resistant PC. Baseline participant characteristics by arm were similar (Table 1).
TABLE 1.
Participant Demographics
Uptake and Genetic Test Results
Of the 662 randomly assigned participants 605 (91.4%) completed their randomized intervention assignment (GC or VE), 597 (90.2%) consented to GT, and 593 (89.5%) completed testing with no statistically significant differences by arm. Of the 498 participants randomly assigned to the VE arm, 461 (93%; 95% CI, 90 to 95) completed the VE visit; of those, 456 (99%; 95% CI, 98 to 100) consented to GT, of whom 453 (99.3%; 95% CI, 98 to 100) completed GT. Of the 164 participants randomly assigned to the GC arm, 144 (88% 95% CI, 82 to 92) completed GC visit; of those, 141 (98%; 95% CI, 94 to 100) consented to GT, of whom 140 (99.3%; 95% CI, 96.1 to 100) completed GT. Differences between arms were not statistically significant. Only five participants without PVs from the VE arm (approximately 1%) asked to speak with a GC.
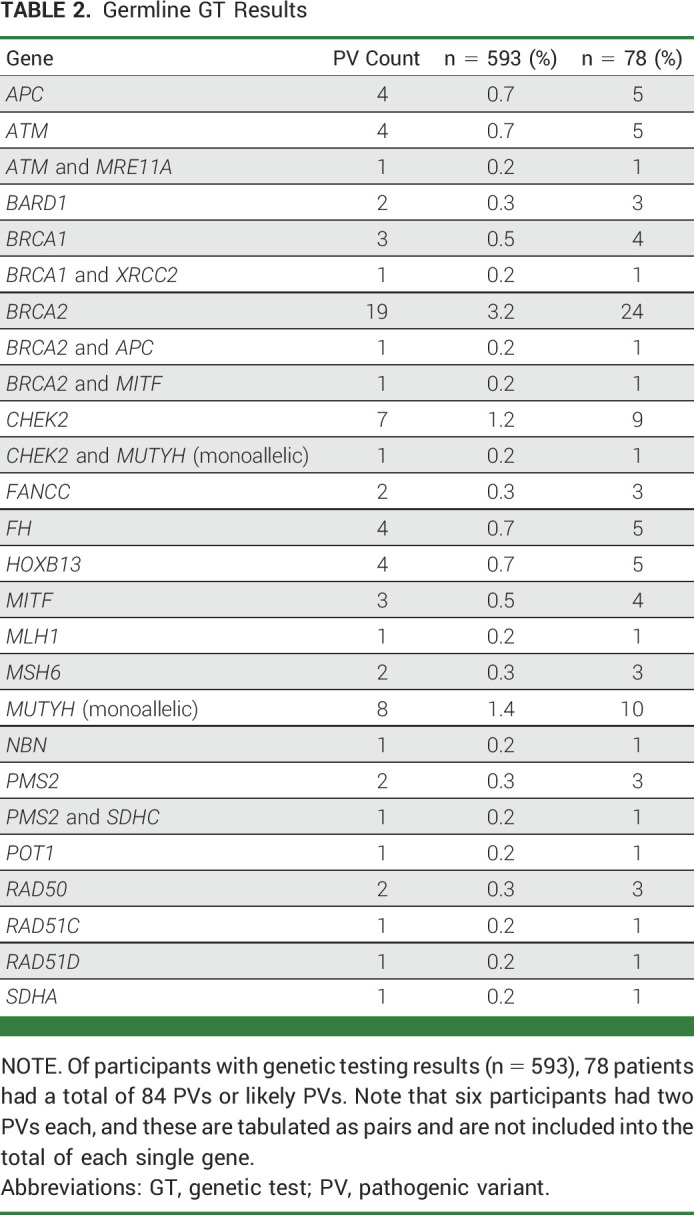
Most participants had negative test results (n = 310, 52%); 203 participants had a variant of uncertain significance (34%). PVs (n = 84) were identified in 78 (13%) participants; an additional two participants had mosaic NF1 results related to clonal hematopoiesis of indeterminate potential. BRCA1/2 PV accounted for 32% of subjects with a positive result (BRCA2:21, BRCA1:4). PVs in other genes traditionally associated with breast and/or ovarian cancer were as follows: CHEK2, n = 8 (one co-occurring with monoallelic MUTYH); ATM, n = 5 (one co-occurring with MRE11A); BARD1, n = 2; RAD50, n = 2; RAD51C, n = 1; RAD51D, n = 1; and NBN, n = 1. PVs in the MMR were identified in PMS2, n = 3 (one co-occurring with SDHC); MSH6, n = 2; and MLH1, n = 1. Results included other PVs, the most frequent of which was monoallelic MUTYH, n = 9 (Table 2).
TABLE 2.
Germline GT Results
Survey Results
Satisfaction
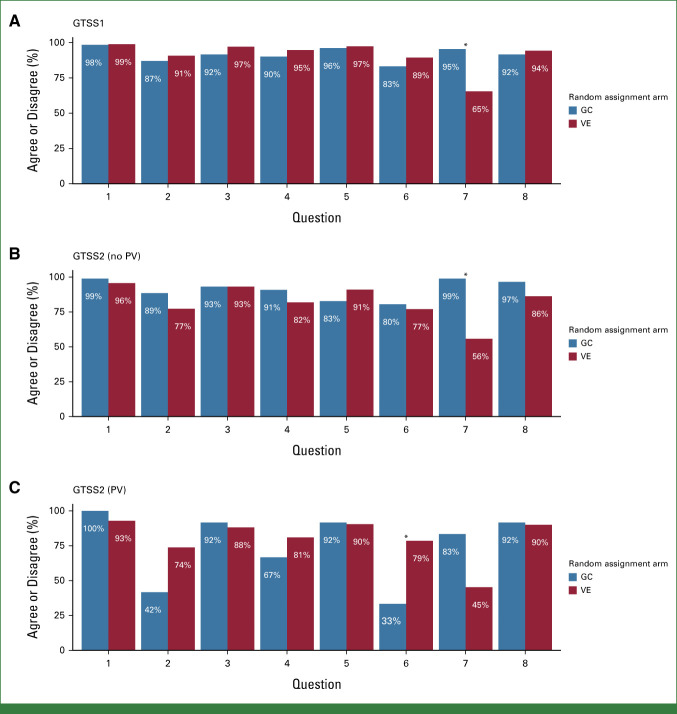
Response rates to GTSS1 (completed immediately after completion of the intervention, before genetic test results) were 99% for VE and 95% for GC. After correcting for multiple comparisons, agreement on one question ([my genetic counselor/the video] answered all my questions and concerns) was found to be significantly different (P < .001) between the VE and GC arms using a Fisher's exact test (Fig 2A) with results favoring the GC arm.
