Abstract
The mycoparasite Humicola fuscoatra NRRL 22980 was isolated from a sclerotium of Aspergillus flavus that had been buried in a cornfield near Tifton, Ga. When grown on autoclaved rice, this fungus produced the antifungal metabolites monorden, monocillin IV, and a new monorden analog. Each metabolite produced a clear zone of inhibition surrounding paper assay disks on agar plates seeded with conidia of A. flavus. Monorden was twice as inhibitory to A. flavus mycelium extension (MIC > 28 μg/ml) as monocillin IV (MIC > 56 μg/ml). Cerebrosides C and D, metabolites known to potentiate the activity of cell wall-active antibiotics, were separated from the ethyl acetate extract but were not inhibitory to A. flavus when tested as pure compounds. This is the first report of natural products from H. fuscoatra.
The sclerotia of Aspergillus flavus Link: Fr. are an important source of inoculum in the disease cycle of this aflatoxin-producing fungus in maize (Zea mays L.) fields (24). Studies of the survival of A. flavus sclerotia buried in sandy field soils in Illinois and Georgia have revealed that they have numerous fungal colonists, including known mycoparasites such as Paecilomyces lilacinus (Thom) Samson (25). In a follow-up study the mycoparasite Humicola fuscoatra Traaen NRRL 22980 was isolated from an A. flavus sclerotium that had been buried for 3 years in a Georgia field planted to corn (26). Mycoparasites negatively impact sclerotium-forming plant-pathogenic fungi by infecting pathogen sclerotia in soil (12). H. fuscoatra parasitizes the oospores of Phytophthora megasperma f. sp. glycinea in soil (19, 21) and has also been detected within, and isolated from, the spores of arbuscular mycorrhizal fungi, such as Glomus fasciculatum and Glomus versiforme, grown in greenhouse pot cultures (5). Mycoparasite invasion of fungal sclerotia may involve antibiosis (3), and therefore, mycoparasites and fungicolous fungi (9) are potentially useful sources of antifungal agents (10, 11, 14). Subrahmanyam and Rao (20) reported that Humicola fuscoatra var. nigra produces an unspecified cell-bound antifungal substance that inhibits phytopathogenic fungi. Our objective was to describe the isolation and characterization of antifungal metabolites produced by H. fuscoatra which inhibit the growth of A. flavus.
MATERIALS AND METHODS
Fungal cultures and fermentation conditions.
H. fuscoatra NRRL 22980 was isolated by the procedure described by Wicklow et al. (25) from a sclerotium of A. flavus that had been buried in soil for 3 years (1989 to 1991) in a Georgia cornfield (Coastal Plains Research Station, Tifton, Ga.). The fungus was grown on several slants of potato dextrose agar (PDA) for 14 days (25°C). A hyphal fragment-spore suspension (propagule density, 106/ml of sterile distilled water) prepared from the potato dextrose agar slants served as the inoculum. Fermentations were carried out in duplicate 3-liter Fernbach flasks, each containing 200 g of rice (Botan Brand; J.F.C. International). Distilled water (200 ml) was added to each flask, and the contents were soaked overnight before being autoclaved at 15 lb/in2 for 30 min. After the flasks had cooled to room temperature, they were inoculated with 3 ml of the hyphal fragment-spore suspension and incubated for 40 days at 25°C.
Isolation and characterization of fungal metabolites.
