Abstract
Cryptosporidiosis is a serious intestinal disease affecting mal-nourished children and immunocompromised individuals with severe fatal diarrhea. Our present work was done to evaluate the possible curative effects of different essential oils (Mint, Thyme, Chamomile and Basil) on Cryptosporidium parvum (C. parvum) in vivo compared with nitazoxanide (NTZ). Seventy immunosuppressed white Albino male mice were allocated in 7 groups as follows: group I infected and not treated (Positive control), group II (GII) treated with NTZ, group III (GIII) treated with Mint essential oil, group IV (GIV) treated with Thyme essential oil, group V (GV) treated with Chamomile essential oil, group VI (GVI) treated with Basil essential oil and group VII (GVII) naïve not infected mice (Negative control). Evaluation was done using parasitological, histopatholgical, serological as well as biochemical methods. All study groups revealed significant reduction (P value < 0.01) in the mean number of C. parvum oocysts in stool. Results of GII were the best with 87.7% reduction in the oocysts count followed by GIII (77.9%), GIV (74.7%), GVI (68.2%) and lastly GV (67.2%). Improvement of the histopathological damage in the small intestine was shown in treated groups. All treated mice showed significant upregulation in the interferon gamma (IFN-γ) levels, significant reduction in the malondialdehyde (MDA) levels and increase in superoxide dismutase (SOD) levels (P value < 0.0001). It is concluded that Mint, Thyme, Chamomile and Basil oils showed promising anti-cryptosporidial, anti-inflammatory and antioxidant functions.
Keywords: Cryptosporidium parvum, Nitazoxanide, Mint, Chamomile, Thyme, Basil
Introduction
Cryptosporidiosis is a zoonotic disease caused by the obligate intracellular intestinal protozoon Cryptosporidium parvum (C. parvum). It affects humans, ruminants and a diversity of many other animals (Craighead et al. 2021). Cryptosporidiosis can result in severe fatal diarrhea in mal-nourished children and immunocompromised individuals (El-Ashkar et al. 2022).
Innate immunity plays a great role to control cryptosporidiosis, which invades enterocytes. This depends mainly on the IFN-γ formation. The enterocytes produce interleukin-18 (IL-18) which synergizes with IL-12 to encourage the innate lymphoid cell (ILC) to produce IFN-γ. IFN-γ acts on enterocytes for early restriction of the parasite growth (Gullicksrud et al. 2022).
Nitazoxanide (NTZ) is the currently used drug in the cryptosporidiosis treatment that was approved by the Food and Drug Administration (FDA). Unfortunately, NTZ has unsatisfactory therapeutic effect in immunosuppressed patients and very low cure rates (56%) in mal-nourished children (Taha et al. 2017). Few other drugs are available for C. parvum treatment such as azithromycin, roxithromycin, paromomycin (Gargala 2008). However, drug toxicity, resistance, limited efficacy and restricted availability in developing countries were reported. Therefore, the urgent need for finding a novel, safe, effective and inexpensive treatment for these patients is still established (Abdelmaksoud et al. 2020). This need stimulated the scientists to investigate the natural medicinal herbs especially ones that had been known to be used traditionally for parasitic infections (Mendonça et al. 2021).
Mentha leaves, also known as mint or peppermint, are widely distributed worldwide with a diversity of uses. They are used in herbal teas, as food additives or as spices (Baser et al. 1999). Mint oil contains numerous nutrients and minerals like manganese, iron, magnesium, folate, calcium, potassium, copper, omega-3 fatty acids, Vitamin C and Vitamin A. Also it is rich in menthol, menthyl acetate, menthone, pulegone, piperitone, limonene, and carvone as volatile bioactive compounds (Kligler and Chaudhary 2007; McKay and Blumberg 2006).
Mint oil is known to have anti-inflammatory, anti-diarrheal, spasmolytic, antiemetic, anti-parasitic, antibacterial, anti-depressive, diaphoretic, analgesic, antioxidant, keratoprotective and hepatoprotective properties (İşcan et al. 2002; Kiran and Patra 2002; Farzaei et al. 2017). It also showed an effect in relieving non-ulcer dyspepsia and irritable bowel syndrome (IBS). Moreover, it showed antimicrobial properties on both gram positive and gram negative bacteria (Mahadevappa et al. 2014). Mint oil acts to strengthen the immune system and it can be used as a mosquito repellent (Iscan et al. 2002). It also showed antifoaming and choleretic effects that support its use in medicinal field (Mahadevappa et al. 2014).
Thyme (Thymus vulgaris) has been known to be used in traditional medication as anti-inflammatory, expectorant, antiseptic, antibacterial, anti-parasitic and antioxidant. These effects are mainly due to the functions of the thymol and carvacrol constituents (Burt 2004). Recent studies have showed its antifungal, antiviral, anti-leishmanial, and anticancer effects (Kowalczyk et al. 2020). Essential oils of thyme and carvacrol were tested against C. parvum in vitro with promising results (Gaur et al. 2018).
Chamomile (Matricaria chamomilla) is traditionally used in medicinal treatment for its analgesic and anti-inflammatory activities. It is widely consumed as a tea (Speisky et al. 2006). It is used to treat wounds, ulcers, eczema and gastrointestinal manifestations like diarrhea and vomiting (Díaz et al. 2014). Many flavonoids had been found in the chamomile composition like apigenin, luteolin, patuletin and quercetin. Other constituents include terpenoids, sequiterpenes and chamazulene (Raal et al. 2012).
Basil (Ocimum basilicum) essential oil main components are terpenes and phenylpropanoids. It also contains linalool, phenolic compounds, alcoholic compounds as well as aldehydes (Mahmoudi et al. 2020; Milenković et al. 2019). Many studies had reported the therapeutic properties of basil essential oil including antimicrobial, insect repelling, antifungal, anti-infammatory, antioxidant and anticancer activities (da Silva et al. 2022).