FIG 2.
GTSS1 and GTSS2 survey responses. (A) On the VE arm, 65% (95% CI, 61 to 70) of respondents agreed with question 7, and on the GC arm, 95 (95% CI, 90 to 98) of respondents agreed with the statement. (B) On the VE arm, 56% (95% CI, 50 to 62) of respondents agreed with question 7, and on the GC arm, 99% (95% CI, 94 to 100) of respondents agreed with the statement. (C) On the VE arm, 79% (95% CI, 63 to 90) of subjects disagreed with question 6, whereas 33% (95% CI, 10 to 65) of respondents disagreed on the GC arm. Q1. The information presented by (my genetic counselor/the video) was informative. Q2. The information presented by (my genetic counselor/the video) was sad or depressing. Q3. The information presented by (my genetic counselor/the video) was confusing or difficult to understand. Q4. The information presented by (my genetic counselor/the video) was distressing. Q5. The information presented by (my genetic counselor/the video) was useful. Q6. The information presented by (my genetic counselor/the video) was worrisome or anxiety inducing. Q7. (My genetic counselor/the video) answered all of my questions and concerns. Q8. (My appointment with my genetic counselor/the video) was about the right length of time. *P ≤ .05. GC, genetic counseling; GTSS, Genetic Testing Satisfaction Survey; PV, pathogenic variant; VE, video education.
On the GTSS2 (completed 1 month after result disclosure), among participants with no PV (VE, n = 276; GC, n = 87), agreement was found to be significantly different (P < .001) between arms on the same question as GTSS1 above again, favoring GC (Fig 2B). However, for participants with PVs (VE, n = 42; GC, n = 12), there was less anxiety with VE (P = .005) on the basis of the question (“The information presented by [my genetic counselor/the video] was worrisome or anxiety inducing”; Fig 2C).
Analysis of differences in answers item by item was conducted for results of the GTSS1 and GTSS2 for 413 participants, 317 (69%) on the VE arm and 96 (67%) on the GC arm using a Fisher-Freeman-Halton test and a Holm's correction for multiple comparisons. Among participants with no PV, there was a significant difference (P < .01) in responses between GTSS1 and GTSS2 as to whether all the participants' questions and concerns had been addressed by their intervention favoring GC.
Psychological Impact
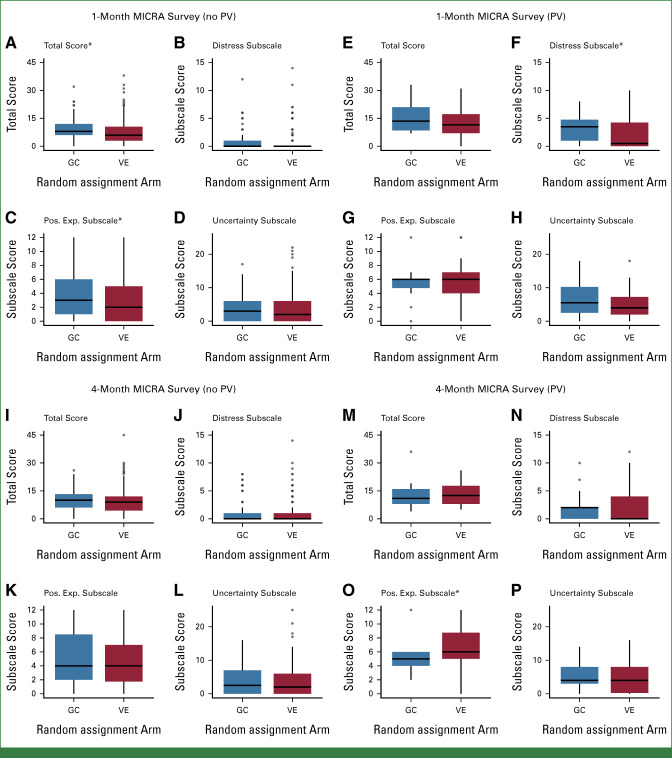
Between-arm differences in the total MICRA score from 1-month post–result disclosure were not statistically significant among participants with PVs. For participants with no PV, the total MICRA score was higher (worse) in the GC arm (P = .02; Fig 3A). Analysis of MICRA subscales by test result found greater distress among participants with PVs in the GC arm (P = .05; Fig 3F) and greater positive experiences among participants with no PV (P = .04) in the GC arm (Fig 3C).
FIG 3.
MICRA survey responses. Among participants with no PV (A-D, I-L), the 1-month total score (A) and the positive experience scales (C) differed by arm. Among participants with a PV (E-H, M-P), the distress subscale (F) at 1-month and the positive experience subscale at 4 months (O) differed by arm. *P ≤ .05. GC, genetic counseling; MICRA, Multidimensional Impact of Cancer Risk Assessment; Pos. Exp., positive experiences; PV, pathogenic variant; VE, video education.
At 4 months postdisclosure, the total MICRA score was not significantly different between the VE and GC arms for participants with or without PVs. The only significant difference in evaluating the subscales was that among participants with a PV, those in the GC arm had more positive experiences (P = .01; Fig 3O).
