Summary
Globally 20 Neglected tropical diseases (NTDs) are prioritized by World Health Organization (WHO), of which 15 are present in the South-East Asia Region (SEAR) with all 11 countries being affected. As the region bears 54% of the global burden, “Finishing the task of eliminating neglected tropical diseases and other diseases on the verge of elimination” was identified as a regional flagship priority in 2014 with focus on lymphatic filariasis (LF), kala-azar, yaws, trachoma, and leprosy. Intensified efforts have been made to raise and sustain political commitment and momentum among partners innovate tools, interventions and strategies to accelerate elimination, and establish the process and support countries to accelerate and validate achievement of elimination targets. Seven countries have verified or validated for having eliminated at least one NTD since 2016, including yaws, LF and trachoma. Between 2010 and 2020, the number of people requiring interventions against NTDs in the South-East Asia Region reduced by 20%. The priorities in the next decade are to strengthen last-mile efforts to eliminate identified NTDs, sustain it and to use the lessons learnt to eliminate other NTDs.
Funding
None.
Keywords: Neglected tropical diseases, NTDs, WHO, South-East Asia, Flagship, Elimination
Introduction
Neglected tropical diseases (NTDs) are a diverse group of disease conditions that usually affect people living without access to adequate sanitation, basic infrastructure, and health services in tropical and sub-tropical regions. NTDs are both a cause and consequence of poverty. NTDs cause physical and psychosocial morbidity and disability, preventing children from attending schools and reducing economic productivity. Globally 20 disease conditions are prioritized as NTDs by WHO, affecting more than one billion people and cost developing economies billions of dollars every year.1
In the WHO South-East Asia Region (SEAR), 15 of these NTDs are present, with at least one NTD endemic in each of the 11 countries of the Region (Table 1). Home to a quarter of the world’s population, the WHO South-East Asia Region bears a substantial burden of NTDs. In 2013, over 700 million people in the Region required interventions against lymphatic filariasis (LF) accounting for 59% of the global estimate.2 Over 72% of the new cases of leprosy reported globally was from the Region.3 In 2014, over 354 million children in the Region required regular deworming against soil-transmitted helminthiases, accounting for 42% of the global burden.4
Table 1.
Neglected tropical diseases prioritized globally and endemic in the WHO South-East Asia Region.
 |
Given this major contribution of the Region to the global burden of NTDs, the Regional Committee of WHO South-East Asia Region declared “Finishing the task of eliminating neglected tropical diseases (NTDs) and other diseases on the verge of elimination” focusing particularly on lymphatic filariasis, kala-azar, yaws, trachoma, and leprosy as one of the Regional Flagship Priorities in 2014. For successful eradication or elimination of communicable diseases, three principal conditions need to be met: availability of effective interventions and strategies to interrupt transmission of the agent, availability of practical diagnostic tools with sufficient sensitivity and specificity to detect levels of infection that can lead to transmission, and an absence of animal reservoir and amplification in the environment.5 These five diseases were chosen because these more or less satisfied these conditions.
Since then, intensified efforts have been made to (i) raise and sustain political commitment and momentum on elimination of these diseases among Member States and donor agencies, (ii) innovate tools, interventions, and strategies to accelerate elimination, and (iii) establish the process and supporting countries to accelerate and validate achievement of elimination targets through global and regional partnerships of Member States, implementing and R&D partners and WHO. The present paper summarizes the major progress and achievements in these priority areas in the last decade, emerging challenges, priorities, and the way forward to finish the task of eliminating NTDs, based on the synthesis of data from annual reports submitted by Member States to WHO on the progress, and proceedings of relevant WHO technical advisory group meetings and peer-reviewed publications.
Progress on elimination of major NTDs
Gaining political commitments at the highest level
The elimination of any communicable disease requires sustained political commitment and adequate funding for disease surveillance, prevention and control measures, healthcare infrastructure, and research and development of new treatments and vaccines. However, NTDs, as its name indicates, continue to be neglected in the global and health agenda and therefore continued advocacy was critical to sustain momentum and availability of sufficient resources for finishing the task of eliminating NTDs. To this end, a series of high-level advocacy efforts were made in the Region in the last decade.
In September 2014 through the Dhaka declaration, Ministers of Health of countries of the WHO South-East Asia committed to institutionalize a cohesive, comprehensive and integrated approach and build partnership and capacity for controlling and eliminating vector-borne diseases in the Region.6 At its side meeting, a memorandum of understanding (MoU) to cooperate and jointly achieve regional kala-azar elimination, originally signed by Bangladesh, India and Nepal in 2005, was renewed and joined by Bhutan and Thailand, to expand and sustain the high-level commitment for Regional collaboration towards kala-azar elimination.7 In 2017, WHO-SEARO further convened a high-level regional meeting of ministers and high-level delegates from the Region as well as partners on “Keeping the Promise: ending NTDs on time in the South-East Asia Region” which concluded with the “Jakarta Call for Action” on accelerating progress towards eliminating NTDs endemic in the South-East Asia Region.8
Such opportunities encouraged Member States, WHO and partner agencies to intensify advocacy efforts for prioritizing NTDs in the national health agendas and also strengthened regional partnership to coordinate and collaborate to eliminate NTDs in the Region. This also led to continuous or increased financial support from international donor agencies, such as Bill and Melinda Gates Foundation (BMGF) for LF and kala-azar elimination, USAID for LF elimination, Sasakawa Health Foundation for leprosy elimination and the Korea International Cooperation Agency (KOICA) for LF elimination in Timor Leste.
Innovating tools, interventions, and strategies to accelerate elimination
The public health interventions recommended by WHO for elimination of five priority NTDs are shown in Table 2. Continuous efforts to develop better tools and strategies and optimize implementation of such interventions on the ground in the last decade have resulted in introduction of a few game changers that have significantly contributed to the progress of elimination of priority NTDs in the region.
Table 2.
