Abstract
Asparagine biosynthesis in soybean (Glycine max [L.] Merr.) nodules has been difficult to demonstrate due to the poor conversion of suspected immediate precursors to asparagine and the instability of the key enzyme asparagine synthetase. The present study was designed to explore the effects of two ammonium assimilation inhibitors on the metabolism of 14CO2 to [14C]asparagine and to demonstrate the existence in nodules of the enzyme asparagine synthetase. When detached nodules were incubated in 14CO2, radioactivity in asparagine (as a percentage of amino acid cpm) increased 10-fold over 4 hours. Vacuum infiltration of 10 mm methionine sulfoximine or 10 mm azaserine prior to 14CO2 incubations decreased both the rate of dark fixation and the radioactivity in the amino acid fraction. These inhibitors also decreased the recovery of label in aspartate and asparagine. These results, plus the sequence of labeling of metabolites from 14CO2, are consistent with a glutamine-dependent synthesis of asparagine from aspartate with oxalacetate as a precursor to aspartate.
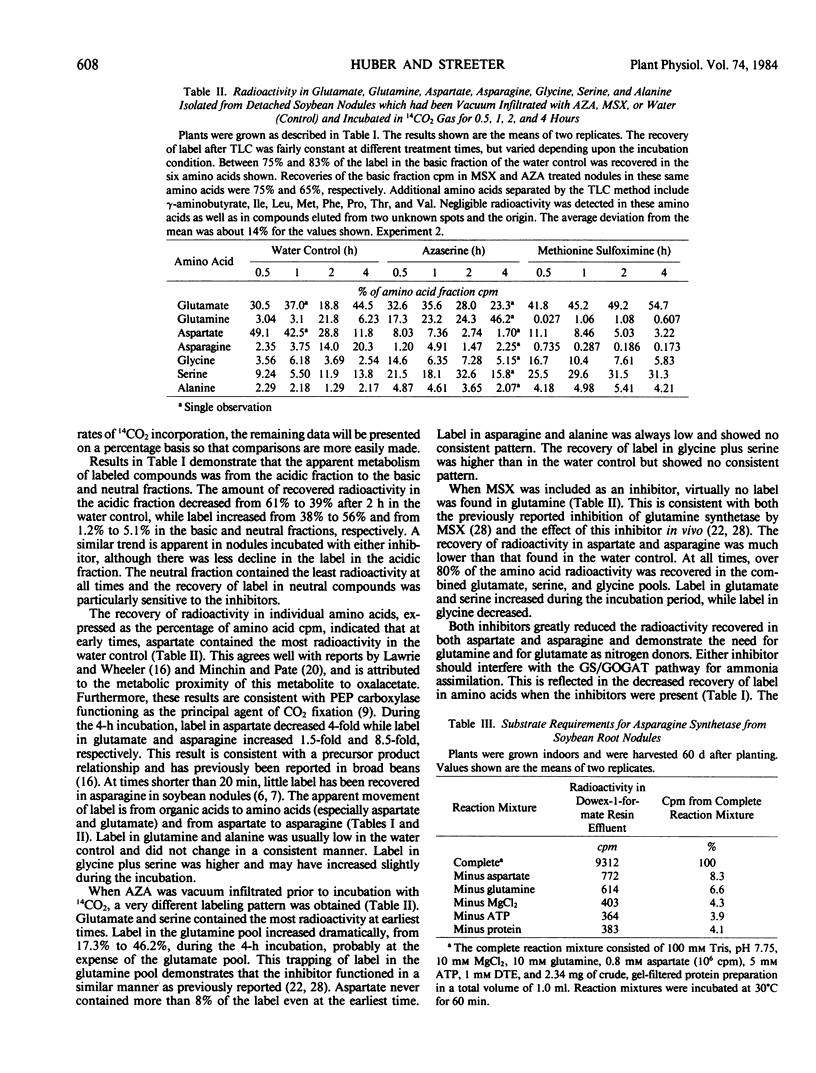
An enzyme catalyzing the ATP- and glutamine-dependent amidation of aspartic acid to form asparagine was isolated from soybean nodules. High levels of sulfhydryl protectants were required and the inclusion of glycerol and substrates in the extraction buffer helped to stabilize the enzyme. Enzyme activity in taproot nodules increased between 38 and 41 days after planting and peaked soon after flower initiation (45 days). The activity then declined to basal levels by 70 days. On a total enzyme activity basis, there was 170-fold more asparagine synthetase activity in the infected zone of the nodule than in the cortex, and 205-fold more activity in the cytosol than the bacteroid fraction. The enzyme has a broad pH maximum around pH 8.25, and the apparent Km values for the substrates aspartate, MgATP, and glutamine are 1.24 mm, 0.076 mm, and 0.16 mm, respectively. Ammonium ion can replace glutamine as the nitrogen donor, but the Km value of the enzyme for ammonium ion is 40-fold higher than that for glutamine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S., Yamaguchi M. Asparagine formation in soybean nodules. Plant Physiol. 1980 Jul;66(1):139–141. doi: 10.1104/pp.66.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Ireland R. J., Lea P. J. Asparagine synthesis in pea leaves, and the occurrence of an asparagine synthetase inhibitor. Plant Physiol. 1983 Sep;73(1):165–168. doi: 10.1104/pp.73.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibach P. H., Mask P. L., Streeter J. G. A rapid one-step method for the isolation of bacteroids from root nodules of soybean plants, utilizing self-generating Percoll gradients. Can J Microbiol. 1981 May;27(5):491–495. doi: 10.1139/m81-072. [DOI] [PubMed] [Google Scholar]
- Reynolds P. H., Boland M. J., Blevins D. G., Schubert K. R., Randall D. D. Enzymes of amide and ureide biogenesis in developing soybean nodules. Plant Physiol. 1982 Jun;69(6):1334–1338. doi: 10.1104/pp.69.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Asparaginase and asparagine transaminase in soybean leaves and root nodules. Plant Physiol. 1977 Aug;60(2):235–239. doi: 10.1104/pp.60.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys. 1973 Aug;157(2):613–624. doi: 10.1016/0003-9861(73)90681-4. [DOI] [PubMed] [Google Scholar]
- Wong P. P., Evans H. J. Poly-beta-hydroxybutyrate Utilization by Soybean (Glycine max Merr.) Nodules and Assessment of Its Role in Maintenance of Nitrogenase Activity. Plant Physiol. 1971 Jun;47(6):750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