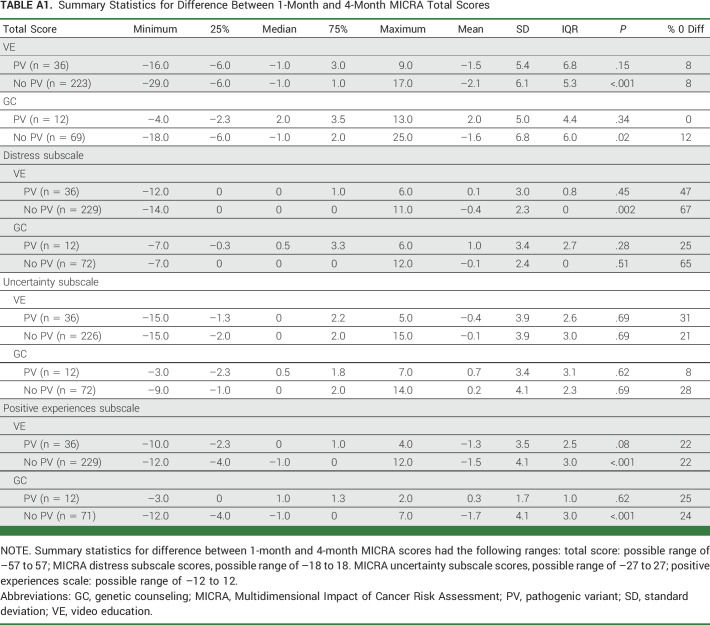
Differences between the total scores and subscales on the 1-month and 4-month MICRA surveys were evaluable for 340 participants who completed the survey at both timepoints (VE, 259; GC, 81; Appendix Table A1, online only). No statistically significant differences were found between the 1-month and 4-month MICRA among participants with a PV, whereas participants with no PV on the VE arm demonstrated more distress (P < .01) and less positive experiences (P < .001).
Knowledge
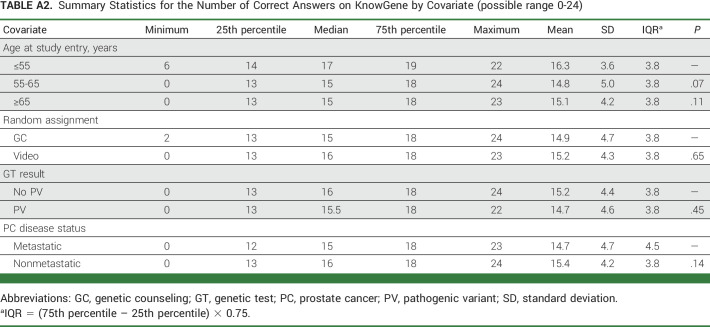
The KnowGene survey was completed by 305 participants on the VE arm (66% of those who completed the intervention) and 93 on the GC arm (65% of those who completed the intervention). The number of questions correctly answered was the outcome variable in a linear regression model with age at study entry (≤55, 55-65, ≥65 years), random assignment arm (VE v GC), PV (v no PV), and mPC (v nonmetastatic) as covariates. None of the covariates were found to be significant, and there were no significant differences in knowledge about multigene panel testing between the VE and GC arms (summary statistics in Appendix Table A2, online only).
Result Communication
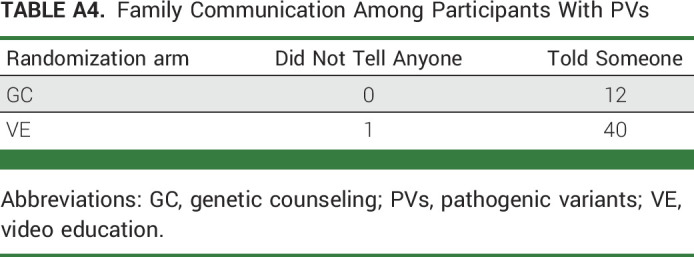
Postintervention, 582 of 595 (98%) responded to the results disclosure survey. There was no difference in intent to disclose results to family members (VE, 99%; GC, 99%; Appendix Table A3, online only). Of 74 participants with PV results who received the FCS, 53 (72%) completed it and there were no significant differences in disclosing results to family (VE, 98%; GC, 100%; Appendix Table A4, online only).
DISCUSSION
GT is an important part of PC care and has been incorporated into the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). There has been low compliance with GT for patients with breast or ovarian cancers, and it is anticipated that uptake in GT for patients with PC will be similarly low or lower.37 Our results indicate that VE is a tool that can be used to expand the reach of limited resources and improve access to cancer GT. Patients with PC randomly assigned to VE or GC both had high levels of GT uptake with slightly better, though nonsignificant, uptake and completion in VE. Both VE and GC demonstrated high satisfaction after their intervention. These findings suggest that for patients with PC, pretest VE without pretest GC can be used to facilitate GT.
Developing testing strategies and infrastructure to maximize identification with germline PVs is especially important in individuals with PC, given the high proportion of HRD genes attributed to PC predisposition. For example, BRCA2 PVs were the most frequent PVs identified in this study (accounting for 2.7% of mPC cases, 3.5% of the tested participants, and 25% of PVs), a finding that has considerable therapeutic implications as PARP inhibitors have been FDA-approved for the treatment of BRCA+ mCRPC.1,38,39 The BRCA2 PV frequency is lower than that reported by Pritchard et al, in which 5.3% of patients with mPC had a germline BRCA2 PV; our cohort was purposefully not limited to mPC, thus accounting for some differences.3 The germline PV spectrum has important management implications.
The psychological outcomes associated with the different pretest approaches were mixed. After result disclosure, participants with PVs who had been in the GC arm had greater distress (at 1 month), but not at 4 months. Participants with no PVs who had been in the GC arm had greater satisfaction specifically with having their questions answered, more positive experiences, and less distress. This finding is not surprising given the tailored, expert communication and empathic exchanges that patients experience with CGCs.25,40 However, only 1% of participants without PVs assigned to VE elected to meet with a CGC post-test despite ample opportunity to do so. Together with the fact that knowledge scores were not compromised in the VE arm, this low uptake of post-test GC may inform the prioritization of GC resources toward supporting patients and families with PVs. A noninferiority four-arm randomized trial of online cancer GT (MAGENTA) assessing the need for individualized pre- or post-test GC found no significant differences in anxiety, depression, or decisional regret in the arms in which pre- and/or post-test GC was omitted as compared with the control arms. Differences in our findings may be explained by study design as interventions were in person (not online) or by study population as our participants were markedly different from the MAGENTA population (males, all had cancer and were approximately 20 years older).25,40
Results from a nonrandomized, patient choice study of patients with PC found no differences in uptake of GT, knowledge, or decision regret with GT between VE and GC, and when offered a choice, patients selected VE.41 This study, together with our data, demonstrates that a truncated approach to GT for this population is reasonable, in particular, as the identification of germline PV has become increasingly important for estimating prognosis and for treatment selection in oncology care.42 While we found that uptake, satisfaction, knowledge, and psychosocial impact were favorable in both VE and GC arms, opportunities for providing tailored and personalized support exist. For example, chatbots or relational agents, which provide an interactive platform, may mitigate these differences.
