Abstract
Helminth infections are a worldwide problem that affects both humans and animals in developing countries. The common pinworm Syphacia muris frequently infects lab rats and can obstruct the creation of unrelated biological experiments. The objective of this study was to examine the in vivo efficacy of silver nanoparticles against S. muris infected Wistar rats. Transmission electron microscopy and X-ray diffraction examinations of silver nanoparticles revealed highly pure polycrystals with a mean size of 4 nm. Rats were divided into group I, the control: received distilled water; groups II and III, the treated: received 2, 4 mg/kg b.w. of Ag NPs, respectively. At the end of the experimental period, all rats were euthanized and dissected for collecting worms. The surface topography of the recovered worms was displayed using light and scanning electron microscopy, and their physiological status was determined using oxidative stress biomarkers. The histological changes in the rat liver, kidney, and spleen were also examined. In the current study, Ag NPs administration revealed substantial alterations in worms collected from treated rats, including shrinkage of lips, peeling and rupture of body cuticles, and disruption of surface annulations. Also, induced a significant increase in malondialdehyde and nitric oxide levels, as well as a decrease in reduced glutathione, glutathione peroxidase and catalase levels compared to control group. Moreover, sections of treated rats' liver, kidney and spleen displayed normal cellular appearance. In conclusion, this is the first in vivo study to evaluate Ag NPs efficacy against S. muris in laboratory rats without significant toxicity.
Keywords: Syphacia muris, Ag NPs, Microscopic examination, Oxidative stress, Histopathology
Introduction
Helminthic infections are found all over the world, affecting both humans and animals, causing harm and economic loss, particularly in tropical and subtropical climates (Khan et al. 2015). Multiple species infections with parasitic helminths, including nematodes, are common in wild rodent populations (Behnke et al. 2001). Syphacia muris, an interesting oxyurid species, infects laboratory rats at a high rate even in well-managed conditions (Pinto et al. 1994). Rodents are used to assess the safety of medications intended for human use (Ayinmode et al. 2015). Pinworm infestations are undesirable due to the possible negative effects on research activities; these parasites might impede the development of protocols and outcome evaluations (Pinto et al. 2001). For example, altered growth of certain strains of rats and mice (Meade and Watson 2014). In addition, Syphacia infections can affect a host's humoral response to nonparasitic antigenic stimuli (Sato et al. 1995). Furthermore, rodent pinworms can promote myelopoiesis and erythropoiesis as well as change bone marrow reactivity (Ilic et al. 2010). Additionally, these worms have the potential to induce zoonotic illnesses (Okorafor et al. 2012; Khalil et al. 2014). Although these worms' infections are less harmful to humans than other infectious agents, cases of heavy worm burdens frequently result in malnutrition, anaemia, stunted growth, and other intestinal diseases in infected hosts (Sueta et al. 2002).
Chemotherapy is the main effective tool to resist and control helminth parasites which has a severe adverse impact on host animals (Wang et al. 2015). Furthermore, drug resistance to anthelminthic drugs is a widespread issue, defined as a heritable decrease in the sensitivity of a parasite population to the action of a medicine (Gang and Hallem 2016). As a result of the health risks connected with the use of chemotherapy to inhibit helminth parasites, the development of nanoparticle-based medicinal formulations as feasible alternatives to anthelmintic drugs has been encouraged (Wang et al. 2015). Nanoparticles (NPs) have unique features due to their small size, shape, and morphology, making them extremely effective against a wide range of parasites (Ali et al. 2014; Delavari et al. 2014). They are also easily generated in large quantities using various methods and are highly biodegradable and biocompatible (Bhatia et al. 2016). Metal nanoparticles (NPs) have recently found numerous applications in the medical field (Zhang et al. 2016). Among metal and metal oxides, Ag NPs and ZnO are a well-known example of nano-sized materials that have been applied as a means of controlling human pathogenic microbes possessing antimicrobial effects and have been frequently used in the field of medicine (Xiang et al. 2017) due to their unique physical, chemical, and biological characteristics (Zhang et al. 2018).
Silver nanoparticles have potent antiparasitic and antibacterial properties. Ag NPs' antiparasitic activity was demonstrated by the inhibition of metabolic activities and cell proliferation in Leishmania spp. promastigotes. Antiviral activities of Ag NPs have also been demonstrated to impede viral replication and prevent virus particles from binding to host cell receptors. These nanoparticles have promising efficacy as anticancer agents and could be a reason that they have attracted more attention as an anthelmintic agent (Wei et al. 2015). Nanoparticles were commonly evaluated in vitro, with only a few studies reporting in vivo effectiveness of various nanoparticles (Ali et al. 2021). It is well known that silver nanoparticles with their unique properties have a wide range of biological uses both in vitro and in vivo. Thus, the goal of this study was to evaluate the anthelmintic potential of silver nanoparticles against Syphacia muris infected Wistar rats through assessing the oxidative stress, morphological changes in the studied worm as well as its impact on host tissues.
Material and methods
Synthesis of silver nanoparticles
Silver nanoparticles were synthesized at the Nanotechnology Center, Cairo University, El-Sheikh Zayed, Egypt. Chemicals used for silver nanoparticle production were silver nitrate (AgNO3) and trisodium citrate (Na3C6H5O7). The method was based on co-precipitation to form silver colloid structures. Fifty ml of 0.001 M AgNO3 was heated to boiling, followed by dropwise addition of 5 ml of 1% trisodium citrate solution. The suspension was mixed and heated until the color changed to pale yellow, then was cooled at room temperature, and kept in the dark until use (Youssef et al. 2020). The reaction is based on the following equation:
Characterization of silver nanoparticles
Transmission electron microscopy (TEM)
Morphology and particle size of Ag NPs were examined using TEM (EM-2100 High-Resolution-Japan) at a magnification 20X and an accelerating voltage 200 kV to assess 2D shape and size. A drop from a dilute sample solution was deposited on an amorphous carbon coated-copper grid and left to dry at room temperature. The resulting monolayer of particles was analyzed using the Image J software programme, and several TEM images were taken (Ross and Dykstra 2003).
X-ray diffraction
Samples were air-dried, powdered, and used for analysis using an XRD Model D8 Discover instrument manufactured by the Bruker Company. The light source was Cu Kα radiation with a current of 35 mA at a voltage of 40 kV. The 2θ angles ranged from 5° to 80° with a scan speed of 0.3°/min. Mean crystal size was estimated from the full width at half maximum (FWHM) and the Scherrer formula according to the following equation:
where D is the mean crystal size, λ is the X-ray wavelength, 0.89 is the shape factor, β is the line broadening at half maximum intensity (FWHM) in radians, and θ is the Bragg angle.
Experimental design
Twenty-one Wistar rats weighing 120–130 g, were purchased from the National Organization for Drug Control and Research, and were transferred to the Laboratory of Parasitology, Zoology Department, Faculty of Science, Cairo University. The experimental animals were kept at room temperature with a 12 h light/dark cycle and were fed standard rodent feed and water ad libitum. Natural S. muris infections were identified in rats after 7 days of acclimatization using perianal cellophane tape, as described by Meade and Watson (2014) to confirm the infection. Rats were divided into three groups (n = 7); group I, untreated control: received distilled water; groups II and III were treated with 2, 4 mg/kg body weight of Ag NPs, respectively, by El Mahdy et al. (2014) intraperitoneal injections daily for 14 days. Syphacia muris were recovered from the caecum and colon of all tested rats after necropsy. All procedures using animals were compliant with the requirements of the Animal Ethics Committee, Faculty of Science, Cairo University, Egypt (Ethical Approval Number: CUIF7519).