So, our present study was performed to evaluate the potential therapeutic effects of different essential oils (Mint, Thyme, Chamomile and Basil) on C. parvum in vivo compared with NTZ.
Aim of the work
Evaluation of the potential curative and therapeutic role of Mint, Thyme, Chamomile and Basil essential oils in treating immunosuppressed mice experimentally infected with C. parvum oocysts in comparison with the reference drug NTZ.
Materials and methods
Animals
Seventy Swiss Albino laboratory-bred male mice were used in this study. Their age was 4–6 weeks old and they measured 20–25 g in weight. All experiments were performed in well—ventilated conditioned places away from direct sunlight, in plastic cages provided with clean beddings.
Experimental design
Seventy mice were divided into seven groups (ten mice each):
Group I (Positive control) immunosuppressed, infected and not treated.
Group II (Drug control) immunosuppressed, infected and treated with NTZ.
Group III (Mint) immunosuppressed, infected and treated with Mint essential oil.
Group IV (Thyme) immunosuppressed, infected and treated with Thyme essential oil.
Group V (Chamomile) immunosuppressed, infected and treated with Chamomile essential oil.
Group VI (Basil) immunosuppressed, infected and treated with Basil essential oil.
Group VII (Negative control) immunosuppressed and non-infected.
Induction of immunosuppression
Mice were immunosuppressed by administration of dexamethasone (Dexazone, Kahira Pharmaceuticals and Chemical Industries Company—Egypt). Each mouse received an oral dose of 0.25 ug/g/day of dexamethazone via esophageal tube. The drug was given for 14 days prior to induction of C. parvum infection. Mice received dexamethazone for the whole experiment period (Rehquel et al. 1998).
C. parvum oocysts identification
C. parvum oocysts were purchased from the Theodore Bilharz Research Institute (TBRI) in Giza, Egypt. Samples were identified by PCR to identify C. parvum. DNA isolation was done by (QIA amp DNA Stool Mini Kit QIAGEN, Hilden, Germany) extraction kit. DNA was then amplified by quantification of 18S ribosomal gene of Cryptosporidium following the manufacturer instructions (Abdelhamed et al. 2019).
Induction of Infection
Infection was performed via oral inoculation of oocysts using esophageal tube. Each mouse received 0.1 ml of the oocysts’ inoculum containing 103 C. parvum oocysts/ml dissolved in 200 μl of phosphate buffered saline (PBS) (Abdelmaksoud et al. 2020). Mice feces were collected daily and examined for C. parvum oocysts to insure mice infection. Mice were confirmed to be infected at the 4th day after inoculation. The treatment started at the 5th day post infection (p.i). All drugs were given orally using esophageal tube.
Treatment
Nitazoxanide (NTZ) (drug control)
Nanazoxid, Utopia Pharmaceutical Company, Egypt was used. It was obtained as 100 mg/5 ml suspension. NTZ was given at a dose of 10 mg/kg/day for successive 14 days (El-Sayed and Fathy 2019).
Essential oils
Mint, Thyme, Chamomile and Basil essential oils were purchased in their commercial pharmaceutical form from a local market in Giza, Egypt. Mint essential oil was given at a dose of 20 mg/kg/day for successive 14 days (Salin et al. 2011). Thyme essential oil was given at a dose of 15 μg/kg/day for successive 14 days (Farrag et al. 2021). Chamomile essential oil was given at a dose of 1000 mg/kg/day for successive 14 days (Corpas-Lopez et al. 2015). Basil essential oil was given at a dose of 500 mg/kg/day for successive 14 days (Uraku et al. 2015).
Mice scarification
Mice were sacrificed at 19th day p.i. They received intraperitoneal anesthetic (500 mg/kg thiopental)—anticoagulant (100 units/ml heparin) according to Liang et al. (1987). Blood samples were collected for assessment of IFN-γ levels in the sera. Parts of jejunum were removed to be subjected to biochemical analysis and ileal parts were dissected for histopathological examination in all groups.
Parasitological evaluation
Stool pellets were collected and stained with Modified Zheil Nelsen (MZN) stain for parasitological examination (Henricksen and Pohlenz 1981). Samples were examined under the oil immersion lens (X 100). Examination was done for confirmation of the mice infection and for counting C. parvum oocysts.
Histopathological evaluation
Ileal segments underwent dissection and fixation in formalin (10%) solution. After that, embedding in paraffin wax blocks was done. Sections were performed and stained with Hematoxylin and Eosin (H&E).
Evaluation of IFN-γ levels
Serum samples were subjected to sandwich Enzyme-Linked Immunosorbent Assay (ELISA) using (Rat IFN Gamma ELISA Kit PicoKine™, Catalog number: EK0374) kit. The steps were done following the manufacturer’s guidlines. The values of IFN-γ were measured from the standard curve. The detection ranged from 31.2 to 2000 pg/ml.
Biochemical analysis
Samples from jejunum were perfused prior to dissection with PBS solution (pH 7.4) with 0.16 mg/ml heparin to remove excess RBCs. After that, tissues were homogenized in 5–10 ml cold buffer (100 mM potassium phosphate (pH 7.0) that contains 2 mM EDTA)/gram tissue. Then centrifuged at 4000 rpm for 15 min at 4 °C, collection and storage of supernatants at − 20 °C was done till use. A volume of 1.0 ml of supernatant was mixed with 0.5 ml ice-cold extraction reagent in a test tube, vortexed for 30 s and then centrifuge at 4000 rpm at 4 °C for 10 min. Then, the uppermost aqueous layer was collected and assayed colorimetrically. The superoxide dismutase (SOD) and malondialdehyde (MDA) levels were measured using (CAT. No. SD 25 21 and CAT. No. MD 25 29 respectively) kits. SOD activity measures were expressed in U/g tissue while MDA value was expressed in nmol/g tissue.
Ethics statement
Our study protocol was approved and carried out according to the guidelines of the Laboratory Animal Centre for Research Ethics Committee at Faculty of Medicine, Tanta University (code number: 35947/10/22).