The high GT completion rates in this study indicate that patients participating in our randomized trial on GT processes are a subset of patients with PC and, in practice, lower GT uptake may occur. Testing uptake would likely be lower in patients without cancer, especially among those with Medicare as the lack of coverage is often a barrier to GT access. Oncologic indications can mitigate some barriers as demonstrated in a study of pharmacogenomic GT among Medicare-enrolled individuals.43 Despite the multisite approach designed to optimize participant geographic and racial diversity, the study population was mostly accrued at one site (DFCI) and therefore comprised predominantly White, English speakers in the United States limiting conclusions in other populations. This also introduces the potential bias of differences between patients who seek care at academic centers compared with community practices who may be more likely to seek novel or experimental diagnostic and treatment approaches. Response may be different with non-US patients and/or non-English speakers, which is the subject of an upcoming trial (ClinicalTrials.gov identifier: NCT05225428). Given the greater frequency of early-onset, aggressive PCs in patients with African ancestry, more data are needed in diverse populations. By design, all test results were disclosed by CGCs, and thus, postdisclosure surveys might have been influenced by these interactions, leading to potentially fewer differences between the two arms. Other considerations in this type of study design are the medical literacy of the patients, the design of the video, and the awareness and ability of GCs in determining and addressing participant literacy.44 Educational attainment and literacy measures were not ascertained for trial participants. While the KnowGene scale was validated in women, this scale is applicable to multigene cancer panel testing and is pending further validation in more diverse patient populations with varied diagnoses. Analysis of cascade testing, impact on PC treatment, and somatic signatures for this cohort is in progress and will be reported separately.
Since the COVID-19 pandemic, there has been an expansion of virtual GC and implementation of technologies including chatbots and VE.45-51 In 2021, leveraging the VE experience from this study, together with increasing GC referrals, the DFCI Division of Cancer Genetics and Prevention implemented clinical, in-person, VE service delivery. During the 18-month period of VE, 2,000 additional patients with cancer were served by this new workflow.
In conclusion, participants randomly assigned to VE or GC both had high levels of uptake of GT with high satisfaction. VE and other paradigms, which promote ease of GT of patients with PC and other cancers, will enable the identification of germline PVs with their downstream implications. However, nuanced differences between GC and VE exist and will be further studied and delineated in ongoing trials in diverse patient populations (ClinicalTrials.gov identifier: NCT04330716, NCT05225428).
ACKNOWLEDGMENT
The authors acknowledge study participants, Dana-Farber PMC team IMAGINE, Dana-Farber Cancer Institute Medical Oncology Grant, and Ambry Genetics for supporting cost of testing and Rosalba Sacca, PhD, MS, CGC; Lillian Werner, MS; Sarfaraz Shaikh and Alison Slack for administrative support.
APPENDIX. METHODS
Genes tested on CancerNext Expanded at the time of this study: AIP, ALK, APC, ATM, BAP1, BARD1, BLM, BRCA1, BRCA2, BRIP1, BMPR1A, CDH1, CDK4, CDKN1B, CDKN2A, CHEK2, DICER1, EPCAM, FANCC, FH, FLCN, GALNT12, GREM1 (duplication/deletion only), HOXB13, MAX, MEN1, MET, MITF (p.E318K alteration only), MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, NF1, NF2, PALB2, PHOX2B, PMS2, POLD1, POLE, POT1, PRKAR1A, PTCH1, PTEN, RAD50, RAD51C, RAD51D, RB1, RET, SDHA, SDHAF2, SDHB, SDHC, SDHD, SMAD4, SMARCA4, SMARCB1, SMARCE1, STK11, SUFU, TMEM127, TP53, TSC1, TSC2, VHL, XRCC2.
Survey Measures
Result Disclosure Survey
The Result Disclosure Survey (RDS) analyzed whether the subject intended to share information about their genetic test results with family members.
Genetic Testing Satisfaction Survey
Answers to the Genetic Testing Satisfaction Survey (GTSS) ranged from disagree strongly to agree strongly on a 5-point Likert scale and were classified as either agree (agree and agree strongly) or disagree (disagree and disagree strongly), with neither agree nor disagree falling into the category that was considered less optimal depending on the question. For questions 1, 5, 7, and 8 “neither agree nor disagree” was grouped in the disagree category as this is the category of less desirable answers, and for questions 2, 3, 4, and 6 it was grouped in the agree category. A Fisher's exact test was used to test differences between arms for the RDS and the GTSS.
Multidimensional Impact of Cancer Risk Assessment
The Multidimensional Impact of Cancer Risk Assessment has been previously validated in men with BRCA1/2 pathogenic variants (PVs)34 and consists of questions split into three subscales: distress, uncertainty, and positive experiences.35 Each subscale was scored, with the total score being the sum of the subscales. Answers were scored on a 0- to 3-point scale to conform with assumptions of the nonparametric test, subscales were scored if at most one question was unanswered in the subscale, and a total score was calculated only if all the subscales for that subject were available. Wilcoxon rank-sum tests were used to test for differences between arms. A Fisher's exact test was used to test for differences between the arms among participants with PVs on the Family Communication Survey, regarding result disclosure to relatives.
KnowGene
The KnowGene scale, a validated measure of knowledge of cancer multigene panel testing, was delivered 4 months after the result disclosure.36 The number of questions correctly answered was summed for each participant and used as the outcome variable in a linear regression model with age at study entry (≤55, 55-65, and ≥65 years), random assignment arm (genetic counseling v video education), PV (v no PV), and metastatic prostate cancer (v nonmetastatic) as covariates.
TABLE A1.
Summary Statistics for Difference Between 1-Month and 4-Month MICRA Total Scores
TABLE A2.
Summary Statistics for the Number of Correct Answers on KnowGene by Covariate (possible range 0-24)
TABLE A3.