Microscopic examination
Parasites were cleared with glycerol for light microscopy examination in temporary mounts as described by Pritchard and Kruse (1982). The recovered nematodes were classified according to Pinto et al (1994). Images were obtained by a LEICA DM 750 microscope equipped with a LEICA ICC 50 HD camera.
Worms were fixed for SEM in a solution of 3% glutaraldehyde, washed in 0.1 M sodium cacodylate buffer (pH 7.4), dehydrated through a graded ethanol series (50%, 60%, 70%, 80%, 90% and 100%), and dried at 30 °C for 30 min using a critical point drier (LEICA, EM CPD300). Dried specimens were mounted on SEM stubs, coated with gold, and examined with a JEOL JSM-5200 SEM (Tokyo, Japan) using an accelerating voltage 25 kV. All body measurements are presented as means in mm ± SD in parentheses.
Determination of oxidative stress biomarkers
Seven worms were homogenized in 50 mM Tris–HCl, pH 8.0 and centrifuged at 3000 rpm for 10 min at 4 °C. Supernatants were kept at − 20 °C until they were used. Levels of malondialdehyde (MDA), a marker for lipid peroxidation, were measured as described by Buege and Aust (1978); reduced glutathione (GSH), the major intracellular thiol utilized by cells for antioxidant protection and an important biochemical marker of oxidative stress was evaluated following Caito and Aschner 2015); catalase activity (CAT), an important enzyme for protection against H2O2-induced stress, was measured as described by Kotze (2003); nitric oxide (NO) was assessed according to Massetti et al. (2004). All oxidative biomarkers were assessed using reagent kits obtained from Bio Diagnostic (Egypt).
Histopathological studies
Host tissues (kidney, liver, and spleen) were isolated and fixed in 10% buffered formalin after being washed with saline. Samples were dehydrated in ascending grades of alcohol, cleared with xylene, and embedded in paraffin. Five µm sections were cut and stained with hematoxylin and eosin (H& E). Sections were examined and photographed using a LEICA DM 750 microscope equipped with a LEICA ICC 50 HD camera.
Statistical analysis
Data were analyzed by one-way ANOVA using Statistical Processor Systems Support, SPSS software, version 20, followed by Least Significant Difference post hoc tests to compare group means. Data are presented as mean ± SE, and p < 0.05 was considered significant.
Results
Characterizations of Ag NPs
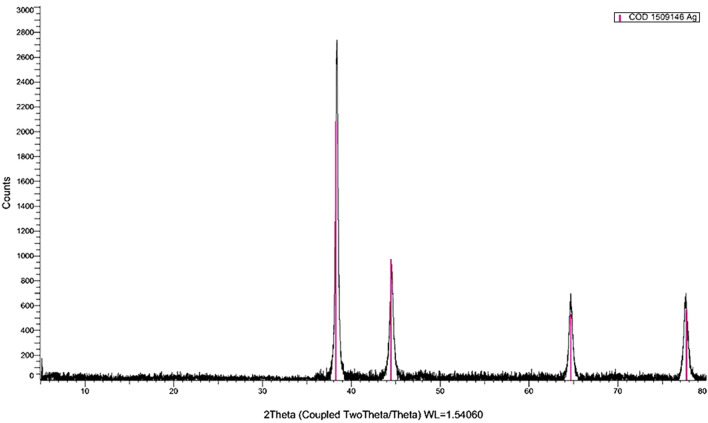
The XRD curve of Ag NPs showed an amorphous state (zero peaks) without any additional peaks, indicating homogeneous chemical composition (Fig. 1). The pattern illustrates the presence of four characteristic peaks for silver nanoparticles at 2θ = 38.3°, 44.5°, 64.8° and 77.8°. Crystallite size, calculated using the Debye–Scherrer equation, was 4 nm; crystallinity was high, and particles displayed a cubic lattice structure. TEM images (Fig. 2) confirmed a spherical outline with a homogenous shape without aggregation or lattices. These images illustrate nano-scale crystallinity.
Fig. 1.
XRD pattern of Ag NPs shows a cubic lattice structure of polycrystals
Fig. 2.
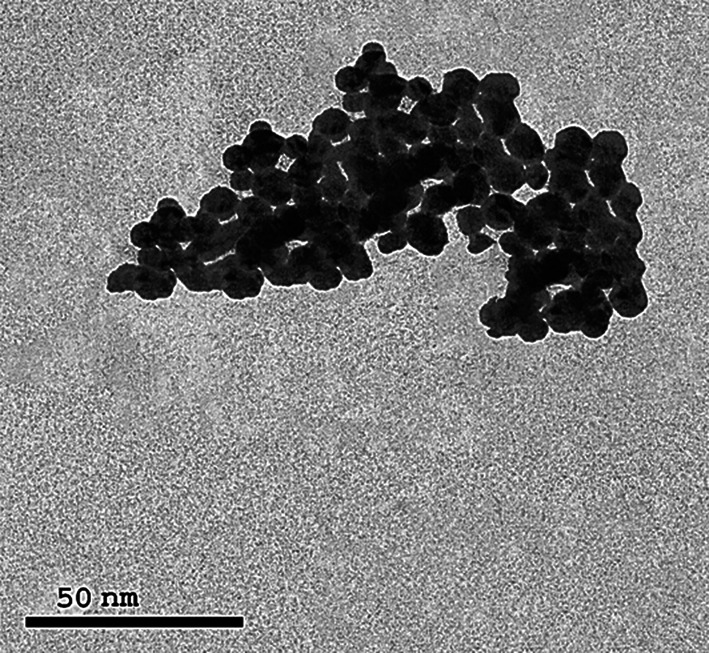
TEM image of Ag NPs
Anthelmintic effects of Ag NPs
As shown in Table 1, silver nanoparticles administration resulted in a significant decrease in the worm burden in treated rats received 2 mg/kg b.w. (n = 48) and 4 mg/kg b.w. (n = 26) with a mean intensity of infection was 6.85 ± 0.46 and 3.71 ± 0.42, respectively, compared to those collected from untreated rats (n = 74, 10.57 ± 1.13). Regarding the worm count, Ag NPs induced significant reduction (P < 0.001) of 35.13% in treated rats receiving 2 mg/kg b.w. and 64.86% in the group receiving 4 mg/kg b.w.
Table 1.
Reduction percentages of S. muris naturally infected Wistar rats (n = 7) after Ag NPs treatment
| Groups | Worm count | Intensity of infection | Percent of reduction |
|---|---|---|---|
| Untreated (infected) | 74 | 10.57 ± 1.13 | 0 |
| 2 mg/kg Ag NPs | 48 | 6.85 ± 0.46* | 35.13% |
| 4 mg/kg Ag NPs | 26 | 3.71 ± 0.42** | 64.86% |
Data are presented as mean ± SE; One-way ANOVA followed by least significant difference post hoc tests to compare group means and p < 0.05 was considered significant
Microscopic examination (based on 5 mature specimens).
