Abstract
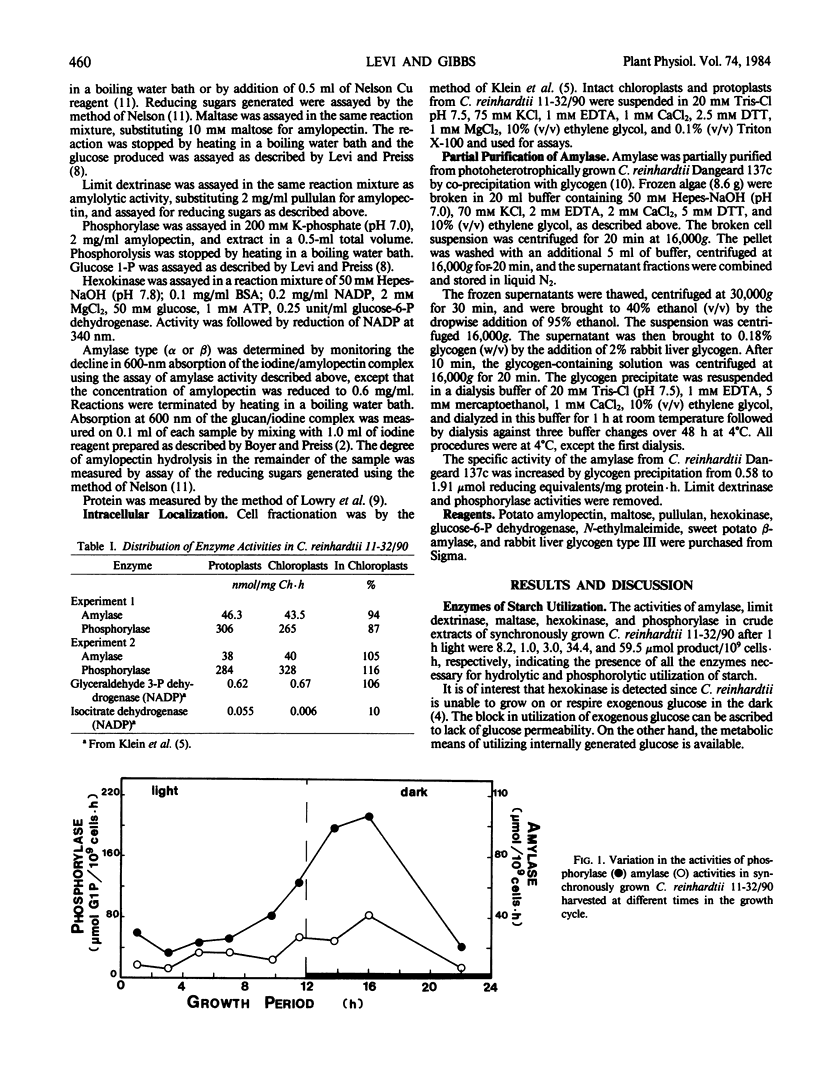
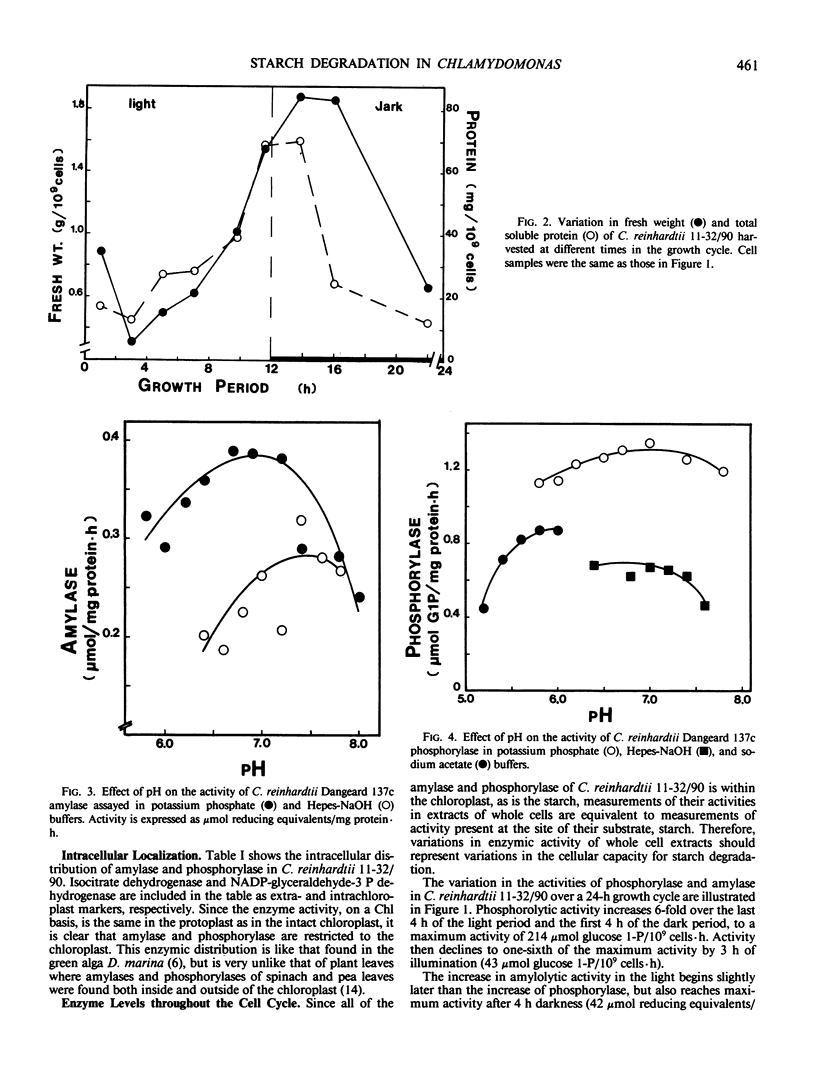
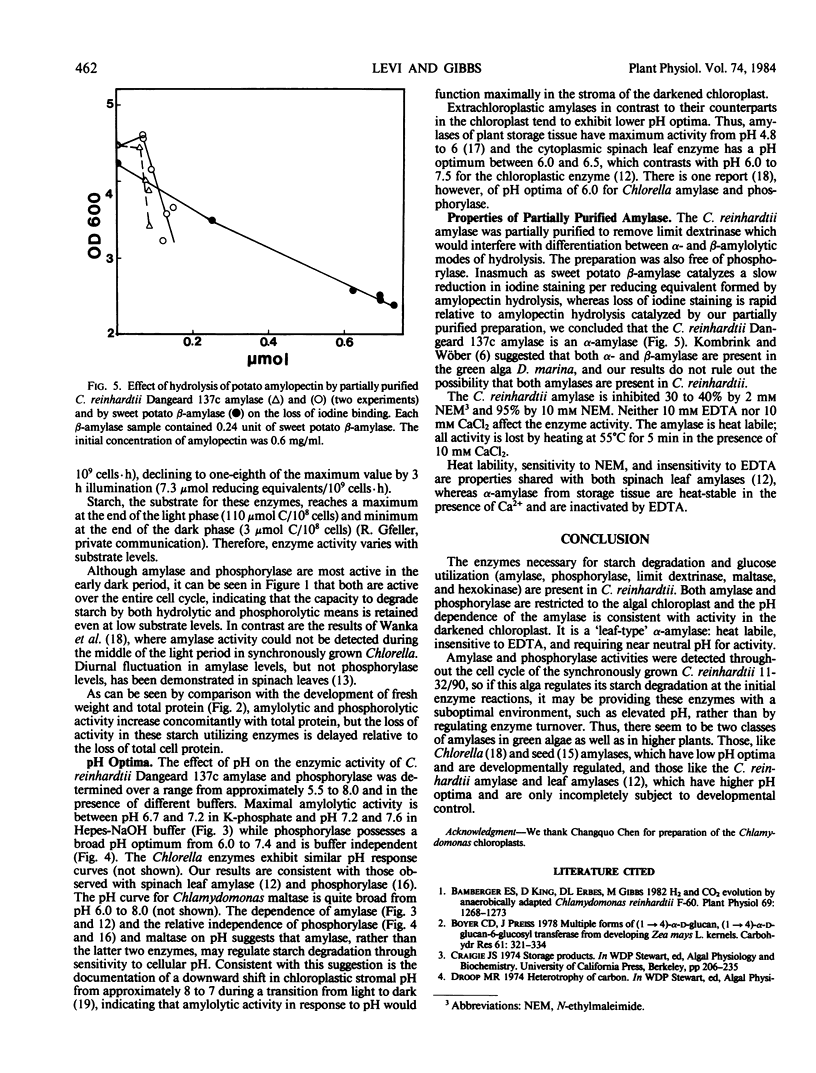
The activities of amylase and phosphorylase were monitored during the 12-hour light/dark synchronous cell cycle of autotrophically grown Chlamydomonas reinhardtii 11-32/90. The activity of amylase increased from 7.3 to 42 micromole reducing equivalents per 109 cells per hour while phosphorylase increased from 43 to 214 micromole glucose 1-phosphate released per 109 cells per hour between the midlight and middark periods. Cellular fractionation indicated that both enzymes were localized solely within the chloroplast. The pH optima for amylase and phosphorylase were 6.7 to 7.6 and 6.0 to 7.4, respectively. The amylase is a heat-labile α-amylase which is insensitive to ethylenetetraaecetate but inhibited by N-ethylmaleimide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., King D., Erbes D. L., Gibbs M. H(2) and CO(2) Evolution by Anaerobically Adapted Chlamydomonas reinhardtii F-60. Plant Physiol. 1982 Jun;69(6):1268–1273. doi: 10.1104/pp.69.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M., Platt-Aloia K. A. Cellular Fractionation of Chlamydomonas reinhardii with Emphasis on the Isolation of the Chloroplast. Plant Physiol. 1983 Jun;72(2):481–487. doi: 10.1104/pp.72.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Preiss J. Starch Degradation in Spinach Leaves: ISOLATION AND CHARACTERIZATION OF THE AMYLASES AND R-ENZYME OF SPINACH LEAVES. Plant Physiol. 1980 Nov;66(5):870–876. doi: 10.1104/pp.66.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz P., Beck E. Diurnal oscillation of amylolytic activity in spinach chloroplasts. Plant Physiol. 1978 Nov;62(5):687–689. doi: 10.1104/pp.62.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Okita T. W., Greenberg E. Characterization of the spinach leaf phosphorylases. Plant Physiol. 1980 Nov;66(5):864–869. doi: 10.1104/pp.66.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