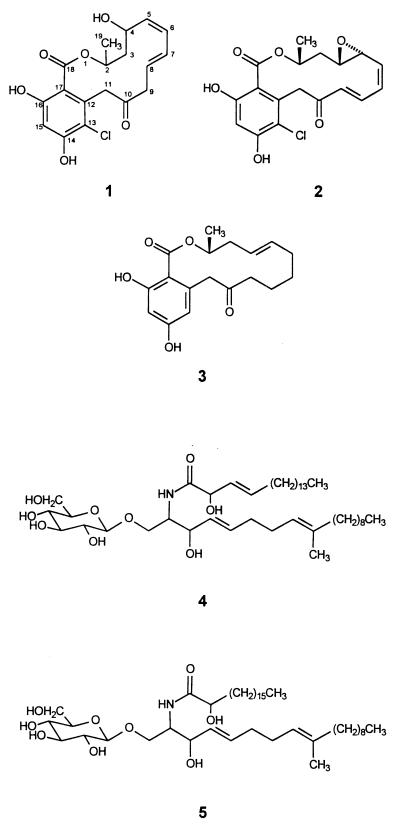
A portion (2 g) of the ethyl acetate extract was dissolved in 80:20 H2O-methanol, and the resulting solution was extracted sequentially with hexane and CHCl3 (50 ml; two times each). The CHCl3 fractions were combined and evaporated to afford a residue (212 mg), which was subjected to fractionation on a Sephadex LH-20 column (42 by 1.5 cm) by eluting it successively with 4:1 CH2Cl2-hexane, 4:1 CH2Cl2-acetone, 3:2 CH2Cl2-acetone, and 4:1 acetone-CH2Cl2 (100 ml each), followed by a 100% methanol wash. The fraction eluting with 4:1 acetone-CH2Cl2 (26 mg) was purified by semipreparative reversed-phase high-performance liquid chromatography (HPLC) (Dynamax 5-μm-particle-size C18 column; 40 to 60% CH3CN in 0.1% HCOOH–H2O in 25 min) to give monorden analog 1 (4.2 mg; retention time [Rt], 13 min). The structure of compound 1 was determined by analysis of 1H nuclear magnetic resonance (NMR), 13C NMR, two-dimensional NMR, and mass spectral data. Compound 1 has the following characteristics: it is a colorless oil; [α]D = −137° (c = 0.5 mg/ml; CHCl3; 24°C); negative ion electrospray ionization mass spectrometry, pseudomolecular ion at m/z 365 {(M-H)−; 100% relative intensity}. 1H NMR (multiplicity; J in hertz; assignment): 6.61 (s; H-15), 6.10 (dd; 10.8, 10.8; H-6), 6.02 (m; H-7), 5.76 (ddd; 15.0, 6.6, 6.6; H-8), 5.27 (dd; 9.6, 9.6; H-5), 4.84 (m; H-2), 4.81 (d; 18; H-11a), 4.64 (m; H-4), 3.81 (d; 18; H-11b), 3.27 (dd; 14.4, 6.6; H-9a), 2.93 (dd; 14.4, 6.6; H-9b), 2.18 (ddd, 12.9, 11.1, 3.6; H-3a), 1.59 (ddd; 12.9, 10.2, 2.4; H-3b), 1.35 (d; 6.0; H3-19). 13C NMR: 203.0 (C-10), 167.1 (C-18), 156.6 (C-14), 135.5 (C-12), 134.5 (C-5), 129.0 (C-6), 128.2 (C-7), 127.8 (C-8), 116.0 (C-17), 114.1 (C-13), 103.8 (C-15), 71.0 (C-2), 64.5 (C-4), 44.6 (C-9), 43.8 (C-11), 43.4 (C-3), 20.4 (C-19). Heteronuclear multiple-bond correlations (H no.→C no.): H-2→C-3, 18, 19; H-3a→C-2, 4, 5; H-3b→C-4, 5; H-5→C-3, 7; H-6→C-4, 8; H-7→C-9; H-8→C6, 9; H-9a→C-8, 10; H-9b→C-8, 10; H-11a→C-10, 12, 13, 17; H-11b→C-10, 12, 13, 17; H-15→C-16, 17; H3-19→C-2, 3.