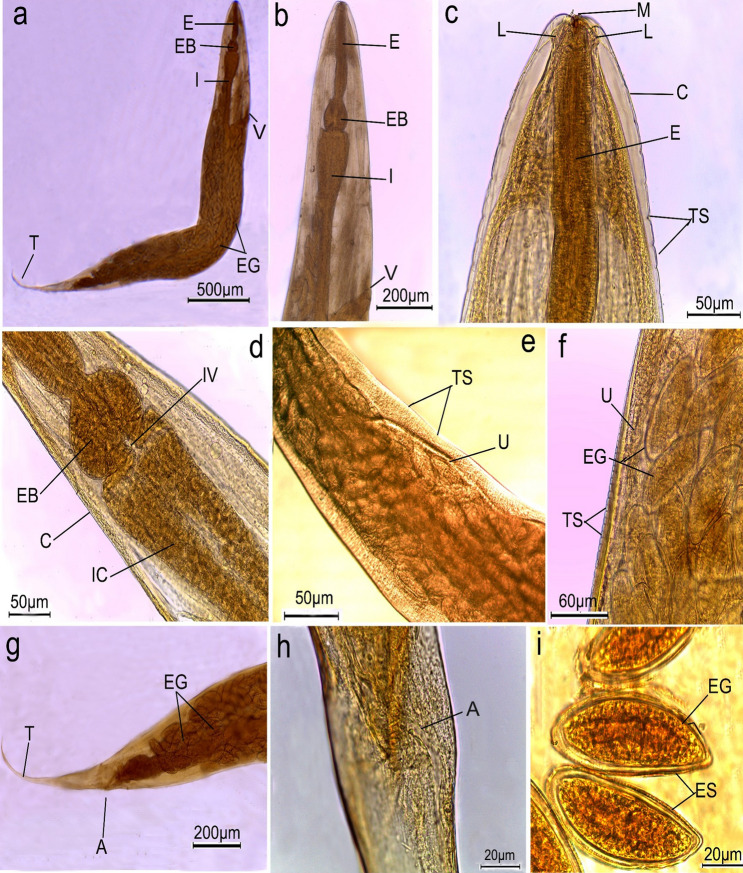
Worms collected from untreated (control) rats displayed typical S. muris characteristics. bodies were 3 to 5 mm long and 0.12 to 0.15 mm wide, with colorless to creamy bodies and narrow posterior extremities. Heads were bulbous with triradiate small mouth openings surrounded by three equal, well-developed fleshy lips (one dorsal and two ventrolateral) that lacked labial papillae (Figs. 3a–c, 5a, b). The vulva is located at the anterior part of the body (Fig. 3a, b). Buccal cavities led to short esophagi which were further subdivided into anterior cylindrical and globular bulbs (Fig. 3b, d). The latter led to simple tubular intestines via intestinal valves (Fig. 3d). The body cuticle was found to be transversally annulated (Figs. 3e, f, 5e). The uterus nearly occupies the entire body, is densely filled with eggs (Fig. 3f, g), and ends with a pointed tail measuring 0.57–0.69 mm long (Figs. 3g, 5j) with anal opening (Fig. 3h). The ova were elliptical, flattened on one side, and surrounded with egg sheathes measuring 70 × 32 µm in length (Fig. 3i). All the collected worms were female.
Fig. 3.
Photomicrographs of female Syphacia muris isolated from control rats cleared with lactophenol. a whole worm showing oesophagus (E), oesophageal bulb (EB), intestine (I), vulva opening (V), eggs (EG) and pointed tail (T); b, c high magnification of anterior region showing mouth (M) surrounded by three lips (L), oesophagus (E), body covered by cuticle (C) with transverse striations (TS) and vulva opening (V); d fore-body region showing oesophageal bulb (EB), intestinal valve (IV) lead to intestinal caeca (IC); e, f mid-body region showing uterus (U), folds containing eggs (EG); g, h posterior end of female worm showing anal opening (A) and terminated with tail tip (T) i high magnification of eggs (EG) surrounded by egg sheath (ES)
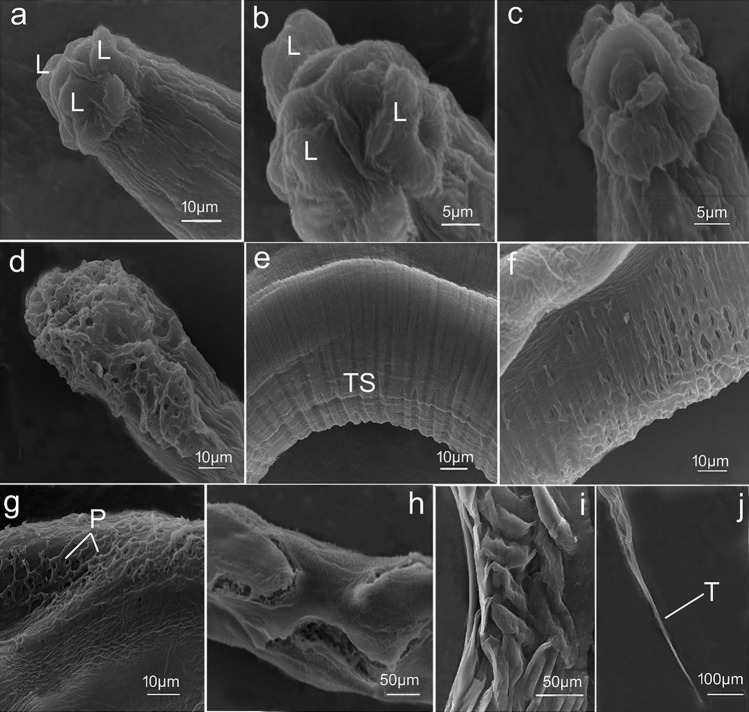
Fig. 5.
Scanning electron micrographs of female Syphacia muris a, b, e, j worms isolated from control rats a, b cephalic region showing three well-developed lips (L), one dorsal and two submedial ventral; e regular transverse striations (TS) of cuticle. j posterior extremity with a sharp tail region (T); c, f, g worms recovered from treated rats received 2 mg/kg Ag NPs; c wrinkling of cephalic region; f disruption and damage of cuticle striations; g appearance of conspicuous pits (P) on the body surface; d, h, i worms recovered from treated rats received 4 mg/kg b.w. Ag NPs; d complete deformation and erosion of the head region; h, i deep injury of the body surface with burst cuticle
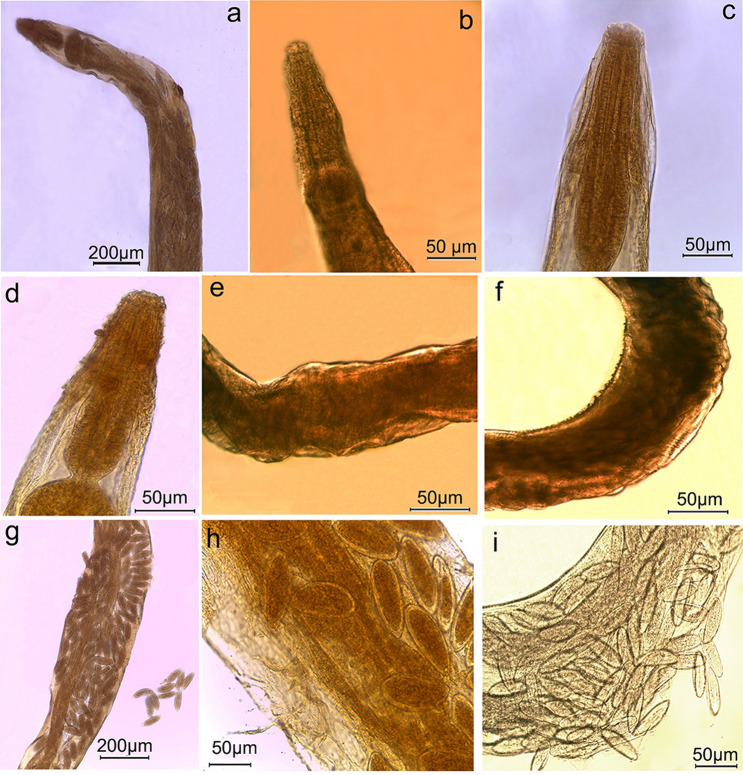
In contrast, nematodes collected from treated rats revealed morphological changes following Ag NPs administration. Worms isolated from treated rats received 2 mg/kg b.w. of Ag NPs had wrinkled and shrunken cephalic region with completely indistinguishable lips (Figs. 4a–c, 5c). In addition, the cuticle was disrupted and damaged (Fig. 5f) with conspicuous pits on body surfaces (Fig. 5g). The cephalic region of worms retrieved from treated rats received 4 mg/kg b.w. Ag NPs revealed complete deformation (Fig. 5d), and body cuticles showed significant surface injury with cuticle layers bursting in some areas (Figs. 4g–I, 5h, i). Furthermore, several nematodes had severely damaged uteri and egg release (Fig. 4i).
