Abstract
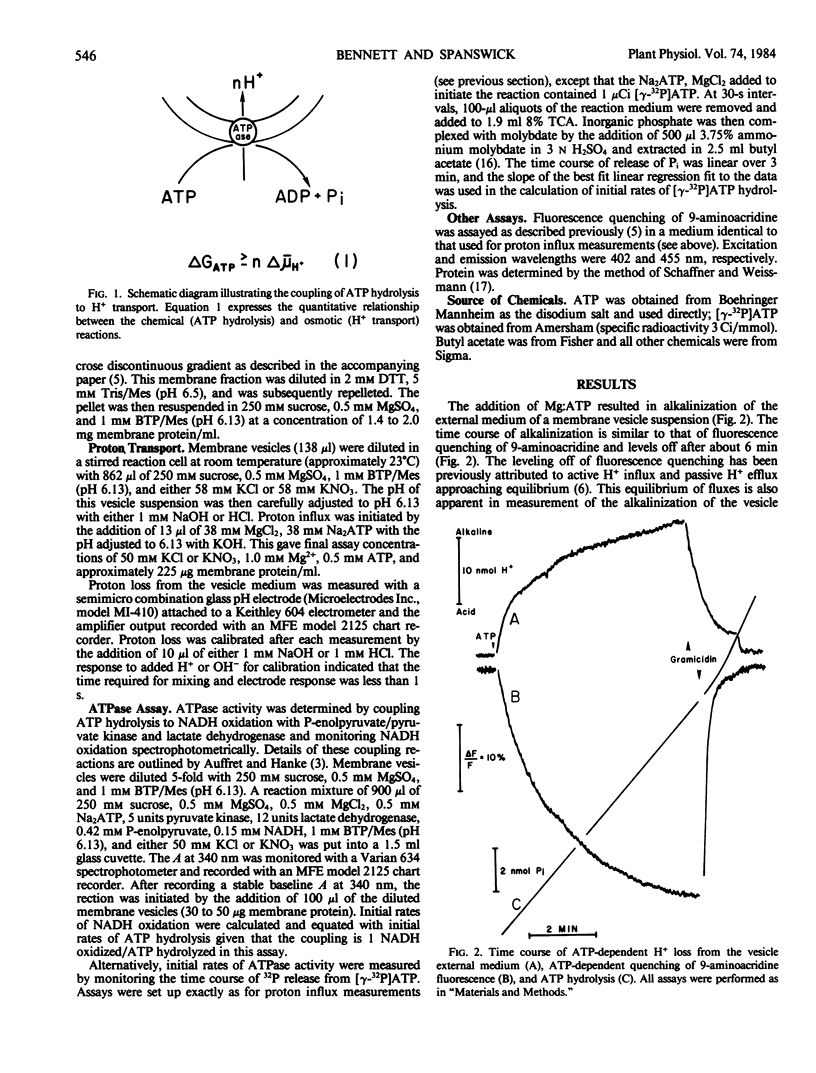
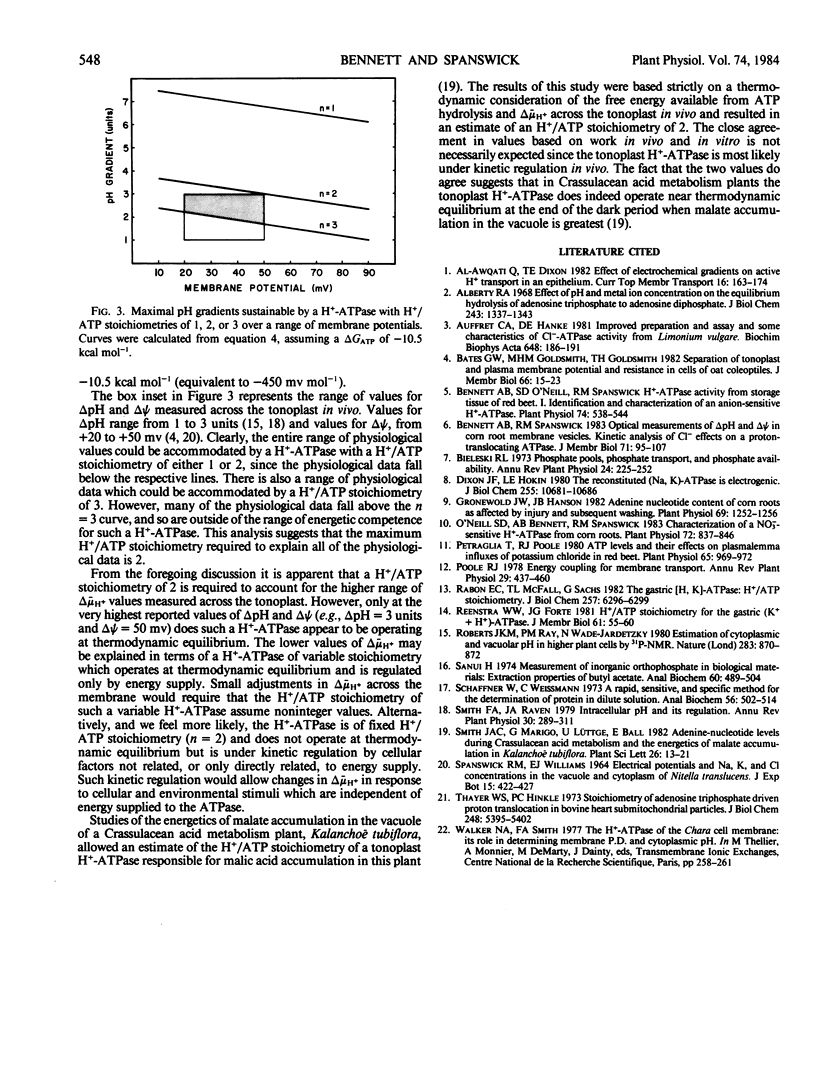
The H+/ATP stoichiometry was determined for an anion-sensitive H+-ATPase in membrane vesicles believed to be derived from tonoplast. Initial rates of proton influx were measured by monitoring the alkalinization of a weakly buffered medium (pH 6.13) following the addition of ATP to a suspension of membrane vesicles of Beta vulgaris L. Initial rates of ATP hydrolysis were measured in an assay where ATP hydrolysis is coupled to NADH oxidation and monitored spectrophotometrically (A340) or by monitoring the release of 32P from [γ-32P]ATP. Inasmuch as this anion-sensitive H+-ATPase is strongly inhibited by NO3−, initial rates of H+ influx and ATP hydrolysis were measured in the absence and presence of NO3− to account for ATPase activity not involved in H+ transport. The NO3−-sensitive activities were calculated and used to estimate the ratio of H+ transported to ATP hydrolyzed. These measurements resulted in an estimate of the H+/ATP stoichiometry of 1.96 ± 0.14 suggesting that the actual stoichiometry is 2 H+ transported per ATP hydrolyzed. When compared with the reported values of the electrochemical potential gradient for H+ across the tonoplast measured in vivo, our result suggests that the H+-ATPase does not operate near equilibrium but is regulated by cellular factors other than energy supply.