Abstract
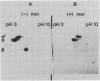
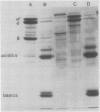
Supplemental methionine in a complete culture medium increased the methionine content of the protein fraction of cultured soybean (Glycine max L. Merrill) cotyledons (Thompson, Madison, Muenster 1981 Phytochemistry 20: 941-945). To explain the observed increase in protein methionine, we have measured the amounts and subunit compositions of 7S and 11S storage proteins and determined the amino acid compositions of the three major protein fractions (2-5S, 7S, 11S) of seeds developed on plants and of cultured cotyledons grown in the presence or absence of supplemental l-methionine. Development of cultured cotyledons was representative of development of seeds on plants. The ratios of 11S to 7S proteins, the subunit contents, and amino acid compositions of their storage protein fractions were similar, but not identical. Supplemental methionine increased the mole percent methionine in each of the three protein fractions of cultured cotyledons and changed the amounts of several other amino acids. Supplemental methionine inhibited expression of the 7S β-subunit gene. Concomitant with the absence of the β-subunit, which contains no methionine, was an increase in the ratio of 11S to 7S proteins, and an increase in the methionine content of the subunits composing these fractions. Inhibition of β-subunit gene expression by methionine in cultured cotyledons provides a reproducible, easily controlled system for the study of eucaryotic gene expression.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton K. A., Thompson J. F., Madison J. T., Rosenthal R., Jarvis N. P., Beachy R. N. The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J Biol Chem. 1982 Jun 10;257(11):6089–6095. [PubMed] [Google Scholar]
- Beachy R. N., Thompson J. F., Madison J. T. Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol. 1978 Feb;61(2):139–144. doi: 10.1104/pp.61.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Higgins T. J., Randall P. J., Spencer D. Regulation of Legumin Levels in Developing Pea Seeds under Conditions of Sulfur Deficiency: Rates of Legumin Synthesis and Levels of Legumin mRNA. Plant Physiol. 1983 Jan;71(1):47–54. doi: 10.1104/pp.71.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creason G. L., Holowach L. P., Thompson J. F., Madison J. T. Exogenous methionine depresses level of mRNA for a soybean storage protein. Biochem Biophys Res Commun. 1983 Dec 28;117(3):658–662. doi: 10.1016/0006-291x(83)91647-9. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Ditta G. S., Breidenbach R. W. Developmental regulation of cloned superabundant embryo mRNAs in soybean. Dev Biol. 1981 Apr 30;83(2):218–231. doi: 10.1016/0012-1606(81)90468-1. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Partial characterization of the acidic and basic polypeptides of glycinin. J Biol Chem. 1979 Oct 10;254(19):9921–9926. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Schuler M. A., Schmitt E. S., Beachy R. N. Closely related families of genes code for the alpha and alpha' subunits of the soybean 7S storage protein complex. Nucleic Acids Res. 1982 Dec 20;10(24):8225–8244. doi: 10.1093/nar/10.24.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Smith I. K. Sulfate transport in cultured tobacco cells. Plant Physiol. 1975 Feb;55(2):303–307. doi: 10.1104/pp.55.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Beta-conglycinin from soybean proteins. Isolation and immunological and physicochemical properties of the monomeric forms. Biochim Biophys Acta. 1977 Feb 22;490(2):370–384. doi: 10.1016/0005-2795(77)90012-5. [DOI] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Major proteins of soybean seeds. A straightforward fractionation and their characterization. J Agric Food Chem. 1976 Nov-Dec;24(6):1117–1121. doi: 10.1021/jf60208a030. [DOI] [PubMed] [Google Scholar]
- Tumer N. E., Thanh V. H., Nielsen N. C. Purification and characterization of mRNA from soybean seeds. Identification of glycinin and beta-conglycinin precursors. J Biol Chem. 1981 Aug 25;256(16):8756–8760. [PubMed] [Google Scholar]
- WOLF W. J., BRIGGS D. R. Ultracentrifugal investigation of the effect of neutral salts on the extraction of soybean proteins. Arch Biochem Biophys. 1956 Jul;63(1):40–49. doi: 10.1016/0003-9861(56)90007-8. [DOI] [PubMed] [Google Scholar]
- Wong K. F., Dennis D. T. Aspartokinase in Lemna minor L: Studies on the in Vivo and in Vitro Regulation of the Enzyme. Plant Physiol. 1973 Feb;51(2):327–331. doi: 10.1104/pp.51.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulka A. Y., Calloway D. H. Nitrogen retention in men fed varying levels of amino acids from soy protein with or without added L-methionine. J Nutr. 1976 Feb;106(2):212–221. doi: 10.1093/jn/106.2.212. [DOI] [PubMed] [Google Scholar]