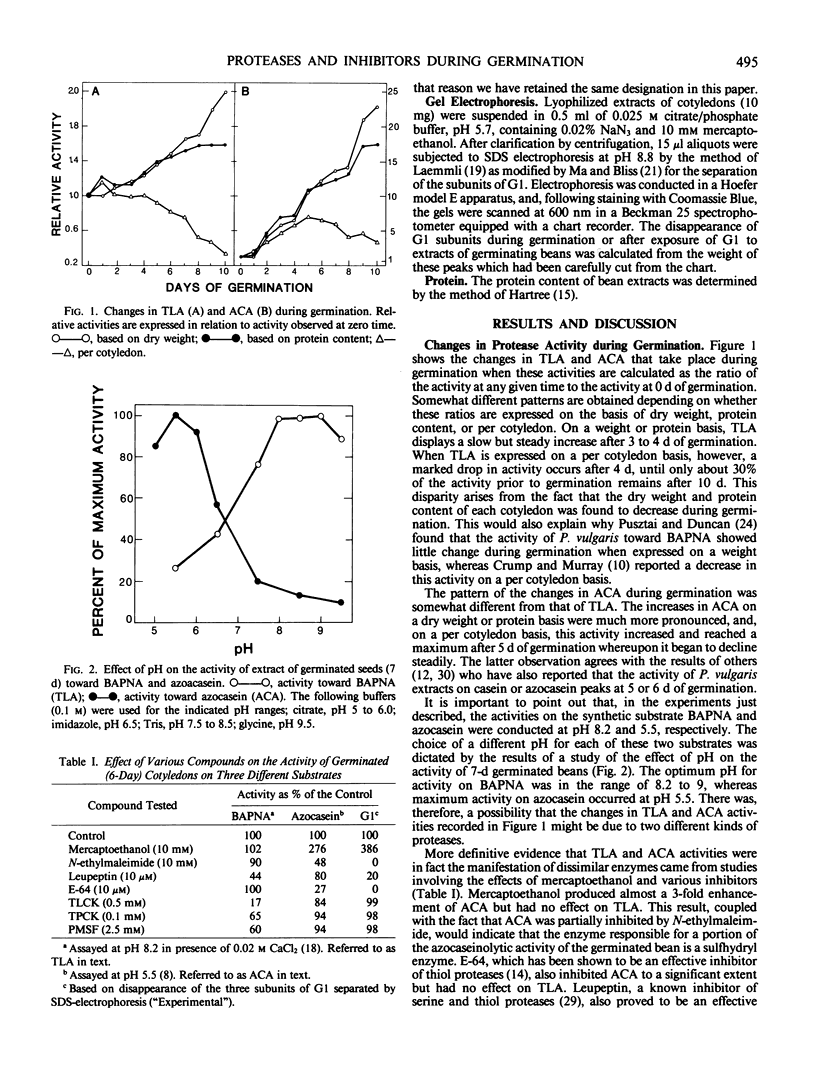
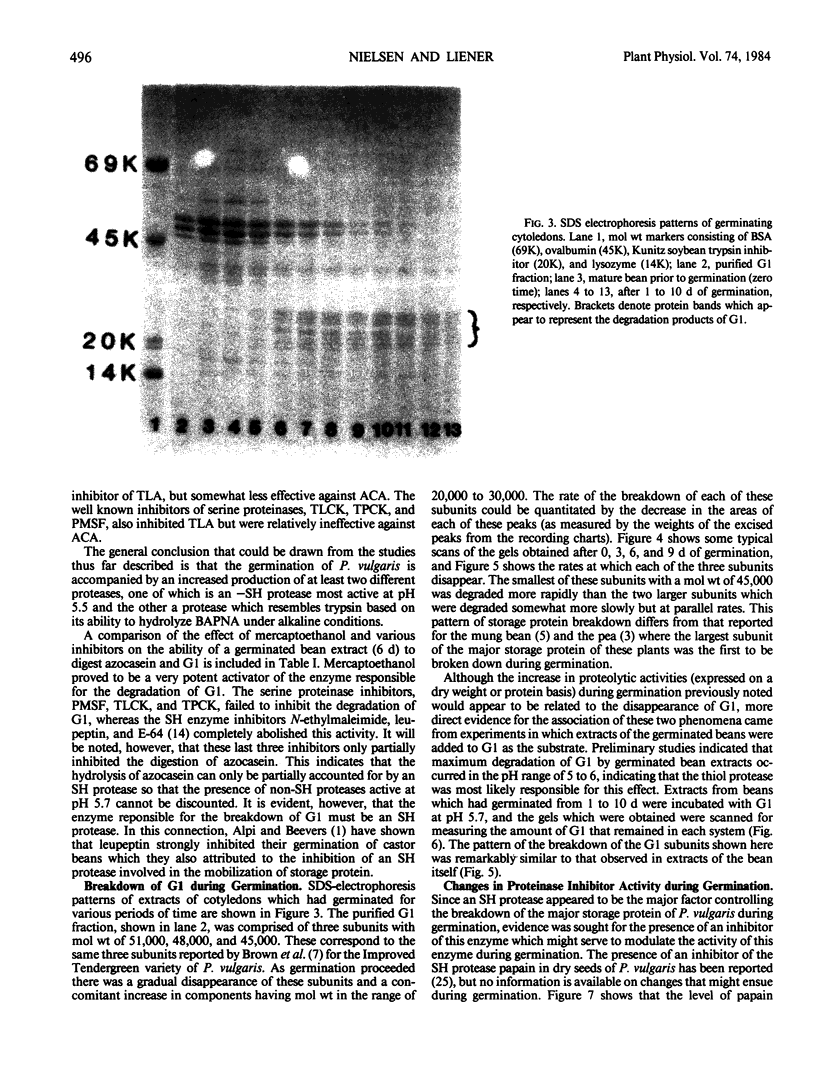
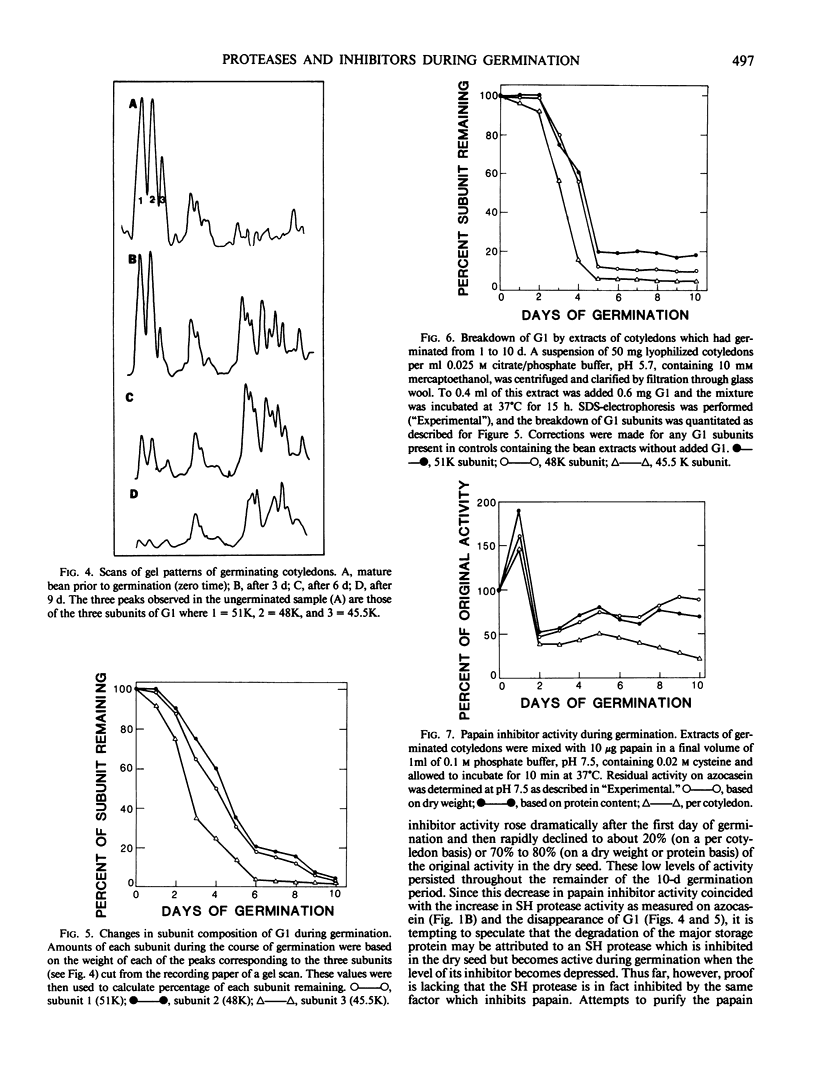
Abstract
Cotyledons from Phaseolus vulgaris L. (var. Improved Tendergreen) were tested for their activity on α-N-benzoyl-dl-arginine-p-nitroanilide (BAPNA) and azocasein during a germination periood of 10 days. Both activities increased throughout germination when activity was expressed on the basis of dry weight or protein. That these two activities were most likely due to the action of different enzymes was indicated by the fact that (a) optimal pH for the hydrolysis of BAPNA and azocasein was 8.2 and 5.5, respectively, and (b) the digestion of azocasein was considerably enhanced by mercaptoethanol and partially inhibited by thiol protease inhibitors, N-ethylmaleimide, and E-64, whereas these same regents caused little change in activity toward BAPNA. The three subunits of the major storage protein, G1, disappeared during germination and were accompanied by the accumulation of lower molecular weight products. The breakdown of G1 by extracts of the germinated beans could be demonstrated in vitro at pH 5 to 6. This activity was enhanced by mercaptoethanol and completely abolished by N-ethylmalemide, leupeptin, and E-64. It is concluded that a thiol protease with an acid pH optimum is primarily responsible for the disappearance of the major storage