Public health interventions recommended by World Health Organization for elimination of priority NTDs of the Region.
| Disease | Preventive chemotherapy | Intensified disease management | Vector control | WASH | Surveillance and M&E |
|---|---|---|---|---|---|
| Lymphatic Filariasis | ✔ | ✔ | ✔ | – | ✔ |
| Kala-azar | – | ✔ | ✔ | – | ✔ |
| Trachoma | ✔ | ✔ | – | ✔ | ✔ |
| Yaws | ✔ | ✔ | – | ✔ | ✔ |
| Leprosy | ✔ | ✔ | – | – | ✔ |
NTDs: neglected tropical diseases; WASH: water, sanitation and hygiene; M&E: monitoring and evaluation.
The first game changer was Triple Drug Therapy of IDA (ivermectin, diethylcarbamazine (DEC) and albendazole) as preferred treatment regimen for accelerating LF elimination. LF is endemic in 9 out of 11 countries in the Region. The primary strategy for interrupting transmission of LF is annual mass drug administration (MDA) targeting the entire population in endemic areas until the prevalence of infection is reduced to a threshold below which transmission is considered no longer sustainable even without interventions, at which point the continuation of MDA is no longer warranted. Conventionally, two drug combination (DEC/albendazole or ivermectin/albendazole) have been used for MDA which does not always kill adult worms while clearing microfilaria circulating in the blood. Therefore, MDA must be given till the adult worms retain fecundity, which is an average of 5–7 years.9,10 In 2016, a study showed superior parasite killing effects of the triple drug therapy, suggesting permanent sterilization or destruction of adult worms followed in 2017 by WHO recommendation on use of triple drug combination for MDA.11 High coverage with one or two rounds of annual MDA of three drugs should be able to reduce the prevalence of infection below the proposed threshold.10
In June 2018, India, one of the first countries in the world to adopt the IDA strategy, initiated its implementation in five districts, with support from WHO, BMGF and several partners.12 Technical committee was established to help identify the target districts, develop implementation guidelines, develop micro-plans, improve social mobilization, and ensure pharmacovigilance. As a result, all five districts achieved effective treatment coverage of over 80%.12 Since then India continues to scale up IDA. Indonesia adopted IDA in 2021, Nepal in 2022 followed by Myanmar in 2023.
The second example is introduction of the rK39 rapid immunochromatographic test (ICT), a field-based rapid diagnostic test, and improved treatment alternatives, including miltefosine, the first effective oral agent for kala-azar, and liposomal amphotericin B, which can be administered as a single dose and boasts an efficacy of over 95%, for accelerating elimination of kala-azar.13 Kala-azar is endemic in Bangladesh, India and Nepal, with sporadic cases also reported in Bhutan, Sri Lanka and Thailand.14 In the last decades, the Special Programme for Research and Training in Tropical Diseases (TDR) and WHO have coordinated and financed research for development of new innovative tools to support the kala-azar elimination initiative in the Indian subcontinent.
The performance of rK39 ICT for antigen detection of kala-azar demonstrated it to be highly sensitive and specific.13 Pentavalent antimony has been the mainstay for kala-azar treatment for more than 6 decades, despite its toxicity, need for parenteral administration in a healthcare setting, and a long course of therapy.14 Development and evaluation of various treatments for kala-azar was supported by TDR and other partners such as DNDi and local research institutes. These led to better treatment alternatives in the last decade, including miltefosine, the first effective oral agent for treatment of kala-azar, and liposomal amphotericin B, which can be given as a single dose and has an efficacy of more than 95%.14,15 In 2012, WHO secured a donation of liposomal amphotericin B from Gilead Sciences. In the same year, Bangladesh adopted single dose liposomal amphotericin B as the first-line treatment for kala-azar, which was soon followed by India and Nepal.16 Availability of these tools were game changers in kala-azar elimination efforts in India sub-continent, by enabling early detection and treatment of cases even in remote communities. This encouraged implementors on the ground to strengthen and innovate active case detection in the last decade.
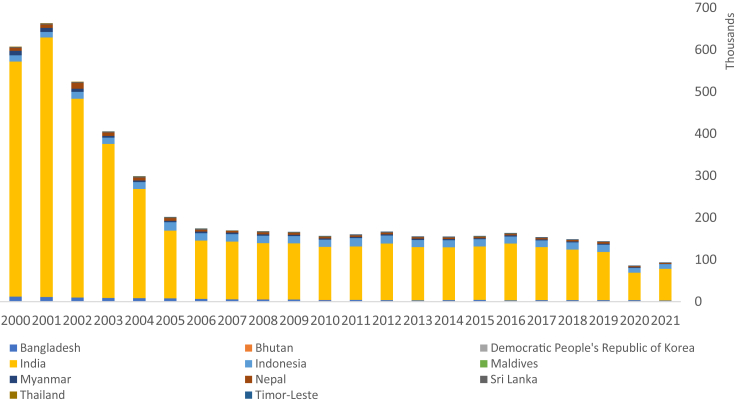
The next gamechanger is single dose rifampicin for post-exposure prophylaxis (SDR-PEP) of leprosy. Leprosy is endemic in 10 Member States of the Region. From the 1980s, a steady decline was observed in the prevalence of leprosy globally, due to the introduction of multidrug therapy (MDT) and shortening of the treatment period. However, the decline has been slow in the past 15 years, indicating the limitation of the current strategy to further reduce transmission of M. leprae (Fig. 1).17 In 2018, a study in Bangladesh showed the effectiveness of SDR-PEP in reducing the risk of leprosy among the treated contacts.18 In the same year, WHO published the Global Leprosy Strategy 2021–2030 “Towards Zero Leprosy”, which identified SDR-PEP as a new approach for achieving the goal of interruption of leprosy transmission, and in 2020 WHO published a technical guidance on contact tracing and PEP of leprosy.19,20 Since then, India, Indonesia and Maldives have been rolling out SDR-PEP as part of the national leprosy programs and other countries in the Region are preparing to do so.