Fig. 4.
Photomicrographs of Syphacia muris isolated from treated rats a, b, c, e, f worms isolated from treated rats received 2 mg/kg b.w. Ag NPs displaying a, b, c shrunken cephalic region with completely indistinguishable lips; e wrinkled body surfaces f cuticle striation becomes rigid with appearance of conspicuous pits on body surfaces; d, g, h, i worms recovered from treated rats received 4 mg/kg b.w. Ag NPs showing d deformation of cephalic region g, h, i transverse annulations of body cuticle are peeled off and eggs become free
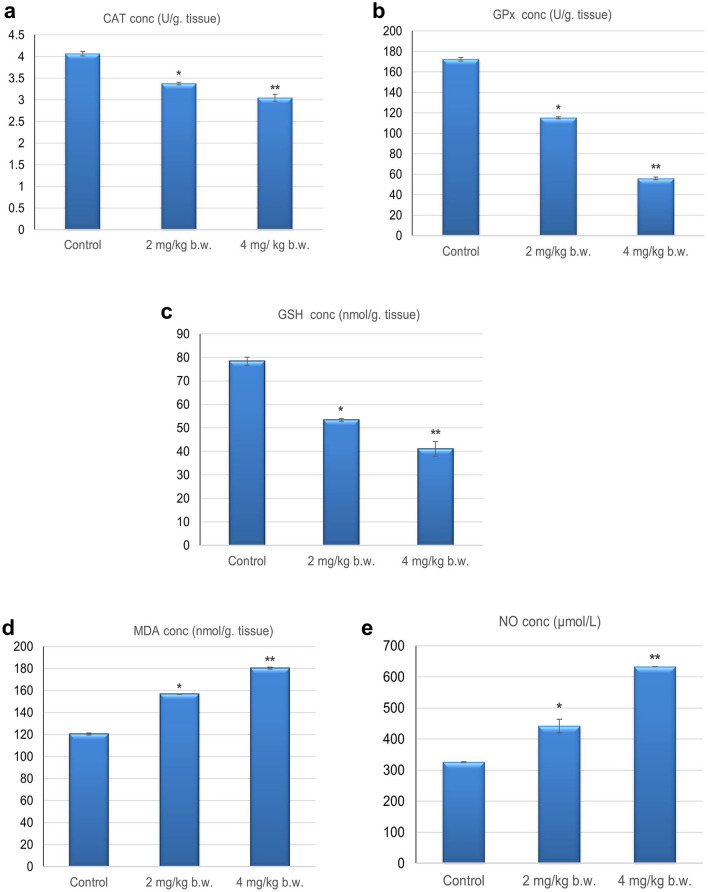
Oxidative stress biomarkers
Worms that were retrieved from rats given 2 and 4 mg/kg of Ag NPs for 14 days showed different effects on the levels of oxidative stress indicators when compared with those collected from untreated rats. Level of antioxidant enzymes, catalase (CAT) (3.37 ± 0.079; 3.04 ± 0.051) and glutathione peroxidase (GPx) (115.14 ± 0.94; 55.80 ± 1.7) were significantly (p < 0.05) decreased as well as a significant decline (p < 0.05) in GSH content (53.38 ± 3.13; 41.04 ± 1.71) was observed in worms recovered from treated rats received low and high doses respectively, compared to those collected from untreated rats as shown in Fig. 6a–c. Furthermore, Ag NPs triggered lipid peroxidation in S. muris, as evidenced by a significant (p < 0.05) increase in malondialdehyde (MDA) level (156.67 ± 0.35; 180.29 ± 1.0). Also, nitric oxide (NO) concentration (441.78 ± 22.8; 632.91 ± 0.01) exhibited a significant increase (p < 0.05) in worms collected from treated rats in a dose-dependent manner as shown in Fig. 6d, e. Thus, nematodes recovered from treated rats suffering nitro-sative stress compared to worms collected from untreated rats.
Fig. 6.
Anthelminthic effects of Ag NPs on oxidative stress markers of Syphacia muris isolated from untreated (control) and treated groups a catalase (CAT); b glutathione peroxidase (GPx); c reduced glutathione (GSH); d malondialdehyde (MDA); e nitric oxide (NO)
Ag NPs impact on host tissues
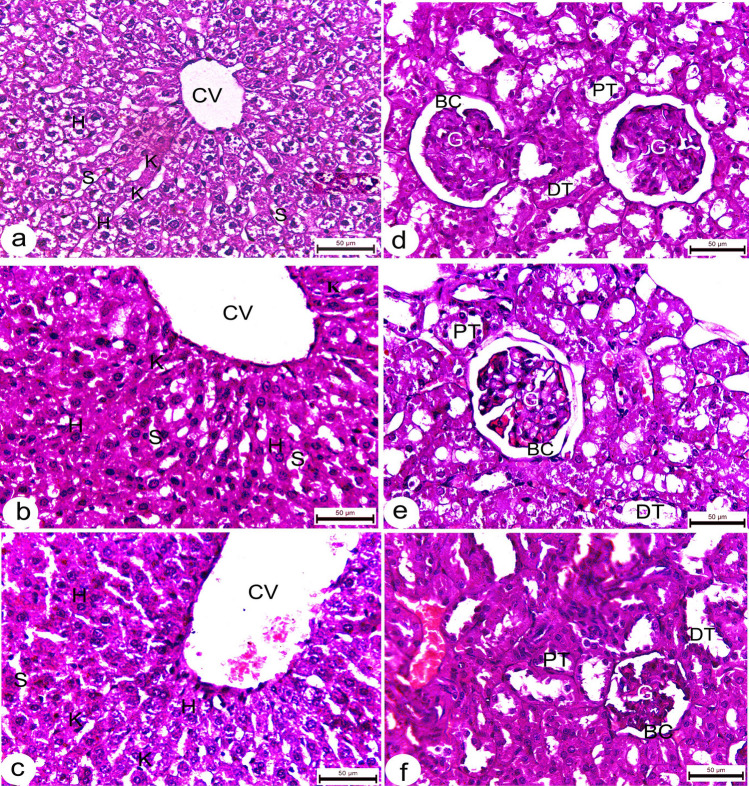
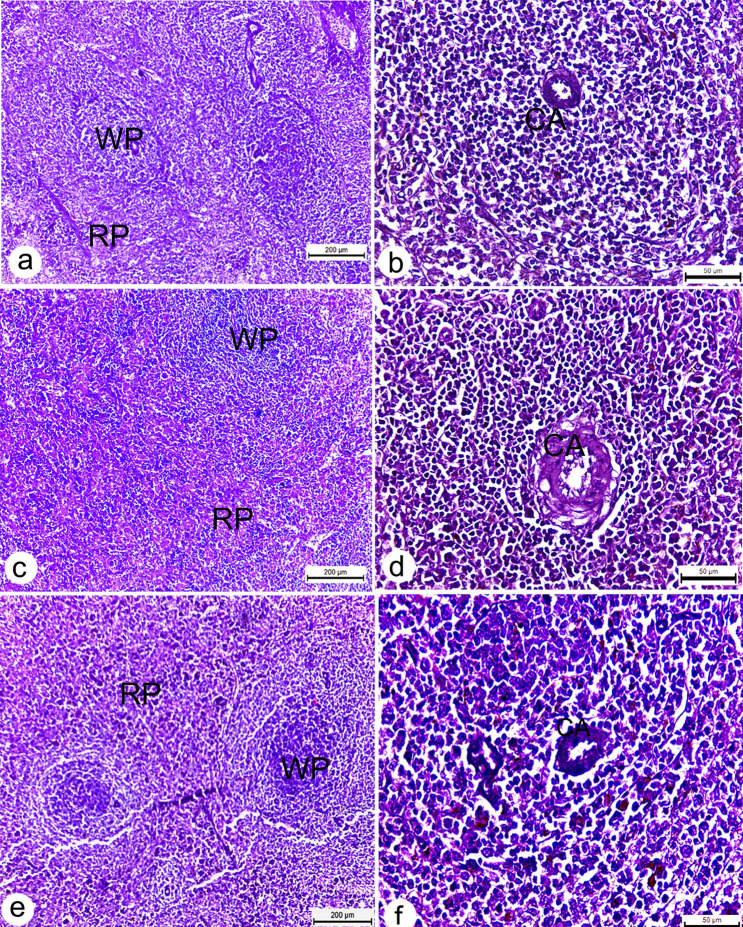
Sections from uninfected rats demonstrated normal histological architecture. Liver displayed a well-organized lobular appearance, including healthy polygonal hepatocytes with homogenous cytoplasm and randomly distributed sinusoids (Fig. 7a). The kidneys showed regularly formed, convoluted tubules with normal hematopoietic tissues and numerous renal corpuscles with well-developed glomeruli (Fig. 7b). Spleens exhibited typical red pulp and dark patches of white pulp structures reflecting predominant small lymphocytes and lymphoid follicles (Fig. 8a, b). Also, sections of treated rats’ tissues showed regular architecture with a normal cellular appearance of the liver (Fig. 7b, c), kidney (Fig. 7e, f) and spleen tissues (Fig. 8c–f).
Fig. 7.
Photomicrographs of liver and kidney tissues of Wistar rats. a liver tissues from untreated rats showing normal hepatic architecture with healthy polygonal hepatocytes (H), well organized hepatic sinusoids (S), Kuppfer cells (K) and central vein (CV); b, c liver section of treated rats received 2.4 mg/kg b.w. Ag NPs showing relatively normal hepatic architecture; d kidney tissues of untreated rats showing regular architecture with typical appearance of glomeruli (G) with abundant Bowman’s capsules (BC), and distal (DT) and proximal tubules (PT); e, f kidney section of treated rats received 2,4 mg/kg b.w. Ag NPs displaying normal cellular distribution. All sections stained with H&E. scale bars = 50 µm
Fig. 8.