Statistical analysis
Data were expressed as mean and standard deviation (± SD). Student t test was performed using GraphPad software for assessment of the statistical significance among all groups. P value was considered significant at < 0.01.
Results
Parasitological evaluation
All study groups (GII, GIII, GIV, GV and GVI) revealed statistically highly significant decrease (P value < 0.01) in the C. parvum oocysts count compared to GI (Positive Control). The best result was shown with GII (treated with NTZ) with percentage reduction 87.7% followed by GIII, GIV, GVI and the least percentage reduction was shown in GV that was 67.2% (Table 1).
Table 1.
The results of C. parvum oocysts (× 103) counting
| Group | Mean ± SD | % Reduction | P value |
|---|---|---|---|
| GI positive control | 25.7 ± 7.6 | 0 | < 0.01 |
| GII (NTZ)* | 3.2 ± 0.7 | 87.7 | |
| GIII (Mint Oil)* | 5.7 ± 2.0 | 77.9 | |
| GIV (Thyme oil)*N | 6.5 ± 0.9 | 74.7 | |
| GV (Chamomile oil)*N | 8.4 ± 1.2 | 67.2 | |
| GVI (Basil oil)*N | 8.1 ± 0.7 | 68.2 |
*Statistically significant (P value < 0.01) compared to GI (Positive control), N statistically significant (P value < 0.01) compared to GII (NTZ treated)
Histopathological evaluation
GVII (negative control)
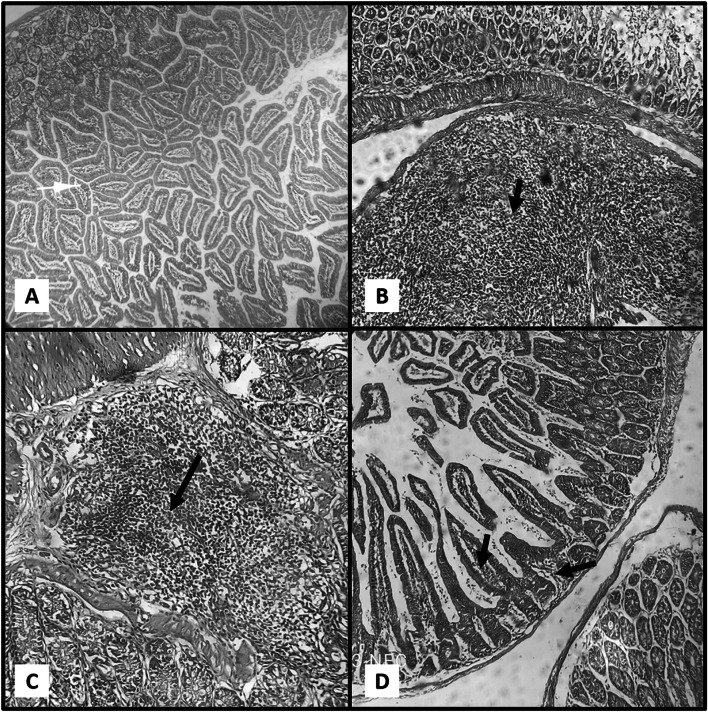
Ileal sections examination of the negative control group showed intestinal villi with preserved architecture (retained average villous/crypt length ratio with intact brush border) and normal mucin secretion Fig. 1a
Fig. 1.
a Section of Ileum in GVII (Negative Control) showing normal villous architecture. b, c Sections of Ileum in GI (Positive Control) showing pathological changes with marked inflammatory infiltrate in the lamina propria ‘black arrows’. d Section of Ileum in GII (NTZ) revealed healing of intestinal villi with slight inflammatory infiltrate ‘black arrows’ (X 10 power—H&E stain)
GI (positive control)
Ileal sections examination of the positive control group (infected non-treated) revealed villous shortening and broadening. There were mucosal ulcerations as well as focal mucin depletion. Lamina propria showed inflammatory cellular infiltrate mainly lymphocytes, macrophages and plasma cells. Also, hyperplasia of lymphoid follicles was seen Fig. 1b and c
GII (NTZ treated) and GIII (mint oil treated)
Ileal sections examination of the NTZ treated and Mint Oil treated groups revealed marked improvement of the histopathological damage resulted from C. parvum infection. The normal architecture of the villi was restored. Healing of the mucosa occurred with intact surface epithelium apart from minimal surface erosions. Lamina propria showed slight inflammatory cellular infiltration Fig. 1d and 2a
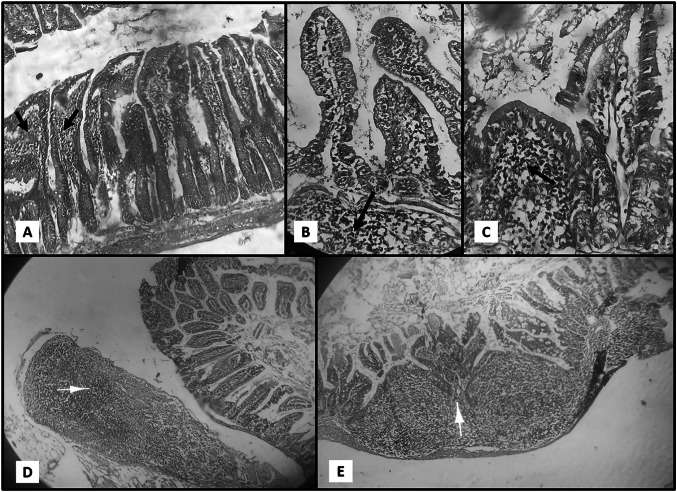
Fig. 2.