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Auffret C. A., Hanke D. E. Improved preparation and assay and some characteristics of Cl--ATPase activity from Limonium vulgare. Biochim Biophys Acta. 1981 Nov 6;648(2):186–191. doi: 10.1016/0005-2736(81)90033-x. [DOI] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. The reconstituted (Na,K)-ATPase is electrogenic. J Biol Chem. 1980 Nov 25;255(22):10681–10686. [PubMed] [Google Scholar]
- Gronewald J. W., Hanson J. B. Adenine nucleotide content of corn roots as affected by injury and subsequent washing. Plant Physiol. 1982 Jun;69(6):1252–1256. doi: 10.1104/pp.69.6.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia T., Poole R. J. ATP Levels and their Effects on Plasmalemma Influxes of Potassium Chloride in Red Beet. Plant Physiol. 1980 May;65(5):969–972. doi: 10.1104/pp.65.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabon E. C., McFall T. L., Sachs G. The gastric [H,K]ATPase:H+/ATP stoichiometry. J Biol Chem. 1982 Jun 10;257(11):6296–6299. [PubMed] [Google Scholar]
- Reenstra W. W., Forte J. G. H+/ATP stoichiometry for the gastric (K+ + H+)-ATPase. J Membr Biol. 1981;61(1):55–60. doi: 10.1007/BF01870752. [DOI] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Stoichiometry of adenosine triphosphate-driven proton translocation in bovine heart submitochondrial particles. J Biol Chem. 1973 Aug 10;248(15):5395–5402. [PubMed] [Google Scholar]
- WILLIAMS M. C., WOODALL J. P., CORBET P. S. NYANDO VIRUS: A HITHERTO UNDESCRIBED VIRUS ISOLATED FROM ANOPHELES FUNESTUS GILES COLLECTED IN KENYA. Arch Gesamte Virusforsch. 1965;15:422–427. doi: 10.1007/BF01241769. [DOI] [PubMed] [Google Scholar]