The remainder of the ethyl acetate extract (7 g) was purified on a silica gel (Fluka no. 60765) vacuum liquid chromatography column (6 by 5 cm) eluting with 1:9 hexane-CH2Cl2 (1,500 ml), followed by a step gradient of methanol-CH2Cl2 in the following ratios and volumes: 1:99 (2,000 ml), 2:99 (1,000 ml), 3:97 (750 ml), 4:96 (500 ml), 8:92 (500 ml), 15:85 (500 ml), and 30:70 (500 ml). The fractions eluting with 1:99 methanol-CH2Cl2 were combined on the basis of their thin-layer chromatography behavior, with 3:2:1 hexane-CHCl3-methanol as the eluent. These combined fractions (1.8 g) were further fractionated on a column of silica gel (Fisher no. D22661264-01; 60 to 200 μ) with a step gradient of 5:95 ethyl acetate-CH2Cl2 (100 ml), 20:80 ethyl acetate-CH2Cl2 (100 ml), 40:60 ethyl acetate-CH2Cl2 (100 ml), 20:80 ethyl acetate-CH2Cl2 (200 ml), 1:99 methanol-CH2Cl2 (100 ml), and 10:90 methanol-CH2Cl2 (200 ml). The fractions eluting with 20:80 and 40:60 ethyl acetate-CH2Cl2 were combined (1,040 mg [wet weight]), and 70 mg of the resulting material was purified by semipreparative reversed-phase HPLC (Dynamax 5-μm-particle-size C18 column; 40 to 80% acetonitrile in 0.1% HCOOH in 20 min) to produce monorden (compound 2; 40 mg; Rt, 11.2 min) and monocillin IV (compound 3; 40 mg; Rt, 11.2 min). Monorden (compound 2) and monocillin IV (compound 3) were identified by comparison of their 1H and 13C NMR chemical shifts and mass spectral data with published values (1, 16).
The fraction eluting from the vacuum liquid chromatography column with 15:85 methanol-CH2Cl2 (367 mg) was fractionated on a silica gel column (Fisher no. D22661264-01; 60 to 200 μ; 38 by 2.2 cm) with a step gradient of methanol-CH2Cl2 in the following ratios and volumes: 1:99 (700 ml), 2:99 (250 ml), 3:97 (200 ml), 5:95 (500 ml), 10:90 (250 ml), 12:88 (400 ml), 15:85 (200 ml), and 50:50 (200 ml). The fraction eluting with 15:85 methanol-CH2Cl2 (85 mg) on trituration with acetone gave an acetone-insoluble portion (38 mg), which was then purified by semipreparative reversed-phase HPLC (Hamilton 10-μm-particle-size PRP-1 column; 50 to 100% CH3CN in 0.1% HCOOH–H2O in 20 min) to produce cerebrosides C (compound 4) and D (compound 5). Cerebroside C (6.1 mg; Rt, 24.4 min) and cerebroside D (6.8 mg; Rt, 24.8 min), obtained by processing 30 mg of the acetone-insoluble material, were identified by comparison of their 1H NMR, 13C NMR, and mass spectral data with published values (18).
Bioassay of extractable residue.
Following incubation, the fermented rice substrate in each Fernbach flask was first fragmented with a large spatula and then extracted three times with ethyl acetate (200 ml each time). The combined ethyl acetate extracts were filtered and evaporated. Following evaporation of the ethyl acetate, approximately 6 mg of the residue was redissolved in ethyl acetate for antifungal activity assays. The remaining dried extract was stored at −20°C.
One- and 0.5-mg equivalents of extractable residue, dissolved in methanol, were pipetted onto individual analytical-grade filter paper disks (13.0-mm diameter) in individual Petri dish lids and dried for 30 min in a laminar flow hood. Up to four disks were placed equidistant from one another on the surface of fresh yeast malt agar (23) containing 22% glycerol, as modified by Nout (15). This agar was seeded with A. flavus (NRRL 6541) conidia to give a final conidial suspension of approximately 100 spores per ml. The bioassay plates were incubated for 4 days at 25°C and examined for the presence of a zone of inhibition surrounding a disk, which is evidence of the inhibition of germination and a measure of fungistatic activity. This bioassay procedure was used to guide the isolation of those H. fuscoatra metabolites which accounted for the antifungal activity. Pure compounds were evaluated for antifungal activity by placing 0.25 mg onto individual paper disks.