Fig. 1.
Reported number of new leprosy cases in the South-East Asia Region, 2000–2021.
Achievement of elimination targets and their validation
In 2015, the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases endorsed standardized processes for confirming and acknowledging success for NTDs targeted for eradication, elimination of transmission, or elimination as a public health problem (Table 3). Subsequently, WHO published the standard operating procedures (SOPs) for validation of elimination of LF and trachoma as a public health problem.24,25 WHO-SEARO also developed the process of validation of elimination of kala-azar as a public health problem in South-East Asia in 2016.26
Table 3.
Operational criteria/definition for global or regional disease-specific eradication, elimination and control of NTDs under the Regional Flagship Priority in the South-East Asia Region.
| Disease | Geographical scope | Operational criteria/definition |
|---|---|---|
| Eradication | ||
| Yaws | Global | Absence of a new, infectious, serologically confirmed indigenous yaws cases for three consecutive years, supported by high coverage of active surveillance.21 |
| Elimination (interruption of transmission) | ||
| Leprosy | Global | There is no more local transmission of M. leprae, evidenced by zero new autochthonous cases among children (less than 15 years old) for at least five years.22 |
| Elimination as a public health problem | ||
| Kala-azar | Regional | i) Annual incidence of kala-azar at the district or sub-district level in all endemic countries in the SE Asia Region: less than one per 10,000 population; and ii) Case-fatality rate due to primary visceral leishmaniasis (VL): less than 1%.14 |
| Lymphatic filariasis | Global | Prevalence of infection with Wuchereria bancrofti, Brugia malayi or Brugia timori less than target thresholds in all endemic areas.23 |
| Trachoma | Global | Reduction in the prevalence of trachomatous trichiasis “unknown to the health system” to less than 0.2% in adults aged 15 years and older; a reduction in the prevalence of the active trachoma sign “trachomatous inflammation-follicular” (TF) in children aged 1–9 years to less than 5% (sustained for at least two years in the absence of intervening antibiotic mass drug administration); and the presence of a system to detect and manage incident cases of trachomatous trichiasis, with evidence of appropriate support for that system.24 |
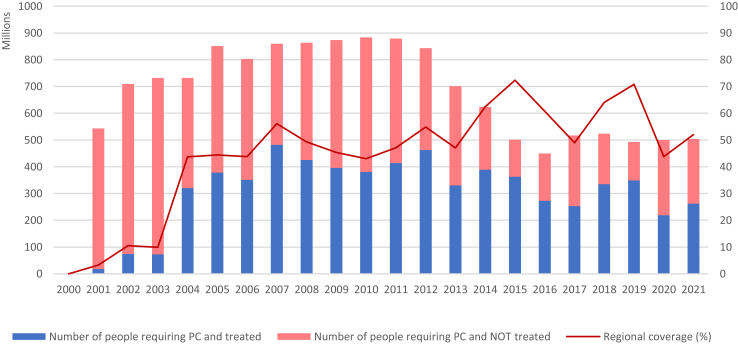
Since the launch of the Global Program to Eliminate Lymphatic Filariasis in 2000, 68% of LF-endemic districts across nine endemic countries in the Region have achieved the target thresholds as seen in transmission assessment surveys (TAS). As a result, the population requiring MDA has come down from 883 million in 2013 to 519 million in 2021 (Fig. 2). The number of people requiring MDA has been stable since 2017 because the districts with persistent transmission have rolled out IDA in a phased manner and also India has changed its implementation units from districts to blocks (sub-districts) and intensified LF elimination efforts. As per the SOP, Maldives and Sri Lanka compiled the dossier to claim achievement of elimination of LF as a public health problem and submitted to WHO, which has been validated by SEARO’s Regional Dossier Review Group in 2016.27,28 In 2017, Thailand too was validated for having eliminated LF as a public health problem.29,30 Bangladesh stopped MDA nationwide in 2016, passed the final TAS as post-MDA surveillance in 2021 and submitted a national dossier to WHO for validation of elimination of LF as a public health problem in December 2022. After a refinement of the dossier as per the guidance from the Regional Dossier Review Group, WHO validated Bangladesh’s claim for having eliminated LF as a public health problem in April 2023. While India, Indonesia, Myanmar and Nepal are progressively expanding IDA, Timor Leste met the criteria to stop MDA through TAS nationwide and moved into the post-MDA surveillance phase in 2021.
Fig. 2.
The number of population requiring MDA for elimination of LF in the WHO South-East Asia Region, 2000–2021.
Trachoma was known to be a public health problem in three countries in the South-East Asia Region, namely India, Myanmar, and Nepal. In 2018, Nepal became the first country in the Region to eliminate trachoma as a public health problem.31 In 2020, Myanmar joined Nepal in eliminating trachoma, despite challenges posed by the onset of the COVID-19 pandemic. India remains the only country in the Region yet to eliminate trachoma. The country, however, is carrying out a nationwide pre-validation survey and, despite the interruption of the pre-validation survey during the COVID-19 pandemic, aims to submit the dossier to WHO for validation of elimination of trachoma as a public health problem in 2023.