a Section of Ileum in GIII (Mint) revealing intestinal improving with slight inflammatory infiltrate ‘black arrows’. b Sections of Ileum in GIV (Thyme) partial healing with mild inflammatory cellular infiltrate in the lamina propria ‘black arrow’. c Section of Ileum in GV (Chamomile) revealing partial healing with some broad villi and mild inflammatory infiltrate in the lamina propria ‘black arrow’. d Section of Ileum in GV (Chamomile) revealing inflammatory infiltrate ‘white arrow’. e Section of Ileum in GVI (Basil) showed minimal healing of intestinal villi with moderate inflammatory infiltrate ‘white arrow’ (X 10 power—H&E stain)
GIV (thyme oil treated) and GV (chamomile oil treated)
Ileal sections examination of the Thyme Oil treated and Chamomile Oil treated groups revealed partial improvement of the histopathological damage resulted from C. parvum infection. The villi were blunted. Partial healing of the mucosa occurred with focal ulcerations. Lamina propria showed mild inflammatory cellular infiltration Fig. 2b c and d
GVI (basil oil treated)
Ileal sections examination of the Basil Oil treated group revealed only slight improvement of the histopathological damage resulted from C. parvum infection. Small intestinal villi were blunt and broad. Intestinal mucosa showed partial healing. Lamina propria showed moderate inflammatory cellular infiltration Fig. 2e
Serological evaluation
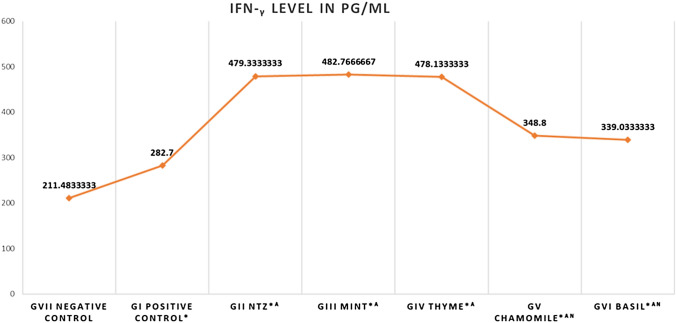
IFN-γ levels showed significant increase in C. parvum—infected mice (282.7 ± 5.8) compared to the negative control ones (211.5 ± 5.5) (P value < 0.0001). All treated mice showed significant upregulation in the IFN-γ with mean levels reaching 482.8 ± 7.7 (P value < 0.0001) Fig. 3
Fig. 3.
IFN-γ levels in all groups in pg/ml. *Statistically significant (P value < 0.0001) compared to GVII (Negative control), A statistically significant (P value < 0.0001) compared to GI (Positive control), N statistically significant (P value < 0.0001) compared to GII (NTZ treated)
Biochemical analysis evaluation
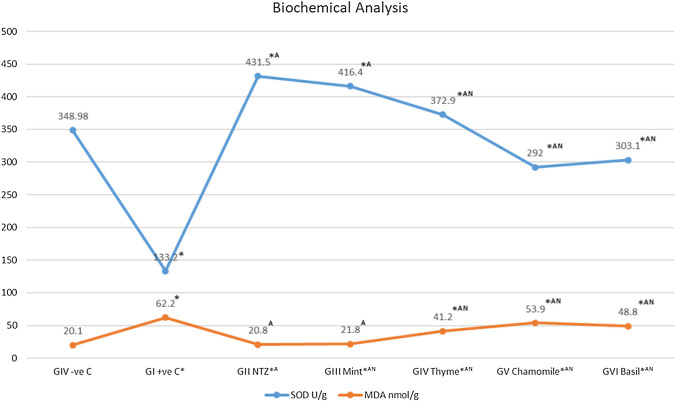
The levels of MDA showed increase in the infected mice with C. parvum oocysts compared to those non- infected (62.2 ± 2.5 vs. 20.1 ± 2). While the levels of SOD were decreased in the infected mice with C. parvum oocysts compared to those non- infected (133.2 ± 3.6 vs. 348.9 ± 9.5). All treated groups of infected mice significantly reduced the MDA levels and increased SOD level compared to the infection control group (P value < 0.0001) Fig. 4
Fig. 4.
SOD (U/g tissue) and MDA (nmol/g tissue) levels in all groups. *Statistically significant (P value < 0.0001) compared to GVII (Negative control), A statistically significant (P value < 0.0001) compared to GI (Positive control), N statistically significant (P value < 0.0001) compared to GII (NTZ treated)
Discussion
C. parvum causes severe fatal diarrhea in immunosuppressed individuals (Farid et al. 2022). Unfortunately, the available drugs for cryptosporidiosis treatment show many drawbacks with increasing resistance. So, the researchers started to search for a new alternative drug preferably from natural source to be less toxic and can be produced easily in adequate quantities (Mendonça et al. 2021).
Our results revealed that treatment with Mint, Thyme, Chamomile and Basil oils could significantly reduce C. parvum oocysts shedding in experimentally immunosuppressed infected mice with percentage reduction in oocysts number 77.9%, 74.7%, 67.2% and 68.2% respectively. However, the best percentage reduction was shown with NTZ (87.7%) and the mean number of shedded oocysts in the NTZ group was statistically significant compared to Thyme, Chamomile and Basil oil groups.
The groups treated with NTZ and Mint oil showed marked improvement in the histopathological damage of the intestinal villi following C. parvum infection. Partial healing of the intestinal damage was shown in the groups treated with Thyme and Chamomile oils while only slight improvement was seen in the group treated with Basil oil.
IFN-γ is the major cytokine that is upregulated in cryptosporidiosis infection to enhance innate as well as acquired immunity. Early C. parvum infection induces secretion of IFN-γ by natural killer (NK) cells, dendritic cells and macrophages (Borad and Ward 2010). In our study, all infected mice with C. parvum oocysts induced cellular inflammatory response in the intestinal tissues with significant upregulation of IFN-γ levels. This was in agreement with (Lean et al. 2002; Tessema et al. 2009; El-Sayed and Fathy 2019). However, the groups treated with Chamomile and Basil oils showed statistically significant decrease in IFN-γ levels compared to the group treated with NTZ.