Results Disclosure Survey
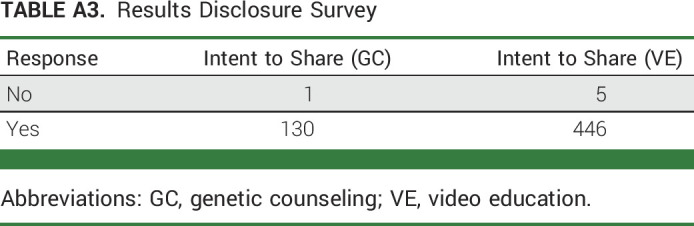
TABLE A4.
Family Communication Among Participants With PVs
Huma Q. Rana
Research Funding: Ambry Genetics, InVitae
Jill E. Stopfer
Honoraria: Ambry Genetics
Consulting or Advisory Role: AstraZeneca
Michelle Weitz
Employment: SimBioSys
Lindsay Kipnis
Honoraria: Invitae
Samantha Culver
Employment: InVitae
Stock and Other Ownership Interests: InVitae
Joanna Mercado
Employment: Genome Medical
Bradley A. McGregor
Consulting or Advisory Role: Seattle Genetics/Astellas, Exelixis, Astellas Pharma, Genentech/Roche, Pfizer, EMD Serono, Eisai, Bristol Myers Squibb, Calithera Biosciences, Merck
Research Funding: Bristol Myers Squibb (Inst), Exelixis (Inst), Calithera Biosciences (Inst), Seattle Genetics/Astellas (Inst), Pfizer/EMD Serono (Inst)
Christopher J. Sweeney
Stock and Other Ownership Interests: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, Genentech/Roche, AstraZeneca, Pfizer, Amgen, Lilly, POINT Biopharma, Cadence Pharma
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Dendreon, Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide. Exelixis: Abiraterone plus cabozantinib combination
Sara Pirzadeh-Miller
Patents, Royalties, Other Intellectual Property: Original inventor of CancerGene Connect—annual royalty distribution
Rebecca Silver
Research Funding: Bayer
Kerry E. Kilbridge
Research Funding: American Cancer Society/Pfizer
Mark M. Pomerantz
Honoraria: Bayer
Xiao X. Wei
Honoraria: OncLive
Consulting or Advisory Role: Novartis
Research Funding: Bristol Myers Squibb
Travel, Accommodations, Expenses: Corvus Pharmaceuticals
Atish D. Choudhury
Employment: LeMaitre Vascular
Honoraria: Journal of Clinical Pathways/Oncology Learning Network, OncLive, Bayer, Targeted Oncology, Aptitude Health, Cancer Network, Clinical Care Options, Great Debates and Updates, Pfizer, Springer
Consulting or Advisory Role: MedaCorp, Clovis Oncology, Dendreon, Bayer, Lilly, Blackstone, Astellas Pharma, AstraZeneca, Blue Earth Diagnostics, Janssen Oncology, Tolmar, Sanofi/Aventis
Research Funding: Janssen (Inst), Bayer
Travel, Accommodations, Expenses: Genentech
Guru P. Sonpavde
Employment: Myriad Genetics
Honoraria: UpToDate
Consulting or Advisory Role: Genentech, Merck, Janssen, Bristol Myers Squibb, Exelixis, EMD Serono, Astellas Pharma, Bicycle Therapeutics, Pfizer, Seagen, Gilead Sciences, Scholar Rock, G1 Therapeutics, Loxo/Lilly, Infinity Pharmaceuticals, Syapse, Lucence, Vial
Speakers' Bureau: Physicans' Education Resource, Onclive, Research to Practice, Medscape, Gilead Sciences, Seagen, Natera, Exelixis, Informação Brasileira de Oncologia
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), QED Therapeutics (Inst), Bristol Myers Squibb (Inst), Predicine (Inst), EMD Serono (Inst), Jazz Pharmaceuticals (Inst), GeneCentric (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Astellas Pharma, QED Therapeutics, Elsevier, Mereo BioPharma, G1 Therapeutics
Christopher Lathan
Consulting or Advisory Role: Lilly, Bristol Myers Squibb Foundation, Bristol Myers Squibb
Carrie Horton
Employment: Ambry Genetics
Travel, Accommodations, Expenses: Ambry Genetics
Jill S. Dolinsky
Employment: Ambry Genetics
Elisabeth I. Heath
Honoraria: Bayer, Sanofi, AstraZeneca, Genzyme, Janssen, Astellas Pharma, Caris Life Sciences, Johnson & Johnson/Janssen, Seagen
Consulting or Advisory Role: Bayer, Sanofi, AstraZeneca, Astellas Pharma, Bristol Myers Squibb, Janssen, Seagen
Speakers' Bureau: Sanofi
Research Funding: Tokai Pharmaceuticals (Inst), Seagen (Inst), Agensys (Inst), Dendreon (Inst), Genentech/Roche (Inst), Millennium (Inst), Celldex (Inst), Inovio Pharmaceuticals (Inst), Celgene (Inst), Zenith Epigenetics (Inst), Merck (Inst), AstraZeneca (Inst), Esanik (Inst), Oncolys BioPharma (Inst), Curemeta (Inst), Bristol Myers Squibb (Inst), eFFECTOR Therapeutics (Inst), Fortis (Inst), Astellas Pharma (Inst), Medivation (Inst), Ignyta (Inst), Synta (Inst), Caris Life Sciences (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Merck Sharp & Dohme (Inst), Plexxikon (Inst), Corcept Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Bayer (Inst), Modra Pharmaceuticals (Inst), Pellficure (Inst), Champions Oncology (Inst), AIQ Solutions (Inst), Novartis (Inst), Janssen Research & Development (Inst), Mirati Therapeutics (Inst), Peloton Therapeutics (Inst), Daiichi Sankyo Inc (Inst), Calibr (Inst), Eisai (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Arvinas (Inst), BioXCel Therapeutics (Inst), Calithera Biosciences (Inst), Corvus Pharmaceuticals (Inst), Exelixis (Inst), Gilead Sciences (Inst), Harpoon Therapeutics (Inst), Roche (Inst), ITeos Therapeutics (Inst), Pfizer (Inst), POINT Biopharma (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences
Other Relationship: Caris Centers of Excellence
Theodora Suzanne Ross
Employment: Merck Sharp & Dohme, AbbVie
Kevin Dale Courtney
Stock and Other Ownership Interests: Regeneron
Consulting or Advisory Role: Novartis
Research Funding: Astellas Pharma (Inst), Lilly (Inst), Stemline Therapeutics (Inst), Clovis Oncology (Inst), Exelixis (Inst), Amgen (Inst), Harpoon Therapeutics (Inst), Pfizer (Inst), Surface Oncology (Inst), Novartis (Inst), Myovant Sciences (Inst), Celgene/Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: My spouse receives patent royalties from Athena Diagnostics, Inc
Judy E. Garber
Consulting or Advisory Role: Novartis, Kronos Bio, GV20 Therapeutics, Belharra Therapeutics, Inc, Earli, Inc
Research Funding: Novartis, Ambry Genetics, InVitae, Amgen
Other Relationship: AACR, Diana Helis Henry Medical Foundation, James P. Wilmot Foundation, Adrienne Helis Malvin Medical Research Foundation, Breast Cancer Research Foundation, Facing our Risk of Cancer Empowered