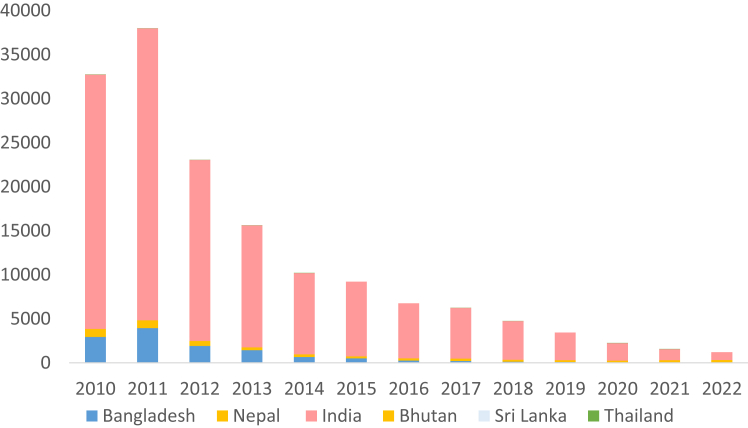
The new cases of kala-azar reported in the Region have reduced by 96.8% between 2011 and 2022 (Fig. 3). By the end of 2022, 772 (99%) implementation units (IU) in the Indian subcontinent (i.e. sub-districts or upazilas in Bangladesh, blocks in India and districts in Nepal) achieved the elimination target and only 3 IUs remained above the elimination threshold.13 Specifically, the elimination target for kala-azar was reportedly achieved in all endemic upazilas of Bangladesh, in 99% of the blocks in India and in 95% of the endemic districts in Nepal.13 Bangladesh has sustained the target of less than one kala-azar case per 10,000 population in all the IUs since 2017 and initiated development of a dossier to stake claim for elimination of kala-azar as a public health problem in 2023, the first country in the world to reach this stage.
Fig. 3.
The reported number of kala-azar cases in the WHO South-East Asia Region, 2010–2022.
India was the first country to be verified for yaws-free status in the world. In 2006, India declared yaws elimination three years after the last case was detected in 2003.32,33 While India sustained post-zero yaws surveillance, they also conducted serological surveys in the formerly endemic districts in 2009–2011, followed by compilation and submission of a dossier for WHO certification. In 2015, WHO convened an International Verification Team (IVT) to verify yaws-free status in India.32 In 2016, WHO certified India as “yaws-free”.
In 2021, Timor Leste conducted an innovative integrated NTD survey, composed of LF transmission assessment survey, screening and serological confirmation of yaws, screening of scabies and stool examination for assessing the prevalence of soil-transmitted helminthiases and taeniasis among over 11,000 school children nationwide. As a result, Timor Leste passed the critical threshold and stopped MDA for LF nationwide. Timor Leste is planning to conduct the next transmission assessment survey to assess whether the country is eligible for validation of elimination of LF as a public health problem in 2023. The integrated NTD survey in 2021 also found no serologically positive yaws cases among over 12,000 children, and another serological survey to verify yaws-free status is due in Timor Leste.
For leprosy too, WHO developed a framework that defines criteria including epidemiological cut-offs for verification of interruption of transmission and elimination of leprosy and also developed the Leprosy Elimination Monitoring Tool to help national programme visualize and assess the progress in leprosy elimination at any sub-national level.22 In 2020, Maldives became the first country to consolidate a Framework for Zero Leprosy in the Maldives with the aim of “100 Leprosy Free Islands by 2023”. There is a need to demonstrate success of leprosy elimination in low-burden countries such as Maldives and Bhutan to motivate other countries in the Region and beyond.
Emerging challenges, opportunities and priorities in the South-East Asia Region
With unprecedented achievements in the last decade, the NTD landscape in the South-East Asia Region is changing fast and new challenges and opportunities are being seen. A new global roadmap for eradication, elimination and control of NTDs—Ending the neglect to attain the Sustainable Development Goals: A roadmap for neglected tropical diseases 2021–2030—was endorsed by the Seventy-third World Health Assembly in 2020.34 There is a need for a new vision and direction to accelerate the control and elimination of NTDs and to sustain gains in the South-East Asia Region in the next decade.
Optimizing ongoing interventions and addressing social and environmental determinants in the last mile of elimination of NTDs through innovation
After a decade of efforts on kala-azar elimination in the South-East Asia Region, the last one percent of the implementation units in the Indian subcontinent is left to achieve the target threshold for elimination as a public health problem. However, despite substantial progress, there remain a series of gaps and challenges. This includes post kala-azar dermal leishmaniasis (PKDL) and kala-azar-HIV co-infection cases that are recognized as important disease reservoir of the parasite but in which the current rK39 RDT has limited use; PKDL cases require long treatment duration, which leads to poor treatment completion and diagnosis and treatment of kala-azar-HIV co-infection require specialized capacity at tertiary health-care facilities.14 Despite a substantial reduction in the overall incidence in the Region, kala-azar outbreak continues to be reported. In Nepal, the disease endemicity is geographically expanding to the hilly and mountainous districts that were considered formerly non-endemic.35 Intensified efforts are needed to strengthen surveillance, enhance active case detection, complete case management, outbreak investigation and vector control.
Regional intensification of efforts for LF elimination has led to substantial achievements but also to programmatic fatigue in many parts of the endemic countries. The presence of a substantial number of individuals who have never been treated during the decade of annual MDA is proof of this.36 These individuals act as a reservoir of transmission in areas where LF transmission continues despite many rounds of annual MDA with reportedly high coverage in India, Indonesia and Nepal.37,38 Identifying and addressing such “never treated” population is a priority to accelerate LF elimination in the coming years. Additionally, a potential contribution of zoonotic transmission to persistent transmission of Brugia species in some countries is an emerging concern, which might need to be addressed through One Health approach.
Despite gradual overall decline in leprosy burden in the Region, proportions of new leprosy cases and those with Grade 2 disability in many countries remain relatively high indicating late case detection and ongoing transmission. There is a critical need to strengthen regional partnership to enhance cross-learning and to revitalize the national leprosy elimination efforts across the Region, and also to facilitate adoption of newer strategies to enhance early case detection and response actions rather than continuing business-as-usual. SDR-PEP strategy is considered as a game changer but so far, adoption of this new strategy in the Region is slow mainly because of the lack of awareness and local evidence about the benefits of SDR-PEP as a preventive measure and resource constraints for procuring single dose rifampicin and rolling out this new strategy.
