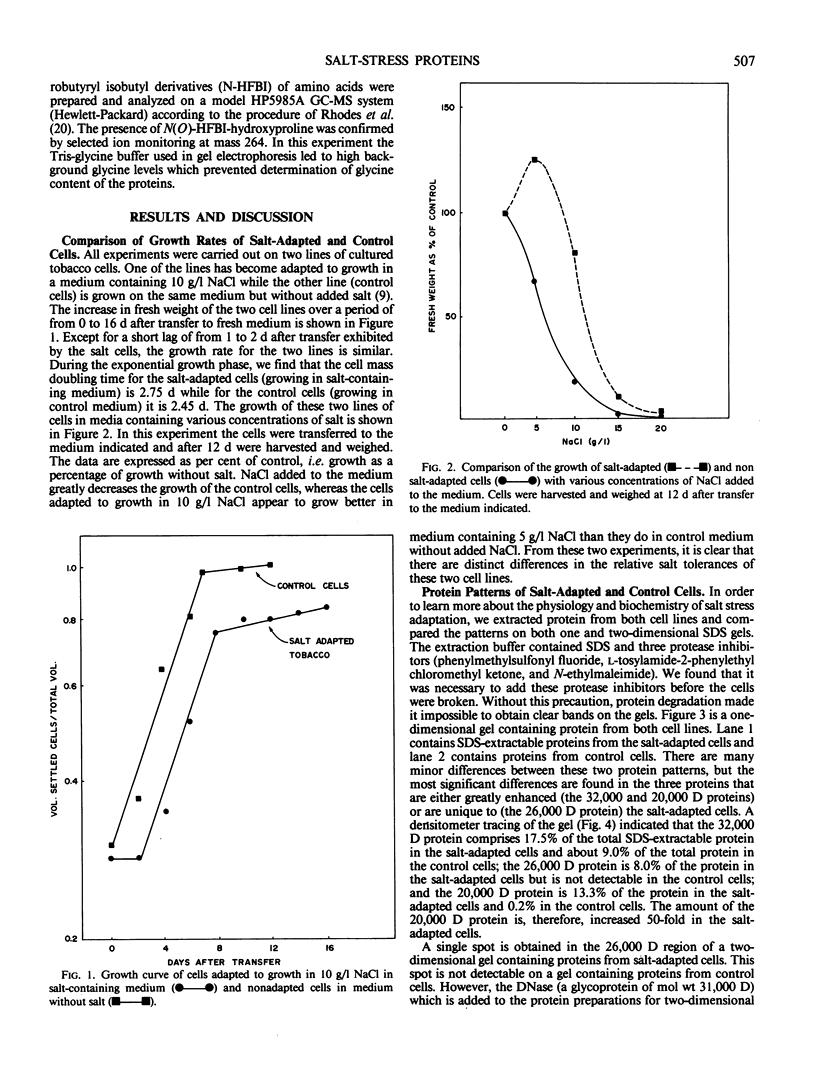
Abstract
The protein pattern of cultured tobacco (Nicotiana tabacum L. var Wisconsin 38) cells that have become adapted to a medium containing 10 grams NaCl per liter was compared to that of unadapted cells on one-dimensional sodium dodecyl sulfate gels. Two protein bands (32,000 and 20,000 daltons) were much more abundant in the salt-adapted cells, and one protein (26,000 daltons) was unique to the salt cells. This protein pattern did not change during the growth cycle of the cells. When salt-adapted cells are transferred to control medium, their ability to grow in the salt-containing medium returns to that of control cells after one passage in the control medium (Hasegawa, Bressan, Handa 1980 Plant Cell Physiol 21: 1347). Within this time the levels of the 32,000 and 20,000 dalton proteins also return to that of the control cells, but the 26,000 dalton protein does not disappear until after at least two passages in control medium. Amino acid analyses of these three proteins revealed that they all contain some hydroxyproline.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S., Parsons M. F. Direct selection in vitro for herbicide-resistant mutants of Nicotiana tabacum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5104–5107. doi: 10.1073/pnas.75.10.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Shepard J. F., Bidney D., Shahin E. Potato protoplasts in crop improvement. Science. 1980 Apr 4;208(4439):17–24. doi: 10.1126/science.208.4439.17. [DOI] [PubMed] [Google Scholar]