Reactive oxygen species (ROS) production in adequate levels is important for maintaining the redox balance, however, overproducing ROS and other free radicals’ initiates oxidative stress leading to cell destruction and death (Poljsak et al. 2013; Di Meo et al. 2016). Antioxidants help protecting humans and animals against infectious diseases by inhibiting and scavenging the free radicals. In our study, it was shown that the experimental infection with C. parvum resulted in increase in the MDA levels and decline levels of SOD. This was agreeing with (Bhagat et al. 2017). All treated groups of infected mice significantly decreased the MDA and elevated the SOD levels in comparison with the infection control group. However, significant improvement in MDA and SOD levels was observed in the NTZ group in comparison with the Thyme, Chamomile and Basil oils groups.
Generally, plant extracts are used for animal feeding as antioxidants to protect them from free radicals’ harm effects (Liu et al. 2018). They act by binding to free radicals to scavenge them, chelating metals, donating atoms of hydrogen atoms and suppressing the action of pro-oxidative enzymes (Brown et al. 2019; Dorman et al. 2003).
In our study, Mint oil showed marked improvement in the small intestinal histopathological changes and elevation in the IFN-γ levels. This was agreeing with Hejna et al. (2021), who revealed that Mint oils have the ability to inhibit pro-inflammatory cytokines production revealing high therapeutic potential and control of the inflammatory response. Also, in the present work, Mint oil succeeded to decrease the MDA levels and elevate the SOD levels in the small intestinal tissues. These effects can be explained by the phenolic and flavonoid constituents of Mint oil that can donate hydrogen or electrons to the free radicals inhibiting formation of hydroxyl peroxide and controlling oxidative stress damage (Djeridane et al. 2006; Krzyzanowska et al. 2011). This was in agreement with Teixeira et al. (2013); Nickavar et al. (2010); Wu et al. (2019); Hejna et al. (2021), who confirmed the antioxidant effects of Mint oil.
In our work, Thyme oil showed partial improving in the small intestinal histopathological damages and significant elevation in the IFN-γ levels. Also, Thyme oil succeeded to decrease the MDA levels and elevate the SOD levels in the small intestinal tissues. However, the anti-oxidant markers levels of the reference drug (NTZ) were significantly better than the levels obtained by Thyme oil treatment.
Thyme was reported to have anti-coccidial effects (Abbas et al. 2012; Remmal et al. 2011). Gaur et al. (2018) had tested the Thyme extract efficacy against C. parvum in vitro with satisfying results. Thyme was reported to have anti-Cryposporidial effects reducing their development and suppressing the parasites’ invasion capacities. These effects owe to the thymol and carvacrol constituents of Thyme. They affect Calcium ions mediated transmitted signals. Also, they inhibit ATP synthesis and enzyme activity (Bessoff et al. 2013; Murphy et al. 2010). Moreover, Kara et al. (2022), had examined Thyme extract against C. parvum in rats and concluded that Thyme extract effectively treated as well as prevented prophylactically the C. parvum infection with no signs of toxicity.
In the present work, Chamomile oil showed partial improving in the small intestinal histopathological pathology and significant elevation in the IFN-γ levels. Also, Chamomile oil succeeded to decrease the MDA levels and elevate the SOD levels in the small intestinal tissues. However, the IFN-γ levels and the anti-oxidant markers levels of the reference drug (NTZ) were significantly better than the levels obtained by Chamomile oil treatment.
Previous studies confirmed the antioxidant and anti-parasitic properties of Chamomile (Lee and Shibamoto 2002). These effects are most probably due to the Chamomile components having anti-inflammatory properties like α-bisabolol, chamazulene and apigenin that act against pro-inflammatory mediators (Srivastava et al. 2009). Bisabolol and bisabolol oxide show anti-5-lipoxygenase effect (Braga et al. 2009). Chamomile extract was shown to inhibit the PGE2 release by inhibiting Cyclooxygenase-2 (COX-2) enzyme activity and decreasing the expression of COX-2 mRNA and proteins (Srivastava et al. 2009). Sabatke et al. (2022) reported that polysaccharides that are present in chamomile tea showed a synergistic effect with NTZ against Giardia intestinalis. It acts synergistically with NTZ to increase its efficacy and decrease its therapeutic dose. They contributed together and inhibited parasitic adhesion to intestinal cells.
Chamomile essential oil was tested against Leishmania promastigotes in vitro. It showed activation of apoptosis, membrane damage and decreased total ATP levels in the mitochondria (Hajaji et al. 2018).
In the present study, Basil oil showed only slight improvement in the small intestinal histopathological changes. It significantly elevated the IFN-γ levels in serum. Also, Basil oil succeeded to decrease MDA levels and elevate SOD levels in the small intestinal tissues. However, the IFN-γ levels and the anti-oxidant markers levels of the reference drug (NTZ) were significantly better than the levels obtained by Basil oil treatment.
Basil oil demonstrated anti-infammatory properties owing to its linalool, eugenol and limonene contents that act to inhibit COX-2 enzyme (Złotek et al. 2016).
Basil oil showed anti-oxidant activities that are explained by the synergistic effects between many constituents rather than be referred to a single compound (Tavallali et al. 2019). The mechanisms of action include sequestration of free radicals, acting as hydrogen donors and chelating metal ions. Moreover, Koroch et al. (2017) reported a methylation behavior with the highest anti-oxidant function was linked to linalool-eugenol. The great anti-oxidant potential of the Basil oil is related to the high proportion of composites that contain a phenol ring with an (OH) group.
Basil oil showed antibacterial activity owing to linalool and other components. Anti-Gram-positive bactericidal action was shown against Bacillus cereus, Listeria monocytogenes and Staphylococcus aureus as well as anti-Gram-negative bactericidal activity was revealed against Pseudomonas aeruginosa and Salmonella spp. (Baldim et al. 2018). Linalool is capable of changing cell permeability due to its higher membrane fluidity facilitating other components entry enhancing its activity in a synergistic manner. Basil essential oil was stated by Stanojevic et al. (2019) to possess high bacterial inhibitory effect against Salmonella enterica, Providencia stuartii, Staphylococci and Streptococci compared to ciprofloxacin and gentamicin.