In intensification of ongoing efforts, it is imperative to secure unwavering political dedication and astute leadership by continuous engagement of Heads of States through national and international forums for advocacy and joint monitoring of elimination progress. This commitment should extend its reach to the grassroots level of governance, facilitated by a well-structured subnational framework designed for the elimination of NTDs. To achieve this, active involvement of communities and civil society is also paramount. Moreover, a comprehensive approach is necessary, one that delves into the environmental and social factors influencing NTD transmission. Inclusion of relevant stakeholders both within and beyond the health sector is essential to this endeavor. The persistence of NTDs can be attributed to enduring behavioral, environmental, and social risk elements, such as inadequate health-seeking behavior, substandard housing, and insufficient environmental hygiene. The effectiveness of public health interventions hinges on a populace that comprehends the urgency, clamors for services, and personally drives the changes required to interrupt disease transmission. A holistic societal approach is vital, underpinned by impactful social and behavioral change communication strategies and the empowerment of communities. This comprehensive approach stands as a pragmatic, sustainable, and cost-effective solution to tackle the social determinants of health that expedite and sustain the battle against NTDs, fostering their eradication, elimination, and control.
Strengthening and sustaining essential NTD interventions and services in the elimination phase through integration and cross-cutting approaches
The last mile of disease elimination can be particularly resource-intensive, demanding intensified efforts and increased allocation of funding to overcome the remaining hurdles. Without a continuous influx of resources, the progress made thus far can be jeopardized, and hard-won gains may be lost. Therefore, prioritizing the enhancement and sustainability of resources in the face of rising costs during the concluding phase of disease elimination is of paramount importance to ensure the success of these multifaceted endeavors. Furthermore, in the pursuit of efficient resource allocation, it is imperative to explore integrated elimination strategies that encompass multiple diseases.39 By harmonizing efforts and leveraging shared resources, such integrated approaches can lead to substantial cost savings, optimize impact, and enhance the overall effectiveness of disease control and elimination initiatives. This approach not only maximizes the utilization of available resources but also fosters synergies among various health programs, ultimately accelerating progress towards achieving comprehensive public health goals.
In counties that achieved elimination of a NTD as a public health problem, efforts are also needed to sustain the elimination status in the post-elimination phase, with a focus on integration of surveillance and response with other disease programme and health system, while accelerating R&D of new tools and strategies to make further progress.34
Kala-azar, LF and trachoma are presently targeted for elimination as a public health problem, as there are no appropriate tools to achieve and/or verify interruption of transmission. Therefore, a system for continued surveillance, case finding, outbreak response and targeted response after validation of such status needs to be established. However, there is a high chance that elimination as a public health problem gets mistaken for elimination of transmission and both donor fatigue and program complacency may shift attention and investment to the next unfinished agenda.13 Therefore, there is a critical need to continue the investment in optimizing, integrating and strengthening post-validation interventions within the primary health care and health system for sustainability, and in accelerating research and development of new tools and strategies to further progress towards interruption of transmission.
For any NTDs, at present, there is limited evidence for WHO to recommend any specific post-validation surveillance strategy. It is, however, clear that post-validation strategy will need to be country-specific and feasible to maintain government and donor commitment, and thus integrated with other existing platforms and mainstreamed in the health system functions. There is an urgent need for countries, with support of research and implementation partners, to generate the evidence needed to determine feasible, cost-effective and sustainable post-validation surveillance options for NTD and build necessary diagnostic, entomological and analytical capacity to sustain implementation of such options.
Expanding focus on controlling all NTDs of public health importance in the region
While Member States in the South-East Asian Region rapidly progresses in elimination of kala-azar, LF, trachoma and yaws as per the Regional Flagship priority, WHO-SEARO and its Member States are progressively expanding its focus to the next unfinished agenda in control and elimination of other NTDs such as schistosomiasis, rabies, snakebite envenoming, dengue and other arboviral diseases and neglected parasitic zoonoses.
The Region is one among the most affected by snakebite envenoming, with nearly 70% of annual global snakebite deaths occurring in South Asia alone. In 2022, WHO SEARO launched the Regional Action Plan for prevention and control of snake-bite envenoming in the South-East Asia Region 2022–2030.40 It aims to reduce the number of deaths and cases of disability associated with snakebite envenoming by 50% in the South-East Asia Region by 2030 through community engagement and empowerment to prevent snakebites and provide effective first-aid, health system strengthening to ensure access to life-saving treatment and care, and coordinated technical support to improve availability of quality, effective, safe and affordable antivenoms.
The South-East Asia Region also bears the highest burden of dog-mediated human rabies in the world. More than 1.4 billion people in the Region are at risk of rabies infection, and approximately 45% of worldwide rabies deaths occur in Asia. In 2019, World Health Organization, Food and Agriculture Organization of the United Nations (FAO), World Organization for Animal Health (OIE) and Global Alliance for Rabies Control (GARC) jointly launched Zero by 30: the global strategic plan to end human deaths from dog-mediated rabies by 2030.41 This target is specifically included in WHO’s new global NTD roadmap 2021–2030. To accelerate elimination of rabies in the Region, WHO-SEARO established the Regional Technical Advisory Group on dog-mediated human rabies in 2023 to guide WHO and Member States in providing evidence-based recommendations on strategies to accelerate the progress in elimination of dog-mediated human rabies in the Region towards the 2030 global elimination targets.
Dengue fever has emerged as the world's most common and rapidly spreading vector-borne disease. Except for the Democratic People's Republic of Korea, around 1.3 billion people from the WHO South-East Asia Region live in dengue-endemic areas.42 The Region accounts for more than half of the worldwide dengue burden, and the presence of all four serotypes has made these nations hyperendemic. To review the Regional dengue situation regularly and advise WHO and Member States on evidence-based strategies to accelerate prevention and control of dengue and other arboviral diseases in the Region, WHO-SEARO re-established the Regional Technical Advisory Group (RTAG) on Dengue and other arboviruses in 2021.42 WHO also launched the Global Arboviral Initiative (GAI) in 2022 as an integrated strategic plan focusing on monitoring risk, pandemic prevention, preparedness, detection and response, and building a coalition of partners to strengthen the coordination, communication, capacity-building, research, preparedness, and response necessary to mitigate the growing risk of epidemics due to these diseases. Guided by the GAI, WHO-SEARO plans to lead development of a new Regional Strategic Plan for the prevention and control of dengue in the South-East Asia Region in 2023.