Mary-Ellen Taplin
Honoraria: Janssen-Ortho, Clovis Oncology, UpToDate, Research to Practice, Pfizer, AstraZeneca, Roivant, AbbVie, Arcus Biosciences, Constellation Pharmaceuticals, Epizyme, Targeted Oncology, Arvinas, Blue Earth Diagnostics, Hengrui Therapeutics, Propella Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Bayer, Best Doctors, Inc, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Pfizer, AstraZeneca, Arcus Ventures
Research Funding: Janssen-Ortho (Inst)
Travel, Accommodations, Expenses: Advanced Prostate Cancer Society
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the Annual Meeting of the American Society of Clinical Oncology (ASCO) virtual scientific program (Chicago, IL) May 29-May 31, 2020.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Huma Q. Rana, Jill E. Stopfer, Diane R. Koeller, Courtney Kokenakes, Donna Rachel Vatnick, Christopher Lathan, Jill S. Dolinsky, Judy E. Garber, Mary-Ellen Taplin
Financial support: Judy E. Garber, Mary-Ellen Taplin
Administrative support: Brian Reys, Guru P. Sonpavde, Judy E. Garber, Mary-Ellen Taplin
Provision of study materials or patients: Bradley A. McGregor, Christopher J. Sweeney, Brian Reys, Kerry E. Kilbridge, Atish D. Choudhury, Guru P. Sonpavde, Olga Kozyreva, Theodora Suzanne Ross, Kevin Dale Courtney, Mary-Ellen Taplin
Collection and assembly of data: Huma Q. Rana, Jill E. Stopfer, Lindsay Kipnis, Diane R. Koeller, Samantha Culver, Joanna Mercado, Bradley A. McGregor, Christopher J. Sweeney, Nancie Petrucelli, Courtney Kokenakes, Sara Pirzadeh-Miller, Brian Reys, Arthur Frazier, Andrew Knechtl, Salman Fateh, Donna Rachel Vatnick, Rebecca Silver, Kerry E. Kilbridge, Mark M. Pomerantz, Atish D. Choudhury, Guru P. Sonpavde, Olga Kozyreva, Theodora Suzanne Ross, Kevin Dale Courtney, Judy E. Garber, Mary-Ellen Taplin
Data analysis and interpretation: Huma Q. Rana, Jill E. Stopfer, Michelle Weitz, Diane R. Koeller, Meghan Underhill-Blazey, Bradley A. McGregor, Christopher J. Sweeney, Rebecca Silver, Xiao X. Wei, Atish D. Choudhury, Guru P. Sonpavde, Carrie Horton, Elisabeth I. Heath, Mary-Ellen Taplin, Rebecca Sue Gelman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pretest Video Education Versus Genetic Counseling for Patients With Prostate Cancer: A Multisite Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Huma Q. Rana
Research Funding: Ambry Genetics, InVitae
Jill E. Stopfer
Honoraria: Ambry Genetics
Consulting or Advisory Role: AstraZeneca
Michelle Weitz
Employment: SimBioSys
Lindsay Kipnis
Honoraria: Invitae
Samantha Culver
Employment: InVitae
Stock and Other Ownership Interests: InVitae
Joanna Mercado
Employment: Genome Medical
Bradley A. McGregor
Consulting or Advisory Role: Seattle Genetics/Astellas, Exelixis, Astellas Pharma, Genentech/Roche, Pfizer, EMD Serono, Eisai, Bristol Myers Squibb, Calithera Biosciences, Merck
Research Funding: Bristol Myers Squibb (Inst), Exelixis (Inst), Calithera Biosciences (Inst), Seattle Genetics/Astellas (Inst), Pfizer/EMD Serono (Inst)
Christopher J. Sweeney
Stock and Other Ownership Interests: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, Genentech/Roche, AstraZeneca, Pfizer, Amgen, Lilly, POINT Biopharma, Cadence Pharma
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Dendreon, Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide. Exelixis: Abiraterone plus cabozantinib combination
Sara Pirzadeh-Miller
Patents, Royalties, Other Intellectual Property: Original inventor of CancerGene Connect—annual royalty distribution
Rebecca Silver
Research Funding: Bayer
Kerry E. Kilbridge
Research Funding: American Cancer Society/Pfizer
Mark M. Pomerantz
Honoraria: Bayer
Xiao X. Wei
Honoraria: OncLive
Consulting or Advisory Role: Novartis
Research Funding: Bristol Myers Squibb
Travel, Accommodations, Expenses: Corvus Pharmaceuticals
Atish D. Choudhury
Employment: LeMaitre Vascular
Honoraria: Journal of Clinical Pathways/Oncology Learning Network, OncLive, Bayer, Targeted Oncology, Aptitude Health, Cancer Network, Clinical Care Options, Great Debates and Updates, Pfizer, Springer
Consulting or Advisory Role: MedaCorp, Clovis Oncology, Dendreon, Bayer, Lilly, Blackstone, Astellas Pharma, AstraZeneca, Blue Earth Diagnostics, Janssen Oncology, Tolmar, Sanofi/Aventis
Research Funding: Janssen (Inst), Bayer
Travel, Accommodations, Expenses: Genentech
Guru P. Sonpavde
Employment: Myriad Genetics
Honoraria: UpToDate
Consulting or Advisory Role: Genentech, Merck, Janssen, Bristol Myers Squibb, Exelixis, EMD Serono, Astellas Pharma, Bicycle Therapeutics, Pfizer, Seagen, Gilead Sciences, Scholar Rock, G1 Therapeutics, Loxo/Lilly, Infinity Pharmaceuticals, Syapse, Lucence, Vial
Speakers' Bureau: Physicans' Education Resource, Onclive, Research to Practice, Medscape, Gilead Sciences, Seagen, Natera, Exelixis, Informação Brasileira de Oncologia
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), QED Therapeutics (Inst), Bristol Myers Squibb (Inst), Predicine (Inst), EMD Serono (Inst), Jazz Pharmaceuticals (Inst), GeneCentric (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Astellas Pharma, QED Therapeutics, Elsevier, Mereo BioPharma, G1 Therapeutics
Christopher Lathan
Consulting or Advisory Role: Lilly, Bristol Myers Squibb Foundation, Bristol Myers Squibb
Carrie Horton
Employment: Ambry Genetics
Travel, Accommodations, Expenses: Ambry Genetics
Jill S. Dolinsky
Employment: Ambry Genetics
Elisabeth I. Heath
Honoraria: Bayer, Sanofi, AstraZeneca, Genzyme, Janssen, Astellas Pharma, Caris Life Sciences, Johnson & Johnson/Janssen, Seagen