NTZ showed the best results in our study. It markedly succeeded in restoring the normal intestinal villous architecture following C. parvum infection. It also significantly elevated the IFN-γ levels in serum, decreased the MDA levels and elevated the SOD levels in the small intestinal tissues.
NTZ is a broad spectrum anti-parasitic drug. It has 2 main components tizoxanide as well as tizoxanide-glucuronide that were evidenced to decrease the C. parvum growth (Gargala et al. 2000). Excretion of NTZ in bile was proved explaining its efficacy against C. parvum induced cholangitis in immunosuppressed patients. NTZ given for 3 days was reported to be effective in immunocompetent patients while only 59% of AIDS patients that were suffering from cryptosporidiosis showed a sustained clinical cure with maintained NTZ. However, 3000 mg/day NTZ and sustained administration for long duration were unsafe (Rossignol 2006).
Conclusion
The present study showed that essential herbal oils represent a promising alternative for cryptosporidiosis treatment. Mint, Thyme, Basil and Chamomile oils reduced C. parvum oocysts shedding and relived the histopathological intestinal changes. The best results were seen with Mint oil followed by Thyme, Chamomile and Basil oils respectively. Furthermore, study oils succeeded to elevate the IFN-γ levels in serum, increase the SOD levels and decrease the MDA levels in intestinal tissues representing anti-inflammatory and anti-oxidant properties.
Authors' contributions
All authors contributed to the study conception and design. Material preparation and experimental work was done by NMT and BM.E. Data collection and analysis were performed by RSZ. EK prepared histopathological figures and analysis. The first draft of the manuscript was written by NMT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors did not receive support from any organization for the submitted work. The authors have no relevant financial or non-financial interests to disclose.
Availability of data and material
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
Authors declare that there are no any conflicts of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Our study protocol was approved and carried out according to the guidelines of the Laboratory Animal Centre for Research Ethics Committee at Faculty of Medicine, Tanta University (code number: 35947/10/22).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas RZ, Iqbal Z, Khan A, Sindhu ZUD, Khan JA, Khan MN, Raza A. Options for integrated strategies for the control of avian coccidiosis. Int J Agric Biol. 2012;14:1014–1020. [Google Scholar]
- Abdelhamed EF, Fawzy EM, Ahmed SM, Zalat RS, Rashed HE. Effect of nitazoxanide, artesunate loaded polymeric nano fiber and their combination on experimental cryptosporidiosis. Iran J Parasitol. 2019;14(2):240. [PMC free article] [PubMed] [Google Scholar]
- Abdelmaksoud HF, El-Ashkar AM, Elgohary SA, El-Wakil ES. Potential therapeutic and prophylactic effects of Asafoetida in murine cryptosporidiosis. J Parasit Dis. 2020;44(3):646–653. doi: 10.1007/s12639-020-01241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldim JL, Silveira JGF, Almeida AP, Carvalho PLN, Rosa W, Schripsema J, Luiz JHH. The synergistic effects of volatile constituents of Ocimum basilicum against foodborne pathogens. Ind Crops Prod. 2018;112:821–829. doi: 10.1016/j.indcrop.2017.12.016. [DOI] [Google Scholar]
- Baser KHC, Kürkçüoglu M, Tarimcilar G, Kaynak G. Essential oils of Mentha species from Northern Turkey. J Essent Oil Res. 1999;11(5):579–588. doi: 10.1080/10412905.1999.9701218. [DOI] [Google Scholar]
- Bessoff K, Sateriale A, Lee KK, Huston CD. Drug repurposing screen reveals FDA approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob Agents Chemother. 2013;57:1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat M, Sood S, Yadav A, Verma P, Manzoor N, Chakraborty D, Katoch R, Sangha N. Alterations in oxidative stress parameters and its associated correlation with clinical disease on experimental Cryptosporidium parvum infection in Swiss albino mice. J Parasit Dis. 2017;41:707–712. doi: 10.1007/s12639-016-0871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borad A, Ward H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010;5:507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga PC, Dal Sasso M, Fonti E, Culici M. Antioxidant activity of bisabolol: inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology. 2009;83(2):110–115. doi: 10.1159/000186049. [DOI] [PubMed] [Google Scholar]
- Brown N, John JA, Shahidi F. Polyphenol composition and antioxidant potential of mint leaves. Food Prod Process Nutr. 2019;1:1. doi: 10.1186/s43014-019-0001-8. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Corpas-Lopez V, Morillas-Marquez F, Navarro-Moll MC, Merino-Espinosa G, Diaz-Saez V, Martin-Sanchez J. (−)-α-Bisabolol, a promising oral compound for the treatment of visceral leishmaniasis. J Nat Prod. 2015;78(6):1202–1207. doi: 10.1021/np5008697. [DOI] [PubMed] [Google Scholar]
- Craighead S, Huang R, Chen H, Kniel KE. The use of pulsed light to inactivate Cryptosporidium parvum oocysts on high-risk commodities (Cilantro, mesclun lettuce, spinach, and tomatoes) Food Control. 2021;126:107965. doi: 10.1016/j.foodcont.2021.107965. [DOI] [Google Scholar]
- da Silva WMF, Kringel DH, de Souza EJD, da Rosa ZE, Dias ARG. Basil essential oil: methods of extraction, chemical composition, biological activities, and food applications. Food Bioproc Tech. 2022;15(1):1–27. doi: 10.1007/s11947-021-02690-3. [DOI] [Google Scholar]