Photomicrographs of splenic sections of Wistar rats. a, b spleen section of untreated rats showing well defined splenic architecture, including healthy lymphoid cells, sinuses and central artery (CA); c–f spleen section from treated rats received 2,4 mg/kg b.w. Ag NPs showing normal histology of both red (RP) and white pulp (WP). All sections stained with H&E. scale bars a, c, e = 200 µm, b, d, f = 50 µm
Discussion
Helminth resistance to multiple anthelmintic drugs is rapidly increasing, raising serious public health concerns (Torres-Acosta et al. 2012). Nanoparticles are arising as leading candidates for the development of new anthelmintic drugs due to their small size, remarkable surface reactivity, and biomedical applications (Adeyemi and Whiteley 2013). They have the ability to cross membranes and generate reactive oxygen species (ROS), resulting in high reactivity and, eventually, the death of infectious agents (Bhardwaj et al. 2012). So, the anthelmintic potential of nanoparticles has been constantly evaluated for parasitic infection control (Ali et al. 2014; Esmaeilnejad et al. 2018).
Silver nanoparticles are a well-known nano-material that displays useful physicochemical characteristics (Zhang et al. 2016). These particles are noted for their impact on bacteria, fungi, viruses, and insects (Franci et al. 2015; Govindarajan and Benelli 2015; Lara et al. 2015; Aderibigbe 2017) as well as their anthelminthic capabilities (Meyer et al. 2010; Lim et al 2012). The present investigation revealed that Ag NPs are effective against Syphacia muris, as evidenced by a significant reduction in worm count (35.13% and 64.86% in treated rats received 2, 4 mg/kg of Ag NPs, respectively), which is consistent with Tomar and Preet (2016), who found that Ag NPs caused 87% mortality in adult Haemonchus contortus. On the same line, Pillai et al. (2012) reported significant anthelmintic activity of Ag NPs causing paralysis as well as death of Pheretima posthuma in a time comparable to the standard drugs, Albendazole and piperazine citrate. Similarly, Roh et al. (2009) showed that Ag NPs exerted considerable toxicity in Caenorhabditis elegans. Also, Allahverdiye et al. (2011) reviewed an inhibition in the proliferation of parasites following metal nanoparticles treatment.
Light and scanning electron microscopic examination showed deformation of surface topography of worms collected from treated rats with extensive damage of the cuticle, deeply injured regions, and bursting of cuticle along the entire worm body. In agreement with Barbosa et al. (2019) who reported that Ag NPs exhibited nematicidal activity, being the only ones able to penetrate the larval’s cuticle, alter in the tegmentum, and consequently, the death of the infecting larvae of Ancylostoma caninum (L3). Also, Chisholm and Xu (2012) showed the toxicity effect of NPs on the normal morphology of parasitic nematode. Similarly, Song et al. (2014) mentioned that during the chronic metal treatment of the nematode Caenorhabditis elegans, the front and the rest of the body were severely wrinkled from vulva to tail compared with the control group which may be related to the direct interaction and absorption of the metal with the body of worms. Moreover, Khan et al. (2015) showed a severe damage in the cuticle of the worms during the study of ZnO NPs on Gigantocotyle explanatum. Furthermore, Dorostkar et al. (2017) showed structural damage of cuticle and hypodermis of Toxocara vitulorum caused by zinc oxide and iron oxide nanoparticles. Because the cuticle is the tough extracellular surface of the worm, which protects the animal from the environment and maintains the morphology and integrity of the body, any changes could result in worm death (Song et al. 2014). This might be because NPs trigger the production generation of hydroxyl ions and ROS, which cause membrane damage of pathogens (Li et al. 2011; Yu et al. 2013).