protein during germination. Although an inhibitor of the plant thiol protease, papain, is present in the mature bean and decreases during germination, its role in the control of the breakdown of the storage protein remains to be elucidated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpi A., Beevers H. Effects of leupeptin on proteinase and germination of castor beans. Plant Physiol. 1981 Oct;68(4):851–853. doi: 10.1104/pp.68.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B., Chrispeels M. J. Partial characterization of a protease inhibitor which inhibits the major endopeptidase present in the cotyledons of mung beans. Plant Physiol. 1976 Jul;58(1):1–6. doi: 10.1104/pp.58.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B., Chrispeels M. J. Purification and characterization of vicilin peptidohydrolase, the major endopeptidase in the cotyledons of mung-bean seedlings. Eur J Biochem. 1977 Jul 15;77(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11661.x. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Ishino K., Ortega M. L. Fractionation and characterization of major reserve proteins from seeds of Phaseolus vulgaris. J Agric Food Chem. 1975 May-Jun;23(3):529–533. doi: 10.1021/jf60199a019. [DOI] [PubMed] [Google Scholar]
- JAFFE W. G., HANNIG K. FRACTIONATION OF PROTEINS FROM KIDNEY BEANS (PHASEOLUS VULGARIS). Arch Biochem Biophys. 1965 Jan;109:80–91. doi: 10.1016/0003-9861(65)90290-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Palmer R., McIntosh A., Pusztai A. The nutrition evaluation of kidney beans (Phaseolus vulgaris). The effect of nutritional value of seed germination and changes in trypsin inhibitor content. J Sci Food Agric. 1973 Aug;24(8):937–944. doi: 10.1002/jsfa.2740240811. [DOI] [PubMed] [Google Scholar]
- Rele M. V., Vartak H. G., Jagannathan V. Proteinase inhibitors from Vigna unguiculata subsp. cylindrica. I. Occurrence of thiol proteinase inhibitors in plants and purification from Vigna unguiculata subsp. cylindrica. Arch Biochem Biophys. 1980 Oct 1;204(1):117–128. doi: 10.1016/0003-9861(80)90013-2. [DOI] [PubMed] [Google Scholar]
- Romero J., Ryan D. S. Susceptibility of the major storage protein of the bean, Phaseolus vulgaris L., to in vitro enzymatic hydrolysis. J Agric Food Chem. 1978 Jul-Aug;26(4):784–788. doi: 10.1021/jf60218a037. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Low-molecular-weight enzyme inhibitors of microbial origin. Annu Rev Microbiol. 1982;36:75–99. doi: 10.1146/annurev.mi.36.100182.000451. [DOI] [PubMed] [Google Scholar]
- Yomo H., Srinivasan K. Protein Breakdown and Formation of Protease in Attached and Detached Cotyledons of Phaseolus vulgaris L. Plant Physiol. 1973 Dec;52(6):671–673. doi: 10.1104/pp.52.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]