- Díaz A, Vargas-Perez I, Aguilar-Cruz L, Calva-Rodríguez R, Treviño S, et al. A mixture of chamomile and star anise has anti-motility and antidiarrheal activities in mice. Rev Bras Farmacogn. 2014;24:419–424. doi: 10.1016/j.bjp.2014.07.016. [DOI] [Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- El-Ashkar AM, Mahmoud S, Sabry H, Guirguis N, El Komi W, Ali E, Abdelmksoud HF. Nitazoxanide, Ivermectin, and Artemether effects against cryptosporidiosis in diabetic mice: parasitological, histopathological, and chemical studies. J Parasit Dis. 2022;46(4):1070–1079. doi: 10.1007/s12639-022-01527-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Fathy GM. Prophylactic and therapeutic treatments' effect of moringa oleifera methanol extract on cryptosporidium infection in immunosuppressed mice. Antiinfect Agents. 2019;17(2):130–137. [Google Scholar]
- Farid A, Yousry M, Safwat G. Garlic (Allium sativum Linnaeus) improved inflammation and reduced cryptosporidiosis burden in immunocompromised mice. J Ethnopharmacol. 2022;292:115174. doi: 10.1016/j.jep.2022.115174. [DOI] [PubMed] [Google Scholar]
- Farrag HMM, Yones DA, Hassanin ESA, Ibraheim ZZ, Hussein EEH (2021) Thymus vulgaris, Mentha piperita and Elettaria cardamomum against Trypanosoma evansi in vitro and in an animal model with new insights for the treatment of trypanosomosis. Ann Parasitol 67(1) [DOI] [PubMed]
- Farzaei MH, Bahramsoltani R, Ghobadi A, Farzaei F, Najafi F. Pharmacological activity of Mentha longifolia and its phytoconstituents. J Tradit Chin Med. 2017;37(5):710–720. doi: 10.1016/S0254-6272(17)30327-8. [DOI] [PubMed] [Google Scholar]
- Gargala G. Drug treatment and novel drug target against cryptosporidium. Parasite. 2008;15:275–281. doi: 10.1051/parasite/2008153275. [DOI] [PubMed] [Google Scholar]
- Gargala G, Delaunay A, Li X, Brasseur PH, Favennec L, Ballet JJ. Efficacy of nitazoxanide, tizoxanide and tizoxanide glucuronide against Cryptosporidium parvum development in sporozoite-infected HCT-8 enterocytic cells. J Antimicrob Chemother. 2000;46(1):57–60. doi: 10.1093/jac/46.1.57. [DOI] [PubMed] [Google Scholar]
- Gaur S, Kuhlenschmidt TB, Kuhlenschmidt MS, Andrade JE. Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol Int. 2018;67:170–175. doi: 10.1016/j.parint.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Gullicksrud JA, Sateriale A, Engiles JB, Gibson AR, Shaw S, Hutchins ZA, Hunter CA. Enterocyte–innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium. Mucosal Immunol. 2022;15(2):362–372. doi: 10.1038/s41385-021-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajaji S, Sifaoui I, López-Arencibia A, Reyes-Batlle M, Jiménez IA, Bazzocchi IL, Piñero JE. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol Res. 2018;117(9):2855–2867. doi: 10.1007/s00436-018-5975-7. [DOI] [PubMed] [Google Scholar]
- Hejna M, Kovanda L, Rossi L, Liu Y. Mint oils: in vitro ability to perform anti-inflammatory, antioxidant and antimicrobial activities and to enhance intestinal barrier integrity. Antioxidants. 2021;10(7):1004. doi: 10.3390/antiox10071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricksen S, Pohlenz J. Staining of Cryptosporidium by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İşcan G, Ki̇ri̇mer N, Kürkcüoǧlu M, Başer HC, Demirci F. Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem. 2002;50(14):3943–3946. doi: 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- Kara E, Yasa Duru S, Gökpinar S, Duru Ö, Sevin S, Şenel Y, Kaya U (2022) Investigation of the prophylactic and therapeutic effectiveness of oral thyme extract in rats experimentally infected with Cryptosporidium parvum. Vet Res Commun 1–11 [DOI] [PMC free article] [PubMed]
- Kiran U, Patra DD. Medicinal and aromatic plant materials as nitrification inhibitors for augmenting yield and nitrogen uptake of Japanese mint (Mentha arvensis L. Var. Piperascens) Bioresour Technol. 2002;3:267–276. doi: 10.1016/s0960-8524(02)00143-8. [DOI] [PubMed] [Google Scholar]
- Kligler B, Chaudhary S. Peppermint oil. Am Fam Phys. 2007;75:1027–1030. [PubMed] [Google Scholar]
- Koroch R, Simon JE, Juliani HR. Essential oil com-position of purple basils, their reverted green varieties (Ocimum basilicum) and their associated biological activity. Ind Crops Prod. 2017;107:526–530. doi: 10.1016/j.indcrop.2017.04.066. [DOI] [Google Scholar]
- Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and thyme essential oil—new insights into selected therapeutic applications. Molecules. 2020;25(18):4125. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowska J, Janda B, Pecio L, Stochmal A, Oleszek W, Czubacka A, Przybys M, Doroszewska T. Determination of polyphenols in Mentha Longifolia and M. Piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J AOAC Int. 2011;94:43–50. doi: 10.1093/jaoac/94.1.43. [DOI] [PubMed] [Google Scholar]
- Lean IS, McDonald V, Pollok RC. The role of cytokines in the pathogenesis of Cryptosporidium infection. Curr Opin Infect Dis. 2002;15:229–234. doi: 10.1097/00001432-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Lee KG, Shibamoto T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J Agric Food Chem. 2002;50(17):4947–4952. doi: 10.1021/jf0255681. [DOI] [PubMed] [Google Scholar]
- Liang YS, Bruce JI, Boyd DA (1987) Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycles. In: Sino American Symposium 1:34e48
- Liu Y, Espinosa CD, Abelilla JJ, Casas GA, Lagos LV, Lee SA. Non-antibiotic feed additives in diets for pigs: a review. Anim Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevappa N, Suvarna DKHPV, Moses V, Chandrashekar S, Cowda S. Study on advanced application of mint oil. J Adv Sci Res. 2014;5(4):1–3. [Google Scholar]