To determine if these H. fuscoatra metabolites might also function to prevent insects feeding on the organism or as toxins, the compounds monorden (compound 2), monocillin IV (compound 3), and cerebroside D (compound 5) were incorporated into a pinto bean-based diet according to previously described methods (6). Briefly, the compounds were dissolved in 125 μl of acetone. The solution was blended into a liquid diet preparation with a vortex mixer. The diet mixtures were allowed to solidify, and the solvent was removed in a fume hood. The final concentrations of the compounds in the diet mixture were 100 ppm. The diet mixture for each treatment was sectioned into 20 pieces, and each piece was placed in an individual well of a tissue culture plate. A newly hatched corn earworm [Helicoverpa zea (Boddie)] larva was placed on each piece of the diet mixture, the plates were sealed, and larva mortality was determined after 7 days. All surviving larvae were individually weighed.
RESULTS
Bioassay for antifungal activity.
The ethyl acetate extract of fermented-rice cultures inoculated with H. fuscoatra NRRL 22980 displayed potent antifungal activity in conventional paper disk assays on agar plates seeded with conidia of A. flavus. Three of the major components, when added to each disk at 250 ppm, showed potent activity, as measured by the zone of inhibition surrounding each disk (diameters: compound 1, 5 mm; compound 2, 13 mm; compound 3, 13 mm), while compounds 4 and 5 were not inhibitory to A. flavus in this bioassay (Fig. 1). The activities of monorden and monocillin IV against A. flavus were also assessed by the application of 5 to 200 μg of metabolite (5.6 to 224 μg of metabolite ml of agar−1) in HPLC-grade methanol to yeast malt glycerol agar blocks of known volume (0.8908 ml) by the method described by Morris et al. (14). Monorden was twice as inhibitory to A. flavus mycelium extension (MIC > 28 μg/ml) as monocillin IV (MIC > 56 μg/ml). Compounds 4 and 5 (identified below) were known metabolites that potentiate the activity of cell wall-active antibiotics (17). The monordens and monocillins do not account for all of the antifungal activity in the initial ethyl acetate extract, and further studies of this extract have identified new antifungal compounds that will be the subject of another report. Assays of fungal compounds 2, 3, and 5 in insect dietary tests showed that, relative to the solvent control, there was no significant mortality and the weights of larvae fed diets containing cerebroside D, monocillin IV, and monorden were reduced by 47, 16, and 0.2%, respectively.
FIG. 1.
Structures of new monorden analog (compound 1), monorden (compound 2), monocillin IV (compound 3), cerebroside C (compound 4), and cerebroside D (compound 5).
DISCUSSION
The occurrence of monordens (compounds 1 and 2) and monocillin IV (compound 3) as major constituents of the fermentation extracts of H. fuscoatra provides an intriguing parallel to their occurrence in Monocillium nordinii (Bourchier) W. Gams, a destructive mycoparasite of pine stem rusts (1). Ayer et al. suggest that monorden may play a key role in the mycoparasitic action of M. nordinii on rust sori. Mycoparasitic fungi in unrelated fungal taxa could benefit from the same classes of antifungal metabolites, and one might anticipate finding evolutionary convergence in pathways leading to their biosynthesis.
M. nordinii has also been isolated from the rhizomorph of Armillariella mellea (8). Another monorden-producing fungus, Cylindrocarpon radicicola Wr. [= Cylindrocarpon destructans (Zinssm.) Scholten], was isolated from mycorrhizal tuberous roots of the saprophytic orchid Dipodium punctatum R. Br. (22). Evans and White (7) reported that radicicol (i.e., monorden) was inhibitory to the growth of numerous fungal strains, including Aspergillus niger van Tiegh. and Eurotium repens De Bary. Cerebrosides C (compound 4) and D (compound 5) were first described from an unidentified species of Pachybasium sp. (= Trichoderma sp. Section Pachybasium [2]) and potentiated the activity of the cell wall-active antibiotic aculeacin against Candida albicans (18). Sitrin et al. also reported that no significant chitin synthetase inhibition occurred in the absence of aculeacin. We have not determined if cerebrosides C and D potentiate the activity of H. fuscoatra-coproduced antifungal metabolites, monordens, or monocillin IV. Assays of monorden and monocillin IV in insect dietary tests against Humicola zea at 100 ppm showed little or no activity. The fungi shown to produce monordens or monocillins are recognized as mycoparasites or antagonists of other fungi (e.g., C. destructans, H. fuscoatra, and M. nordinii), while Neocosmospora tenuicristata Ueda et Udagawa was isolated from marine sludge (4). These fungal taxa are not recognized as having any negative interactions with insects.