The antioxidant system was developed to combat the negative impacts of free radicals, such as protein, lipid, and DNA oxidation (Omar et al. 2023). An imbalance between the production of free radicals and the antioxidant system can result in cell death and tissue malfunction (Adefegha et al. 2022). In the current study, following Ag NPs administration, the levels of oxidative stress markers in worms isolated from treated rats were altered. Malondialdehyde (MDA) is an end product of lipid peroxidation that has been used as an indicator of oxidative stress (Jabir and Shaker 2020). Level of MDA was increase in worms recovered from treated rats compared to those from control rats. On the same line, Ahamed et al. (2010) showed that Ag NPs increased MDA levels and altered membrane structure by promoting lipid peroxidation. Also, Zhang et al. (2020) found that the exposure of Caenorhabditis elegans to silica nanoparticles produced an increase of free radicals, including peroxide (H2O2), superoxide anion (O2), and hydroxyl radicals (OH), resulting in elevated MDA levels. Nitric oxide is extremely reactive, with several oxidative molecules producing a variety of reactive nitrogen species (RNS). This has the potential to attack biological systems, causing severe irreversible destruction of biomolecules as well as cellular damage to many organs (Hsieh et al. 2014). In this study, both concentrations of Ag NPs caused a significant increase in nitric oxide (NO) levels in worms collected from treated rats. Excess NO reacts with superoxide to form peroxynitrite, a powerful oxidant linked to a variety of diseases (Lotfy et al. 2022; Salt et al. 2003). This is consistent with the findings of Dorostkar et al. (2017), who found an increase in NO levels in Toxocara vitulorum after treatment with zinc oxide and iron oxide nanoparticles. Furthermore, Unfried et al. (2007) found that increasing NO levels destroy subcellular architecture, resulting in worm paralysis, which is supported by our microscopic findings.
Helminth parasites have a potent antioxidant system, including CAT and GPx enzymes, to protect themselves from oxidative stress caused by the host (Khan et al. 2015). The most crucial defense antioxidants are catalase and GPx, which turn existing free radicals into less dangerous molecules that assist the body fight them and limit the generation of new free radical species, and their absence is highly reactive and toxic to the parasite (Chiumiento and Bruschi 2009). In accordance with previous studies by Brophy et al. (1995) we found that S. muris collected from treated rats had a considerable decline in the activity of both antioxidant enzymes, CAT and GPx. Furthermore, it was noted by Mamdouh et al. (2022) and Youssef et al. (2022) that reduced antioxidant enzyme activities were indicators of oxidative stress brought on by excessive ROS formation. As a result, suppressing or disrupting this parasite defense system would be an effective target that could aid in the eradication of the infection. Also, GSH is the most abundant non-enzymatic molecule and a primary component of detoxification pathways, shields the cell from the harmful effects of endogenous and external oxidants (Kiran et al. 2019). Concentrations of GSH were significantly decreased in S. muris collected from treated rats, which is consistent with Xiong et al. (2011) and Ali et al. (2012) who suggested that a decrease in GSH content may be a common response to metal nanoparticle exposure.
In the present study, sections of liver, kidney, and spleen of Ag NPs treated rats appeared normal with typical cellular architecture as seen in untreated rats. According to previous research, nanoparticles enter the systemic circulation of mammals with low toxicity and risk (Hillyer and Albrecht 2001; Lansdown 2006). On the same line, Kuhnel and Nickel (2014) stated that silver nanoparticles revealed little health effects. Similarly, Stebounova et al. (2011) reported minimal adverse effects of silver nanoparticles. Additionally, recent research on the toxicity of nano-silver in human tissues has shown that it can be used as a safe pharmaceutical agent (Hussein et al. 2020). Moreover, neither Ji et al. (2007) nor Sung et al. (2011) discovered any evidence of harm caused by silver nanoparticles. Also, Hagiu et al. (2015) discussed the use of silver nanoparticles in reconstructive surgery, degenerative disorders, and anti-aging therapy. Furthermore, Mclaughlin et al. (2016), Urie et al. (2018) and El-Aassar et al. (2020) reported that metallic nanoparticles facilitate tissue repair and regeneration. Overall, Ag NPs exhibit anthelmintic effects on S. muris infected laboratory Wistar rats via oxidative/nitrosative stress and morphological alterations with no evidence of an adverse impact on host tissues as suggested by Cromwell et al. (2014) who reported that Ag NPs possess nematicidal activity that may provide an alternative to high-risk synthetic nematicides.
Conclusion
The current study revealed that Ag NPs possessed potential anthelmintic activity against Syphacia muris inducing irreversible damage to the structural architecture of the cuticle, which might disrupt its physiological processes and integrity of the body and caused an overproduction of reactive oxygen species and a deficiency in the antioxidant defense system, and offering an alternative for the classical anthelmintic drugs. This is the first study introduces in vivo evaluation of Ag NPs efficacy against S. muris without notable toxicity in laboratory rats. Thus, we can say that Ag NPs is a promising anthelminthic drug against infectious agents.
Acknowledgements
The Faculty of Science, Cairo University, Egypt, supports this work. The authors extend their appreciation to members of the Zoology Department for helping to complete this work.
Author contributions
MF and SH have guided the development of ideas, MF and NA prepared different figures required, MF and HA wrote and revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Declarations
Conflict of interest
All authors assisted with the design and conduct of the studies. There were no conflicting interests that could have influenced the conduct and reporting of these studies.
Ethics approval and consent to participate
All procedures of using laboratory animals in this study met the Ethics of Research Committee regulations at the Faculty of Science, Cairo University and received the Approval Number: CUIF7519.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adefegha SA, Dada FA, Oyeleye SI, Oboh G. Effect of oral berberine administration on the renal profiles of adenosine deaminase, arginase, and nitric oxide in streptozotocin-induced diabetic nephropathy of rats. Comp Clin Path. 2022;31:255–263. doi: 10.1007/s00580-022-03329-1. [DOI] [Google Scholar]
- Aderibigbe BA. Metal-based nanoparticles for the treatment of infectious diseases. Molecules. 2017;22(1370):1–37. doi: 10.3390/molecules22081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi OS, Whiteley CG. Interaction of nanoparticles with arginine kinase from Trypanosoma brucei: kinetic and mechanistic evaluation. Int J Biol Macromol. 2013;62:450–456. doi: 10.1016/j.ijbiomac.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Posgai R, Gorey TJ, Nielsen M, Hussain SM, Rowe JJ. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol Appl Pharmacol. 2010;242(3):263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Ali D, Alarifi S, Kumar S, Ahmed M, Siddiqui MA. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat Toxicol. 2012;124(125):83–90. doi: 10.1016/j.aquatox.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Ali M, Afzal M, Verma M, Bhattacharya SM, et al. Therapeutic efficacy of poly (lactic-co-glycolic acid) nanoparticles encapsulated ivermectin (nano-ivermectin) against brugian filariasis in experimental rodent model. Parasitol Res. 2014;113:681–691. doi: 10.1007/s00436-013-3696-5. [DOI] [PubMed] [Google Scholar]