- Mahmoudi H, Marzouki M, M’Rabet Y, Mezni M, Ouazzou AA, Hosni K. Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities. Arab J Chem. 2020 doi: 10.1016/j.arabjc.2020.06.003. [DOI] [Google Scholar]
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother Res. 2006;20(8):619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- Mendonça FL, Carvalho JG, Silva RJ, Ferreira LC, Cerqueira DM, Rogge HI, Facury-Filho EJ. Use of a natural herbal-based feed additive containing isoquinoline alkaloids in newborn calves with cryptosporidiosis. Vet Parasitol. 2021;300:109615. doi: 10.1016/j.vetpar.2021.109615. [DOI] [PubMed] [Google Scholar]
- Di Meo S Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 1245049 [DOI] [PMC free article] [PubMed]
- Milenković L, Stanojević J, Cvetković D, Stanojević L, Lalević D, Šunić L, Fallik E, Ilić ZS. New technology in basil production with high essential oil yield and quality. Ind Crops Prod. 2019;140:111718. doi: 10.1016/j.indcrop.2019.111718. [DOI] [Google Scholar]
- Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BGK, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CLMJ, White AC, Merritt EA, Van Voorhis WC, Maly DJ. Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med Chem Lett. 2010;1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickavar B, Alinaghi A, Kamalinejad M. Evaluation of the antioxidant properties of five mentha species. Iran J Pharm Sci. 2010;10:203–209. [Google Scholar]
- Poljsak B, Šuput D, Milisav I (2013) Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 956792 [DOI] [PMC free article] [PubMed]
- Raal A, Orav A, Püssa T, Valner C, Malmiste B, et al. Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries. Food Chem. 2012;131:632–638. doi: 10.1016/j.foodchem.2011.09.042. [DOI] [Google Scholar]
- Rehquel T, David A, Belwett N, Manuel S, Carmona P. C. parvum infection in experimentally infected mice: infection dynamics and effect of immunosuppression. Folkia Parasitol. 1998;45:101–107. [PubMed] [Google Scholar]
- Remmal A, Achahbar S, Bouddine L, Chami N, Chami F. In vitro destruction of Eimeria oocysts by essential oils. Vet Parasitol. 2011;182:121–126. doi: 10.1016/j.vetpar.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Rossignol JF. Nitazoxanide in the treatment of acquired immune deficiency syndrome-related cryptosporidiosis: results of the United States compassionate use program in 365 patients. Aliment Pharmacol Ther. 2006;24(5):887–894. doi: 10.1111/j.1365-2036.2006.03033.x. [DOI] [PubMed] [Google Scholar]
- Sabatke B, Chaves PFP, Cordeiro L, Ramirez MI. Synergistic effect of polysaccharides from chamomile tea with nitazoxanide increases treatment efficacy against Giardia intestinalis. Life. 2022;12(12):2091. doi: 10.3390/life12122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin O, Tormakangas L, Leinonen M, Saario E, Hagstrom M, Ketola RA, Vuorela PM. Corn mint (Mentha arvensis) extract diminishes acute Chlamydia pneumoniae infection in vitro and in vivo. J Agric Food Chem. 2011;59(24):12836–12842. doi: 10.1021/jf2032473. [DOI] [PubMed] [Google Scholar]
- Speisky H, Rocco C, Carrasco C, Lissi EA, López-Alarcón C. Antioxidant screening of medicinal herbal teas. Phytother Res. 2006;20(6):462–467. doi: 10.1002/ptr.1878. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85(19–20):663–669. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic LP, Stanojevic JS, Savic VL, Cvetkovic DJ, Kolarevic A, Marjanovic-Balaban Z, Nikolic LB. Peppermint and basil essential oils: chemical composition, in vitro antioxidant activity and in vivo estimation of skin irri-tation. JEOBP. 2019 doi: 10.1080/0972060x.2019.1661793. [DOI] [Google Scholar]
- Taha NM, Yousof HA, El-Sayed SH, Younis AI, Negm MSI. Atorvastatin repurposing for the treatment of cryptosporidiosis in experimentally immunosuppressed mice. Exp Parasitol. 2017;181:57–69. doi: 10.1016/j.exppara.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Tavallali V, Kiani M, Hojati S. Iron nano-complexes and iron chelate improve biological activities of sweet basil (Ocimum basilicum L.) Plant Physiol Biochem. 2019;144:445–454. doi: 10.1016/j.plaphy.2019.10.021. [DOI] [PubMed] [Google Scholar]
- Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JM, Saraiva JA, Nunes ML. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crop Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- Tessema TS, Schwamb B, Lochner M, Förster I, Jakobi V, Petry F. Dynamics of gut mucosal and systemic Th1/Th2 cytokine responses in interferon-gamma and interleukin-12p40 knockout mice during primary and challenge Cryptosporidium parvum infection. Immunobiology. 2009;214:454–466. doi: 10.1016/j.imbio.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Uraku AJ, Okaka ANC, Ibiam UA, Agbafor KN, Obasi NA, Ajah PM, Nwalo FN. Antiplasmodial activity of ethanolic leaf extracts of Spilanthes uliginosa, Ocimum basilicum (Sweet Basil), Hyptis spicigera and Cymbopogon citratus on mice exposed to plasmodium berghei Nk 65. Int J Biochem Res. 2015;6(1):28. doi: 10.9734/IJBCRR/2015/9806. [DOI] [Google Scholar]
- Wu Z, Tan B, Liu Y, Dunn J, Martorell Guerola P, Tortajada M, Cao Z, Ji P. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules. 2019;24:2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Złotek U, Michalak-majewska M, Szymanowska U. Efect of jasmonic acid elicitation on the yield, chemical composition, and antioxidant and anti-infammatory properties of essential oil of lettuce leaf basil (Ocimum basilicum L.) Food Chem. 2016;213:1–7. doi: 10.1016/j.foodchem.2016.06.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.