Schistosomiasis in the Region is confirmed to be endemic only in a small region in Indonesia - two districts in Central Sulawesi, with about 20,000 populations at-risk. Sustained efforts through the Integrated Schistosomiasis Control Programme implemented in the last 3 decades helped reduce the prevalence of schistosomiasis substantially. Indonesia launched the 2018–2025 National Roadmap for Schistosomiasis Eradication Programme, focused on integrated control programme encompassing mass drug administration, veterinary public health and vector (snail) control with environmental management. Having sustained the low prevalence in the last many years, the country is moving towards interruption of transmission.
In 2021, WHO launched a Strategic Framework for integrated control and management of skin-related NTDs, advocating for an integrated approach for diagnosis, control and management of skin NTDs.34 Integrated approach not only improve efficiency and cost-effectiveness of interventions and service delivery but also reduce stigma and discrimination and improve community and patients acceptance of interventions and services. Skin-related NTDs of public health importance in the Region includes scabies and mycetoma in addition to yaws, leprosy, cutaneous leishmaniasis and PKDL and LF. Scabies is widely prevalent across the Region whereas mycetoma has been reported in India and Thailand, but limited data on true burden and geographical distribution of these diseases exist.43,44 WHO recommends MDA using ivermectin as a public health control strategy against scabies, and the progressive scale up of triple drug therapy MDA using ivermectin for elimination of LF in the Region provides an opportunity to assess and bring ancillary impacts on scabies.45 WHO-SEARO is developing a Regional integrated skin NTD toolkit by regional adaptation of the Global Strategic Framework to work with Member States and partners to scale up integrated skin NTD approach across the Region.
Finally, one of the most neglected NTDs are other parasitic zoonoses such as taeniasis/cysticercosis, echinococcosis and foodborne trematodiases. Taenia solium is known to be the cause of over 30% of epilepsy cases through neurocysticercosis in many endemic areas where adequate sanitation is lacking and people and roaming pigs live in close proximity.46 Human infection with Echinococcus granulosus leads to the development of one or more hydatid cysts located most often in the liver and lungs. Echinococcosis is often expensive and complicated to treat and may require extensive surgery and/or prolonged drug therapy. Opisthorchis viverrini, the liver fluke, one of the parasites causing foodborne trematodiasis, is classified as carcinogenic agents as they may cause bile duct cancer (cholangiocarcinoma). All such diseases are reported in some countries in the South-East Asia Region but data on disease endemicity and their public health importance remains limited. Control and prevention of these diseases require intersectoral collaboration among the public health, animal health, food safety and WASH sectors. In 2018, the Regional Tripartite, composed of WHO-SEARO, WHO WPRO, FAO and WOAH in the Asia Pacific jointly organized the Meeting to accelerate prevention and control of neglected foodborne parasitic zoonoses to bring together national focal points from various sectors.47 Following the meeting, the Regional Tripartite closely collaborated and developed a series of resource materials to accelerate disease mapping in a standardized manner and control and prevention through One Health approach targeting human health, animal health, environment and food safety sectors.48
Again, the effective control and ultimate elimination of such diseases necessitate a comprehensive, multisectoral approach that encompasses a range of integrated actions. These encompass robust surveillance mechanisms, facilitating real-time disease monitoring and forecast utilizing disaggregated data, and seamless data sharing across sectors. Additionally, strategies encompassing veterinary public health, an expansion of water supply and sanitation coverage, and the augmentation of vaccine and antisera accessibility are vital components. Emphasizing food safety and implementing vector control interventions alongside impactful social and behavioral change communication strategies are imperative for safeguarding vulnerable and affected populations.
However, the significance of holistic multisectoral engagement extends beyond the immediate containment and elimination of NTD transmission. It's essential to recognize that numerous NTDs result in lingering morbidities and disabilities that persist even after elimination targets have been achieved by individual countries. Thus, the provision of public health interventions and services must be fortified through collaboration with other programmes and sectors. This includes reinforcing vector control efforts, bolstering water and sanitation initiatives, promoting health education, and enhancing disability and psychosocial care provisions. Such endeavors must be underpinned by unwavering and sustained multisectoral partnerships.
The way forward
The WHO South-East Asia Region has a unique opportunity to demonstrate continued success in the control and elimination of NTDs that would fundamentally change the global NTD landscape. Being the Regional Flagship Priority in the South-East Asia Region, Member States have accorded strong commitment and high priority to them within their national public health agenda. The Region holds strong R&D potential based on the size of the population and the wealth of entities advancing R&D in technology and medicines.49,50 Countries in the Region also have relatively higher health system capacity compared to developing nations in many other Regions.
A new global roadmap for eradication, elimination and control of NTDs—Ending the neglect to attain the Sustainable Development Goals: A roadmap for neglected tropical diseases 2021–2030—set out updated global targets and milestones to prevent, control, eliminate or eradicate 20 diseases and disease groups as well as cross-cutting targets aligned with the Sustainable Development Goals.34 A Regional Strategic Framework for Sustaining, Accelerating and Innovating to end NTDs in the South-East Asia 2023–2030 is being developed by WHO SEARO in collaboration with Member States and partners by adapting the Roadmap 2030 in the context of the South-East Asia Region. It is intended to guide and coordinate efforts among Member States, WHO and partners in the South-East Asia Region to sustain progress, accelerate actions, and innovate approaches to effectively implement the Roadmap 2030 in the Region, encompassing all 15 NTDs of public health importance in the Region. Most importantly, the Regional Strategic Framework proposes the strategic priorities of action at country level under each of the three strategic pillars of the Roadmap: (i) strengthen country ownership, leadership and stewardship, (ii) accelerate programmatic actions, and (iii) intensify integrated and cross-cutting approaches. These strategic priorities can be accomplished only through partnerships and cooperation among Member States, WHO, academia, industry and public and private institutions. With NTDs being diseases of those who are left behind, their elimination will be one more step towards achieving UHC and creating a more equitable world. Together, we can and we will, finish the task of eliminating NTDs.