The best-known mycoparasitic fungi (e.g., Coniothyrium minutans Campbell, Gliocladium virens J. H. Miller et al., Talaromyces flavus (Klocker) Stolk & Samson, Trichoderma harzianum Rifai, and Verticillium biguttatum Gams) are those which have been used, or at least proposed, as agents for biocontrol of soilborne plant-pathogenic fungi because of their antifungal effects on hosts (12). A few of these fungi are known to produce antifungal agents (10, 11, 14). While the general concept that mycoparasites may produce antifungal agents is not new, the number of cases investigated from a chemical standpoint is surprisingly small. We have shown that H. fuscoatra, a mycoparasite of A. flavus sclerotia, produces several antifungal agents effective against A. flavus.
Opportunistic fungal infections of humans (including invasive pulmonary aspergillosis caused by Aspergillus fumigatus Fresenius and A. flavus) have become increasingly common in recent years (17). Unfortunately, only a relatively small number of antifungal therapeutic drugs are available, and all of them suffer from serious limitations and/or cause significant side effects (13). We suggest that screening mycoparasites and other fungal colonists of A. flavus sclerotia for antifungal activity could lead to the identification of novel and quite useful compounds.
ACKNOWLEDGMENTS
This research was supported by grants from the National Science Foundation (CHE-9211252) and Biotechnology Research and Development Corporation.
REFERENCES
- 1.Ayer W A, Lee S P, Tsuneda A, Hiratsuka Y. The isolation, identification, and bioassay of the antifungal metabolites produced by Monocillium nordinii. Can J Microbiol. 1980;26:766–773. [Google Scholar]
- 2.Bissett J. A revision of the genus Trichoderma. III. Section Pachybasium. Can J Bot. 1991;69:2373–2417. [Google Scholar]
- 3.Chet I, Inbar J, Hadar Y. Fungal antagonists and mycoparasites. In: Wicklow D T, Soderstrom B E, editors. The mycota. IV. Environmental and microbial relationships. Berlin, Germany: Springer Verlag; 1997. pp. 165–184. [Google Scholar]
- 4.Cutler H G, Arrendale R F, Springer J P, Cole P D, Roberts R G, Hanlin R T. Monorden from a novel source, Neocosmospora tenuicristata, stereochemistry and plant growth regulatory properties. Agric Biol Chem. 1987;51:3331–3338. [Google Scholar]
- 5.Daniels B A, Menge J A. Hyperparasitization of vesicular-arbuscular mycorrhizal fungi. Phytopathology. 1980;70:584–588. [Google Scholar]
- 6.Dowd P F. Synergism of aflatoxin B1 toxicity with the co-occurring fungal metabolite kojic acid to two caterpillars. Entomol Exp Appl. 1988;47:69–71. [Google Scholar]
- 7.Evans G, White N H. Radicicolin and radicicol, two new antibiotics produced by Cylindrocarpon radicicola. Trans Br Mycol Soc. 1966;49:563–576. [Google Scholar]
- 8.Gams W. Cephalosporium-artige Schimmelpilze (Hyphomyces). Stuttgart, Germany: Gustav Fischer Verlag; 1971. [Google Scholar]
- 9.Hawksworth D L. A survey of the fungicolous conidial fungi. In: Cole G T, Kendrick B, editors. Biology of conidial fungi. I. New York, N.Y: Academic Press; 1981. pp. 171–244. [Google Scholar]
- 10.Huang Q, Tezuka Y, Hatanaka Y, Kikuchi T, Nishi A, Tubaki K. Studies on metabolites of mycoparasitic fungi. III. New sesquiterpene alcohol from Trichoderma koningii. Chem Pharm Bull. 1995;43:1035–1038. doi: 10.1248/cpb.43.1663. [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, Tezuka Y, Hatanaka Y, Kikuchi T, Nishi A, Tubaki K. Studies on metabolites of mycoparasitic fungi. III. Minor peptaibols of Trichoderma koningii. Chem Pharm Bull. 1995;43:1663–1667. doi: 10.1248/cpb.43.1663. [DOI] [PubMed] [Google Scholar]
- 12.Jeffries P. Mycoparasitism. In: Wicklow D T, Soderstrom B E, editors. The mycota. IV. Environmental and microbial relationships. Berlin, Germany: Springer Verlag; 1997. pp. 149–164. [Google Scholar]
- 13.Koltin Y. Targets for antifungal drug discovery. Annu Rep Med Chem. 1990;25:141–148. [Google Scholar]
- 14.Morris R A C, Ewing D F, Whipps J M, Coley-Smith J R. Antifungal hydroxymethyl-phenols from the mycoparasite Verticillium biguttatum. Phytochemistry. 1995;39:1043–1048. [Google Scholar]
- 15.Nout, M. J. R. 1996. Personal communication.