- Ali R, Ahmad N, Mussarat S, Majid A, Alnomasy SF, Khan SN. Nanoparticles as alternatives for the control of Haemonchus contortus: a systematic approach to unveil new anti-haemonchiasis agents. Front Vet Sci. 2021;8:789977. doi: 10.3389/fvets.2021.789977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiye AM, Abamor ES, Bagirova M, Tafailovich M. Antimicrobial effect of TiO2 and Ag2O nanoparticles against drug resistant bacteria and Leishmania parasites. Future Microbiol. 2011;6(8):933–940. doi: 10.2217/FMB.11.78. [DOI] [PubMed] [Google Scholar]
- Ayinmode A, Ndudim NF, Obebe OO. Prevalence of gastrointestinal parasites of rodents in Ibadan, Nigeria. Niger Vet J. 2015;36(2):1158–1164. [Google Scholar]
- Barbosa ACMS, Silva LPC, Ferraz CM, Tobias FL, de Araújo JV, Loureiro B, Braga GMAM, Veloso FBR, Soares FEF, Fronza M, Braga FR. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int J Nanomed. 2019;14:2341–2348. doi: 10.2147/IJN.S193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke J, Bajer A, Sinski E, Wakelin D. Interactions involving intestinal nematodes of rodents: experimental and field studies. Parasitology. 2001;122(S1):S39–S49. doi: 10.1017/S0031182000016796. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Saudagar P, Dubey V. Nanobiosciences: a contemporary approach in antiparasitic drugs. Mol Cell Pharmacol. 2012;4:97–103. [Google Scholar]
- Bhatia D, Mittal A, Malik DK. Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians. 3 Biotech. 2016;6:196. doi: 10.1007/s13205-016-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy PM, Patterson LH, Pritchard DL. Secretory nematode SOD–offensive or defensive? Int J Parasitol. 1995;25(7):856–865. doi: 10.1016/0020-7519(95)00005-m. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Caito SW, Aschner M. Quantification of glutathione in Caenorhabditis elegans. Curr Protoc Toxicol. 2015;64:6–18. doi: 10.1002/0471140856.tx0618s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm AD, Xu S. The Caenorhabditis elegans epidermis as a model skin II: differentiation and physiological roles. Wiley Interdiscip Rev Dev Biol. 2012;1(6):879–902. doi: 10.1002/wdev.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumiento L, Bruschi F. Enzymatic antioxidant systems in helminth parasites. Parasitol Res. 2009;105(3):593–603. doi: 10.1007/s00436-009-1483-0. [DOI] [PubMed] [Google Scholar]
- Cromwell WA, Yang J, Starr JL, Jo YK. Nematicidal effects of silver nanoparticles on root-knot nematode in Bermudagrass. J Nematol. 2014;46(3):261–266. [PMC free article] [PubMed] [Google Scholar]
- Delavari M, Dalimi A, Ghaffarifar F, Sadraei J. In vitro study on cytotoxic effects of ZnO nanoparticles on promastigote and amastigote forms of Leishmania major. Iran J Parasitol. 2014;9(1):6–13. [PMC free article] [PubMed] [Google Scholar]
- Dorostkar A, Ghalavand M, Nazarizadeh A, Tat M, Hashemzadeh SM. Anthelmintic effects of zinc oxide and iron oxide nanoparticles against Toxocara vitulorum. Int Nano Lett. 2017;7(2):157–164. doi: 10.1007/s40089-016-0198-3. [DOI] [Google Scholar]
- El Mahdy MM, Salah Eldin TA, Aly HS, Mohammed FF, Shaalana MI. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp Toxicol Pathol. 2014;67:21–29. doi: 10.1016/j.etp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- El-Aassar MR, Ibrahim OM, Fouda MMG, El-Beheri NG, Agwa MM. Wound healing of nanofiber comprising polygalacturonic/hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohydr Polym. 2020;238:116–175. doi: 10.1016/j.carbpol.2020.116175. [DOI] [PubMed] [Google Scholar]
- Esmaeilnejad B, Samiei A, Mirzaei Y, Farhang-Pajuh F. Assessment of oxidative/nitrosative stress biomarkers and DNA damage in Haemonchus contortus, following exposure to zinc oxide nanoparticles. Acta Parasitol. 2018;63:563–571. doi: 10.1515/ap-2018-0065. [DOI] [PubMed] [Google Scholar]
- Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang SS, Hallem EA. Mechanisms of host seeking by parasitic nematodes. Mol Biochem Parasitol. 2016;208(1):23–32. doi: 10.1016/j.molbiopara.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan M, Benelli G. Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organism. Parasitol Res. 2015;115:925–935. doi: 10.1007/s00436-015-4817-0. [DOI] [PubMed] [Google Scholar]
- Hagiu BA, Vrinceanu N, Postolache P (2015) The hypothesis regarding the regenerative action of silver nanoparticles. In: E-Health and Bioengineering Conference (EHB), pp 1–4. 10.1109/EHB.2015.7391524
- Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 2001;90(12):1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- Hsieh HJ, Liu CA, Huang B, Tseng H, Wang D. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci. 2014;21(1):3. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein J, El-Naggar ME, Fouda MMG, Morsy OM, et al. The efficiency of blackberry loaded Ag NPs, Au NPs and Ag& Au NPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. Int J Biol Macromol. 2020;159:1084–1093. doi: 10.1016/j.ijbiomac.2020.05.115. [DOI] [PubMed] [Google Scholar]
- Ilic V, Krstic A, Katic-Radivojevic S, Jovcic G, Milenkovic P, Bugarski D. Syphacia obvelata modifies mitogen-activated protein kinases and nitric oxide synthases expression in murine bone marrow cells. Parasitol Int. 2010;59:82–88. doi: 10.1016/j.parint.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Jabir AF, Shaker AS (2020) Roles of superoxide dismutase (SOD), malondialdehyde (MDA), 8-iso-prostaglandinF2a (8-iso-PGF2a) as oxidative stress in development and progression of Brest cancer in Iraqi females patients. Al-Qadisiyah J Pure Sci 25(1):1–4. 10.29350/jops.2020.25.1.1053
- Ji JH, Jung JH, Kim SS, Yoon JU, et al. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague–Dawley rats. Inhal Toxicol. 2007;19:857–871. doi: 10.1080/08958370701432108. [DOI] [PubMed] [Google Scholar]
- Khalil AI, Lashein GH, Morsy GH, Abd El-Mottaleb DI. Oxyurids of wild and laboratory rodents from Egypt. Life Sci J. 2014;11(3):94–107. [Google Scholar]
- Khan YA, Singh BR, Ullah R, Shoeb M, Naqv AH, Abidi SM. Anthelmintic effect of biocompatible zinc oxide nanoparticles (ZnO NPs) on Gigantocotyle explanatum, a neglected parasite of Indian water buffalo. PLoS ONE. 2015;10(7):0133086. doi: 10.1371/journal.pone.0133086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran T, Karaman U, Arici Y, Yildiz S. Comparison of malondialdehyde, nitric oxide, adenosine deaminase and glutathione levels in patients with Entamoeba coli, Enterobius vermicularis, Giardia intestinalis, Demodex spp. positive, hydatid cyst and Toxoplasma gondii serum positive. Ann Med Res. 2019;26:1420. doi: 10.5455/annalsmedres.2019.05.236. [DOI] [Google Scholar]
- Kotze AC. Catalase induction protects Haemonchus contortus against hydrogen peroxide in vitro. Int J Parasitol. 2003;33:393–400. doi: 10.1016/s0020-7519(03)00012-2. [DOI] [PubMed] [Google Scholar]
- Kuhnel D, Nickel C. The OECD expert meeting on ecotoxicology and environmental fate—towards the development of improved OECD guidelines for the testing of nano-materials. Sci Total Environ. 2014;472:347–353. doi: 10.1016/j.scitotenv.2013.11.055. [DOI] [PubMed] [Google Scholar]
- Lansdown ABG. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- Lara HH, Romero-Urbina DG, Pierce C, et al. Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J Nanobiotech. 2015;13(91):2–12. doi: 10.1186/s12951-015-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol. 2011;45(5):1977–1983. doi: 10.1021/es102624t. [DOI] [PubMed] [Google Scholar]