Contributors
AY and SR conceptualized the outline and content of the manuscript; AY reviewed, analyzed and drafted the manuscript including tables and figures; SR, APD, MJ and ZL reviewed and provided comments; AY and SR synthesized and finalized the manuscript. All authors approved the final draft of the manuscript.
Declaration of interests
We declare no competing interests. AY, ZW, MJ and SR are staff members of WHO. APD is a Vice Chancellor of AIPH University. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of WHO or AIPH University. The country names used do not imply the expression of any opinion whatsoever on the part of WHO or AIPH University concerning the legal status of any country, territory, city, or area, or of its authorities.
References
- 1.Fitzpatrick C.N.U., Lenk U., de Vlas S.J., Bundy D.A.P. Disease control priorities. 3rd ed. vol. 6. The International Bank for Reconstruction and Development/The World Bank; Washington (DC): 2017. An investment case for ending neglected tropical diseases. [PubMed] [Google Scholar]
- 2.WHO Global programme to eliminate lymphatic filariasis: progress report, 2021. Wkly Epidemiol Rec. 2021;41(97):513–524. [Google Scholar]
- 3.WHO . World Health Organization; Geneva: 2023. Global Health Observatory Data Repository: Leprosy (Hansen’s disease)[Internet]https://www.who.int/data/gho/data/themes/topics/leprosy-hansens-disease [cited 2023 May 12]. Available from: [Google Scholar]
- 4.WHO Schistosomiasis and soil-transmitted helminthiases: progress report. Wkly Epidemiol Rec. 2021;97(48):621–632. [Google Scholar]
- 5.Dowdle W.R. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76(Suppl 2):22–25. [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . WHO Regional Office for South-East Asia; Delhi: 2015. Meeting of Ministers of Health of the WHO South-East Asia Region: report of the thirty-second meeting, Dhaka, Bangladesh, 9 Sept 2014. [Google Scholar]
- 7.Alves F., Bilbe G., Blesson S., et al. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev. 2018;31(4) doi: 10.1128/CMR.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . WHO Regional Office for South-East Asia; Delhi: 2020. Evaluation of implementation of Regional Flagship Areas in the WHO South-East Asia Region 2014-2018. [Google Scholar]
- 9.Tisch D.J., Michael E., Kazura J.W. Mass chemotherapy options to control lymphatic filariasis: a systematic review. Lancet Infect Dis. 2005;5:514–523. doi: 10.1016/S1473-3099(05)70192-4. [DOI] [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: 2017. Guideline ‒ Alternative mass drug administration regimens to eliminate lymphatic filariasis. [PubMed] [Google Scholar]
- 11.Thomsen E.K., Sanuku N., Baea M., et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis. 2016;62:334–341. doi: 10.1093/cid/civ882. [DOI] [PubMed] [Google Scholar]
- 12.Tripathi B., Roy N., Dhingra N. Introduction of triple-drug therapy for accelerating lymphatic filariasis elimination in India: lessons learned. Am J Trop Med Hyg. 2022;106(5_Suppl):29–38. doi: 10.4269/ajtmh.21-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirve S., Kroeger A., Matlashewski G., et al. Towards elimination of visceral leishmaniasis in the Indian subcontinent—translating research to practice to public health. PLoS Negl Trop Dis. 2017;11(10) doi: 10.1371/journal.pntd.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . WHO Regional Office for South-East Asia; Delhi: 2022. Regional Strategic Framework for accelerating and sustaining elimination of kala-azar in the South-East Asia Region: 2022–2026. [Google Scholar]
- 15.Sundar S., Murray H.W. Availability of miltefosine for the treatment of kala-azar in India. Bull World Health Organ. 2005;83(5):394–395. [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . WHO Regional Office for South-East Asia; Delhi: 2011. Report of the Fourth Regional Technical Advisory Group Meeting on Elimination of Kala-azar, 12–14 July 2011, Kathmandu, Nepal. [Google Scholar]
- 17.ter Ellen F., Tielens K., Fenenga C., et al. Implementation approaches for leprosy prevention with single-dose rifampicin: a support tool for decision making. PLoS Negl Trop Dis. 2022;16(10) doi: 10.1371/journal.pntd.0010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moet F.J., Pahan D., Oskam L., Richardus J.H. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: cluster randomised controlled trial. BMJ. 2008;336(7647):761–764. doi: 10.1136/bmj.39500.885752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . WHO Regional Office for South-East Asia; Delhi: 2021. Towards zero leprosy. Global leprosy (Hansen’s Disease) strategy 2021–2030. [Google Scholar]
- 20.WHO . WHO Regional Office for South-East Asia; Delhi: 2020. Leprosy/Hansen disease: Contact tracing and post-exposure prophylaxis. [Google Scholar]
- 21.WHO . World Health Organization; Geneva: 2018. Eradication of yaws – procedures for verification and certification of interruption of transmission. [Google Scholar]
- 22.WHO . WHO Regional Office for South-East Asia; Delhi: 2021. Task Force on definitions, criteria and indicators for interruption of transmission and elimination of leprosy Report of the final meeting, Chengalpattu, India, 24-26 March 2021. [Google Scholar]
- 23.WHO . World Health Organization; Geneva: 2012. Lymphatic filariasis: monitoring and epidemiological assessment of mass drug administration - A manual for national elimination programmes. [Google Scholar]
- 24.WHO . World Health Organization; Geneva: 2017. Validation of elimination of trachoma as a public health problem. [Google Scholar]
- 25.WHO . World Health Organization; Geneva: 2017. Validation of elimination of lymphatic filariasis as a public health problem. [Google Scholar]
- 26.WHO . WHO Regional Office for South-East Asia; Delhi: 2016. Process of validation of elimination of kala-azar as a public health problem in South-East Asia. [Google Scholar]