Consulting or Advisory Role: Bayer, Sanofi, AstraZeneca, Astellas Pharma, Bristol Myers Squibb, Janssen, Seagen
Speakers' Bureau: Sanofi
Research Funding: Tokai Pharmaceuticals (Inst), Seagen (Inst), Agensys (Inst), Dendreon (Inst), Genentech/Roche (Inst), Millennium (Inst), Celldex (Inst), Inovio Pharmaceuticals (Inst), Celgene (Inst), Zenith Epigenetics (Inst), Merck (Inst), AstraZeneca (Inst), Esanik (Inst), Oncolys BioPharma (Inst), Curemeta (Inst), Bristol Myers Squibb (Inst), eFFECTOR Therapeutics (Inst), Fortis (Inst), Astellas Pharma (Inst), Medivation (Inst), Ignyta (Inst), Synta (Inst), Caris Life Sciences (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Merck Sharp & Dohme (Inst), Plexxikon (Inst), Corcept Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Bayer (Inst), Modra Pharmaceuticals (Inst), Pellficure (Inst), Champions Oncology (Inst), AIQ Solutions (Inst), Novartis (Inst), Janssen Research & Development (Inst), Mirati Therapeutics (Inst), Peloton Therapeutics (Inst), Daiichi Sankyo Inc (Inst), Calibr (Inst), Eisai (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Arvinas (Inst), BioXCel Therapeutics (Inst), Calithera Biosciences (Inst), Corvus Pharmaceuticals (Inst), Exelixis (Inst), Gilead Sciences (Inst), Harpoon Therapeutics (Inst), Roche (Inst), ITeos Therapeutics (Inst), Pfizer (Inst), POINT Biopharma (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences
Other Relationship: Caris Centers of Excellence
Theodora Suzanne Ross
Employment: Merck Sharp & Dohme, AbbVie
Kevin Dale Courtney
Stock and Other Ownership Interests: Regeneron
Consulting or Advisory Role: Novartis
Research Funding: Astellas Pharma (Inst), Lilly (Inst), Stemline Therapeutics (Inst), Clovis Oncology (Inst), Exelixis (Inst), Amgen (Inst), Harpoon Therapeutics (Inst), Pfizer (Inst), Surface Oncology (Inst), Novartis (Inst), Myovant Sciences (Inst), Celgene/Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: My spouse receives patent royalties from Athena Diagnostics, Inc
Judy E. Garber
Consulting or Advisory Role: Novartis, Kronos Bio, GV20 Therapeutics, Belharra Therapeutics, Inc, Earli, Inc
Research Funding: Novartis, Ambry Genetics, InVitae, Amgen
Other Relationship: AACR, Diana Helis Henry Medical Foundation, James P. Wilmot Foundation, Adrienne Helis Malvin Medical Research Foundation, Breast Cancer Research Foundation, Facing our Risk of Cancer Empowered
Mary-Ellen Taplin
Honoraria: Janssen-Ortho, Clovis Oncology, UpToDate, Research to Practice, Pfizer, AstraZeneca, Roivant, AbbVie, Arcus Biosciences, Constellation Pharmaceuticals, Epizyme, Targeted Oncology, Arvinas, Blue Earth Diagnostics, Hengrui Therapeutics, Propella Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Bayer, Best Doctors, Inc, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Pfizer, AstraZeneca, Arcus Ventures
Research Funding: Janssen-Ortho (Inst)
Travel, Accommodations, Expenses: Advanced Prostate Cancer Society
No other potential conflicts of interest were reported.
REFERENCES
- 1.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Mateo J, Porta N, Bianchini D, et al. : Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 21:162-174, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pritchard CC, Mateo J, Walsh MF, et al. : Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 375:443-453, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute : Cancer stat facts: Prostate cancer, 2023. https://seer.cancer.gov/statfacts/html/prost.html
- 5.Katz SJ, Ward KC, Hamilton AS, et al. : Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol 36:1218-1224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer LA, Anderson ME, Lacour RA, et al. : Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: Missed opportunities. Obstet Gynecol 115:945-952, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellcross CA, Peipins LA, McCarty FA, et al. : Characteristics associated with genetic counseling referral and BRCA1/2 testing among women in a large integrated health system. Genet Med 17:43-50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demsky R, McCuaig J, Maganti M, et al. : Keeping it simple: Genetics referrals for all invasive serous ovarian cancers. Gynecol Oncol 130:329-333, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Wright JD, Chen L, Tergas AI, et al. : Underuse of BRCA testing in patients with breast and ovarian cancer. Am J Obstet Gynecol 214:761-763, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Randall TC, Armstrong K: Health care disparities in hereditary ovarian cancer: Are we reaching the underserved population? Curr Treat Options Oncol 17:39, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong K, Micco E, Carney A, et al. : Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA 293:1729-1736, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Pagan JA, Su D, Li L, et al. : Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med 37:524-530, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Kurian AW, Abrahamse P, Furgal A, et al. : Germline genetic testing after cancer diagnosis. JAMA 330:43-51, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bednar EM, Oakley HD, Sun CC, et al. : A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment. Gynecol Oncol 146:399-404, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villegas C, Haga SB: Access to genetic counselors in the southern United States. J Pers Med 9:33, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Society of Genetic Counselors. (2020) 2020 Professional Status Survey. https://www.nsgc.org/Policy-Research-and-Publications/Professional-Status-Survey.