- Lim D, Roh JY, Eom HJ, Hyun JW, Choi J. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ Toxicol Chem. 2012;31:585–592. doi: 10.1002/etc.1706. [DOI] [PubMed] [Google Scholar]
- Lotfy BMM, Mousa MR, El-Shehry MSFE et al (2022) Therapeutic potency of Gallium verum extract on ethanol-induced gastric ulcer in rats. Biointerface Res Appl Chem 12:6010–6020. 10.33263/BRIAC125.60106020
- Mamdouh S, Mohamed AS, Mohamed HA, Fahmy WS. Zn contamination stimulate agonistic behavior and oxidative stress of crayfishes (Procambarus clarkii) J Trace Elem Med Biol. 2022;69:126895. doi: 10.1016/j.jtemb.2021.126895. [DOI] [PubMed] [Google Scholar]
- Massetti M, Locci T, Cecchettini A, et al. Nitric oxide synthase immunoreactivity in the nematode Trichinella britovi. Evidence for nitric oxide production by the parasite. Int J Parasitol. 2004;34:715–721. doi: 10.1016/j.ijpara.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Mclaughlin S, Podrebarac J, Ruel M. Nano-engineered biomaterials for tissue regeneration: What has been achieved so far? Front Mater. 2016;3:1–28. doi: 10.3389/fmats.2016.00027. [DOI] [Google Scholar]
- Meade TM, Watson J. Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. J Am Assoc Lab Anim Sci. 2014;53:661–667. [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Lord CA, Yang XY, et al. Intracellular uptake and associated toxicity of silver nano articles in Caenorhabditis elegans. Aquat Toxicol. 2010;100:140–150. doi: 10.1016/j.aquatox.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Okorafor KA, Odaibo AB, Eleng IE, Okete JA. Occurrence and prevalence of ecto- and gastrointestinal parasites in cane rats (Thryonomyss winderianus) from Oyo State, south-western Nigeria. Eur J Zoo Res. 2012;1(3):70–76. [Google Scholar]
- Omar TY, Elshenawy HIA, Abdelfattah MA, Al Shawoush AM, Mohamed AS, Saad DY (2023) Biointerfrence between zinc oxide/alginate nanocomposites and freshwater bivalve. Biointerface Res Appl Chem 13(3):277. 10.33263/BRIAC133.27
- Pillai RK, John SS, Joseph CT, Chandramohanakumar N, Balagopalan M. Vermifugal activity of biofabricated silver nanoparticles. Res J Recent Sci. 2012;1:47–51. [Google Scholar]
- Pinto RM, Vicente JJ, Noronha D, Gonçalves L, Gomes DC. Helminth parasites of conventionally maintained laboratory mice. Mem Inst Oswaldo Cruz. 1994;89:33–40. doi: 10.1590/s0074-02761994000100007. [DOI] [PubMed] [Google Scholar]
- Pinto RM, Goncalves L, Noronha D, Gomes DC (2001) Worm burdens in outbred and inbred laboratory rats with morphometric data on Syphacia muris (Yamaguti, 1935) Yamaguti, 1941 (Nematoda, Oxyuroidea). Mem Inst Oswaldo Cruz. Rio de Janeiro 96(1):133–136. 10.1590/s0074-02762001000100016 [DOI] [PubMed]
- Pritchard MH, Kruse GOW (1982) The collection and preservation of animal parasites. Technical Bulletin No. 1, The Harold W. Manter Laboratory. University of Nebraska Press, Lincoln. 141
- Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43(10):3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Ross LE, Dykstra M (2003) Biological electron microscopy: theory, techniques and troubleshooting. Springer, USA
- Salt IP, Morrow VA, Brandie FM, Connell JM, Petrie JR. High glucose inhibits insulin-stimulated nitric oxide production without reducing endothelial nitric-oxide synthase Ser1177 phosphorylation in human aortic endothelial cells. J Biol Chem. 2003;278:18791–18797. doi: 10.1074/jbc.M210618200. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ooi HK, Nonaka N, Oku Y, Kamiya M. Antibody production in Syphacia obvelata-infected mice. J Parasitol. 1995;81:559–562. doi: 10.2307/3283853. [DOI] [PubMed] [Google Scholar]
- Song S, Guo Y, Zhang X, Zhang X, Zhang J, Ma E. Changes to cuticle surface ultrastructure and some biological functions in the nematode Caenorhabditis elegans exposed to excessive copper. Arch Environ Contam Toxicol. 2014;66(3):390–399. doi: 10.1007/s00244-013-9991-4. [DOI] [PubMed] [Google Scholar]
- Stebounova LV, Adamcakova Dodd A, Kim JS, et al. Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Part Fibre Toxicol. 2011;8:5. doi: 10.1186/1743-8977-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueta T, Miyoshi I, Okamura T, Kasai N. Experimental eradication of pinworms (Syphacia obvelata and Aspiculuris tetraptera) from mice colonies using ivermectin. Exp Anim. 2002;51:367–373. doi: 10.1538/expanim.51.367. [DOI] [PubMed] [Google Scholar]
- Sung JH, Ji JH, Song KS, Lee JH, et al. Acute inhalation toxicity of silver nanoparticles. Toxicol Ind Health. 2011;27:149–154. doi: 10.1177/0748233710382540. [DOI] [PubMed] [Google Scholar]
- Tomar RS, Preet S. Evaluation of anthelmintic activity of biologically synthesized silver nanoparticles against the gastrointestinal nematode, Haemonchus contortus. J Helmin. 2016;91(4):1–8. doi: 10.1017/S0022149X16000444. [DOI] [PubMed] [Google Scholar]
- Torres-Acosta JF, Mendoza-de-Gives P, Aguilar-Caballero AJ, Cuéllar-Ordaz JA. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Vet Parasitol. 2012;189:89–96. doi: 10.1016/j.vetpar.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Unfried K, Albrecht C, Klotz LO, et al. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1(1):52–71. doi: 10.1080/00222930701314932. [DOI] [Google Scholar]
- Urie R, Ghosh D, Ridha I, Rege K. Inorganic nanomaterials for soft tissue repair and regeneration. Annu Rev Biomed Eng. 2018;20:353–374. doi: 10.1146/annurev-bioeng-071516-044457. [DOI] [PubMed] [Google Scholar]
- Wang Q, Rosa BA, Jasmer DP, Mitreva M. Pan-Nematoda transcriptomic elucidation of essential intestinal functions and therapeutic targets with broad potential. Ebio Medicine. 2015;2(9):1079–1089. doi: 10.1016/j.ebiom.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Lu J, Xu H, Patel A, Chen ZS, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20:595–601. doi: 10.1016/j.drudis.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Li J, Liu X, Cui Z, Yang X, Yeung KWK, Pan H, Wu S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater Sci Eng C Mater Biol Appl. 2017;79:629–637. doi: 10.1016/j.msec.2017.05.115. [DOI] [PubMed] [Google Scholar]
- Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ. 2011;409(8):1444–1452. doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Youssef FS, Elbanna HA, Elzorba HY, et al. Synthesis and characterization of florfenicol-silver nanocomposite and its antibacterial activity against some gram positive and gram-negative bacteria. Int J Vet Sci. 2020;9(3):324–330. [Google Scholar]
- Youssef A, Baiomy A, Fahmy SR, Mohamed AS, Saad D, Desoky R (2022) Potential anti-osteoporotic effect of Allolobophora caliginosa extract in orchiectomized rats. Pharm. Sci. Asia 49:138–146. 10.29090/psa.2022.02.21.144
- Yu KN, Yoon TJ, Tehrani AM, Kim JE, Park SJ, Jeong MS, et al. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol In Vitro. 2013;27:1187–1195. doi: 10.1016/j.tiv.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Zhang F, You X, Zhu T, Gao S, Wang Y, Wang R, Yu H, Qian B. Silica nanoparticles enhance germ cell apoptosis by inducing reactive oxygen species (ROS) formation in Caenorhabditis elegans. J Toxicol Sci. 2020;45(3):117–129. doi: 10.2131/jts.45.117. [DOI] [PubMed] [Google Scholar]
- Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shen W, Xue J, Liu Y, et al. Recent advances in synthetic methods and applications of silver nanostructures. Nano-Scale Res Lett. 2018;13(1):54. doi: 10.1186/s11671-018-2450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]