- 27.Samir H., Mohamed N., Saleem S., Aditama T.Y. In: Elimination of infectious diseases from the South-East Asia Region. SpringerBriefs in public health. Singh P.K., editor. Springer; Singapore: 2021. Unburdening the poor: elimination of lymphatic filariasis in the Maldives. [Google Scholar]
- 28.Dilhani Samarasekera S., Pendse R. In: Elimination of infectious diseases from the South-East Asia Region. SpringerBriefs in public health. Singh P.K., editor. Springer; Singapore: 2021. Lymphatic filariasis elimination in Sri Lanka: overcoming the odds. [Google Scholar]
- 29.Rojanapanus S., Toothong T., Boondej P., et al. How Thailand eliminated lymphatic filariasis as a public health problem. Infect Dis Poverty. 2019;8:38. doi: 10.1186/s40249-019-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thammapalo S., Kertesz D. In: Elimination of infectious diseases from the South-East Asia Region. SpringerBriefs in public health. Singh P.K., editor. Springer; Singapore: 2021. Elimination of lymphatic filariasis in Thailand: a model for best practices. [Google Scholar]
- 31.Shrestha M.P., Mishra S.K., Vandelaer J. In: Elimination of infectious diseases from the South-East Asia Region. SpringerBriefs in public health. Singh P.K., editor. Springer; Singapore: 2021. Trachoma elimination in Nepal: bringing light, preventing darkness. [Google Scholar]
- 32.WHO . World Health Organization; Geneva: 2018. Yaws eradication[Internet]https://www.who.int/news-room/facts-in-pictures/detail/yaws-eradication [cited 2023 May 12]. Available from: [Google Scholar]
- 33.Narain J.P., Jain S.K., Bora D., Venkatesh S. Eradicating successfully yaws from India: the strategy & global lessons. Indian J Med Res. 2015;141(5):608–613. doi: 10.4103/0971-5916.159542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO . World Health Organization; Geneva: 2020. Ending the neglect to attain the sustainable development goals: a roadmap for neglected tropical diseases 2021-2030. [Google Scholar]
- 35.Shrestha M., Khatri-Chhetri M., Poudel R.C., et al. Molecular evidence supports the expansion of visceral leishmaniasis towards non-program districts of Nepal. BMC Infect Dis. 2019;19:444. doi: 10.1186/s12879-019-4083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krentel A., Fischer P.U., Weil G.J. A review of factors that influence individual compliance with mass drug administration for elimination of lymphatic filariasis. PLoS Negl Trop Dis. 2013;7(11) doi: 10.1371/journal.pntd.0002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Titaley C.R., Worrell C.M., Ariawan I., et al. Assessment of factors related to individuals who were never treated during mass drug administration for lymphatic filariasis in Ambon City, Indonesia. PLoS Negl Trop Dis. 2022;16(11) doi: 10.1371/journal.pntd.0010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojha C.R., Joshi B., Kc K.P., et al. Impact of mass drug administration for elimination of lymphatic filariasis in Nepal. PLoS Negl Trop Dis. 2017;11(7) doi: 10.1371/journal.pntd.0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espinal M.A., Alonso M., Sereno L., et al. Sustaining communicable disease elimination efforts in the Americas in the wake of COVID-19. Health Policy. 2022;13 doi: 10.1016/j.lana.2022.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO . WHO Regional Office for South-East Asia; Delhi: 2022. Regional Action Plan for prevention and control of snakebite envenoming in the South-East Asia 2022–2030. [Google Scholar]
- 41.WHO . World Health Organization; Geneva: 2018. Zero by 30: the global strategic plan to end human deaths from dog-mediated rabies by 2030. [Google Scholar]
- 42.WHO . WHO Regional Office for South-East Asia; Delhi: 2022. Meeting report: Virtual Meeting of Regional Technical Advisory Group for dengue and other arbovirus diseases, New Delhi, India, 4-6 October 2021. [Google Scholar]
- 43.Karimkhani C., Colombara D.V., Drucker A.M., et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(12):1247–1254. doi: 10.1016/S1473-3099(17)30483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Sande W.W. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7(11) doi: 10.1371/journal.pntd.0002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO informal consultation on a framework for scabies control, meeting report, WHO Regional Office for the Western Pacific, Manila, Philippines, 19–21 February 2019. World Health Organization Regional Office for the Western Pacific; Manila: 2020. https://apps.who.int/iris/bitstream/handle/10665/333154/9789240008069-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- 46.Taeniasis/cysticercosis: fact sheet. WHO; Geneva: 2022. https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis [Google Scholar]
- 47.Meeting to accelerate prevention and control of neglected foodborne parasitic zoonoses in selected Asian countries, Luang Prabang, Lao People's Democratic Republic, 16–18 October 2018: meeting report. World Health Organization Regional Office for the Western Pacific; Manila: 2020. https://apps.who.int/iris/bitstream/handle/10665/333060/RS-2018-GE-50-LAO-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- 48.FAO, WHO, WOAH collaboration strengthens capacity on neglected parasitic zoonoses. World Organization for Animal Health; Paris: 2022. https://rr-asia.woah.org/en/news/fao-oie-who-tripartite-collaboration-strengthens-capacity-on-neglected-parasitic-zoonoses/ [Google Scholar]
- 49.Thomas Z., Saha G.K., Gopakumar K.M., Ganguly N.K. Can India lead the way in neglected diseases innovation? BMJ. 2019;364 doi: 10.1136/bmj.k5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hotez P.J., Bottazzi M.E., Strych U., et al. Neglected tropical diseases among the association of southeast Asian nations (ASEAN): overview and update. PLoS Negl Trop Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003575. [DOI] [PMC free article] [PubMed] [Google Scholar]