- 17.Stoffel EM, McKernin SE, Brand R, et al. : Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol 37:153-164, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Paller CJ, Antonarakis ES, Beer TM, et al. : Germline genetic testing in advanced prostate cancer; practices and barriers: Survey results from the Germline Genetics Working Group of the prostate cancer clinical trials consortium. Clin Genitourin Cancer 17:275-282.e1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manahan ER, Kuerer HM, Sebastian M, et al. : Consensus guidelines on genetic` testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol 26:3025-3031, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurian AW, Ward KC, Hamilton AS, et al. : Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol 4:1066-1072, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szymaniak BM, Facchini LA, Giri VN, et al. : Practical considerations and challenges for germline genetic testing in patients with prostate cancer: Recommendations from the Germline Genetics Working Group of the PCCTC. JCO Oncol Pract 16:811-819, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoll K, Kubendran S, Cohen SA: The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet 178:24-37, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. : Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol 32:618-626, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peshkin BN, Kelly S, Nusbaum RH, et al. : Patient perceptions of telephone vs. in-person BRCA1/BRCA2 genetic counseling. J Genet Couns 25:472-482, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayes N, Bowen DJ, Coffin T, et al. : MAGENTA (making genetic testing accessible): A prospective randomized controlled trial comparing online genetic education and telephone genetic counseling for hereditary cancer genetic testing. BMC Cancer 19:648, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Interrante MK, Segal H, Peshkin BN, et al. : Randomized noninferiority trial of telephone vs in-person genetic counseling for hereditary breast and ovarian cancer: A 12-month follow-up. JNCI Cancer Spectr 1:pkx002, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradbury A, Patrick-Miller L, Harris D, et al. : Utilizing remote real-time videoconferencing to expand access to cancer genetic services in community practices: A multicenter feasibility study. J Med Internet Res 18:e23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradbury AR, Patrick-Miller LJ, Egleston BL, et al. : Randomized noninferiority trial of telephone vs in-person disclosure of germline cancer genetic test results. J Natl Cancer Inst 110:985-993, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro E, Goh C, Olmos D, et al. : Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 31:1748-1757, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axilbund JE, Hamby LA, Thompson DB, et al. : Assessment of the use and feasibility of video to supplement the genetic counseling process: A cancer genetic counseling perspective. J Genet Couns 14:235-243, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Robson ME, Bradbury AR, Arun B, et al. : American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol 33:3660-3667, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Pesaran T, Karam R, Huether R, et al. : Beyond DNA: An integrated and functional approach for classifying germline variants in breast cancer genes. Int J Breast Cancer 2016:1-10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradbury A, Patrick-Miller L, Harris D, et al. : Utilizing remote real-time videoconferencing to expand access to cancer genetic services in community practices: A multicenter feasibility study. J Med Internet Res 18:e23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumish HS, Steinfeld H, Koval C, et al. : Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J Genet Couns 26:1116-1129, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cella D, Hughes C, Peterman A, et al. : A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol 21:564-572, 2002 [PubMed] [Google Scholar]
- 36.Underhill-Blazey M, Stopfer J, Chittenden A, et al. : Development and testing of the KnowGene scale to assess general cancer genetic knowledge related to multigene panel testing. Patient Educ Couns 102:1558-1564, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Mohler JL, Antonarakis ES, Armstrong AJ, et al. : Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 17:479-505, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Abida W, Patnaik A, Campbell D, et al. : Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol 38:3763-3772, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain M, Mateo J, Fizazi K, et al. : Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med 383:2345-2357, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Swisher EM, Rayes N, Bowen D, et al. : Remotely delivered cancer genetic testing in making genetic testing accessible (MAGENTA) trial: a randomized clinical trial. JAMA Oncol. 38, 2020. Published online September 14, 2023. doi10.1001/jamaoncol.2023.3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo J, McDougall C, Bowler N, et al. : Pretest genetic education video versus genetic counseling for men considering prostate cancer germline testing: A patient-choice study to address urgent practice needs. JCO Precis Oncol 5:1377-1386, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonarakis ES, Lu C, Luber B, et al. : Germline DNA-repair gene mutations and outcomes in men with metastatic castration-resistant prostate cancer receiving first-line abiraterone and enzalutamide. Eur Urol 74:218-225, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young J, Bhattacharya K, Ramachandran S, et al. : Rates of genetic testing in patients prescribed drugs with pharmacogenomic information in FDA-approved labeling. Pharmacogenomics J 21:318-325, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilbridge KL, Fraser G, Krahn M, et al. : Lack of comprehension of common prostate cancer terms in an underserved population. J Clin Oncol 27:2015-2021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson CH, Ulm M, Blackburn P, et al. : Video-assisted genetic counseling in patients with ovarian, fallopian and peritoneal carcinoma. Gynecol Oncol 143:109-112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidlen T, Schwartz M, DiLoreto K, et al. : Patient assessment of chatbots for the scalable delivery of genetic counseling. J Genet Couns 28:1166-1177, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Cragun D, Weidner A, Tezak A, et al. : A web-based tool to automate portions of pretest genetic counseling for inherited cancer. J Natl Compr Cancer Netw 18:841-847, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Bibault JE, Chaix B, Guillemasse A, et al. : A chatbot versus physicians to provide information for patients with breast cancer: Blind, randomized controlled noninferiority trial. J Med Internet Res 21:e15787, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazareth S, Hayward L, Simmons E, et al. : Hereditary cancer risk using a genetic chatbot before routine care visits. Obstet Gynecol 138:860-870, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato A, Haneda E, Suganuma N, et al. : Preliminary screening for hereditary breast and ovarian cancer using a chatbot augmented intelligence genetic counselor: Development and feasibility study. JMIR Form Res 5:e25184, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch BM, Allen CG, Ritchie JB, et al. : Using a chatbot to assess hereditary cancer risk. JCO Clin Cancer Inform 4:787-793, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]