- 16.Nozawa K, Nakajima S. Isolation of radicicol from Penicillium luteoaurantium and meleagrin, a new metabolite, from Penicillium meleagrinum. J Nat Prod. 1979;42:374–377. [Google Scholar]
- 17.Seeliger H P R, Tintelnot K. Epidemiology of aspergillosis. In: Vanden Bosche H, Mackenzie D W R, Cauwenbergh G, editors. Aspergillus and aspergillosis. New York, N.Y: Plenum Press; 1988. pp. 23–34. [Google Scholar]
- 18.Sitrin R D, Chan G, Dingerdissen J, DeBrosse C, Mehta R, Roberts G, Rottschaeffer S, Staiger D, Valenta J, Snader K M, Stedman R J, Hoover J R E. Isolation and structure determination of Pachybasium cerebrosides which potentiate the antifungal activity of aculeacin. J Antibiot. 1988;41:469–480. doi: 10.7164/antibiotics.41.469. [DOI] [PubMed] [Google Scholar]
- 19.Sneh B, Humble S J, Lockwood J L. Parasitism of oospores of Phytophthora megasperma var. sojae, P. cactorum, Pythium sp., and Aphanomyces euteiches in soil by Oomycetes, Chytridiomycetes, Hyphomycetes, Actinomycetes, and Bacteria. Phytopathology. 1977;67:622–628. [Google Scholar]
- 20.Subrahmanyam A, Rao A N. Studies on thermomycology-antimicrobial activity of Humicola fuscoatra var. nigra Tf25. Hind Antibiot Bull. 1986;28:44–48. [PubMed] [Google Scholar]
- 21.Sutherland E D, Baker K K, Lockwood J L. Ultrastructure of Phytophthora megasperma f. sp. glycinea oospores parasitized by Actinoplanes missouriensis and Humicola fuscoatra. Trans Br Mycol Soc. 1984;82:726–729. [Google Scholar]
- 22.White N H, Chilvers G A, Evans G. Antifungal activity of Cylindrocarpon radicicola Wr. Nature. 1962;195:406–407. [Google Scholar]
- 23.Wickerham L J. Taxonomy of yeasts. Technical Bulletin 1029. Washington, D.C: United States Department of Agriculture; 1951. [Google Scholar]
- 24.Wicklow D T, Horn B W, Burg W R, Cole R J. Sclerotium dispersal of Aspergillus flavus and Eupenicillium ochrosalmoneum from maize during harvest. Trans Br Mycol Soc. 1984;83:299–303. [Google Scholar]
- 25.Wicklow D T, Wilson D M, Nelsen T C. Survival of Aspergillus flavus sclerotia and conidia buried in soil in Illinois or Georgia. Phytopathology. 1993;83:1141–1147. [Google Scholar]
- 26.Wicklow, D. T., and D. M. Wilson. Unpublished data.