Abstract
The Cyclin-dependent kinase 1, as a serine/threonine protein kinase, is more than a cell cycle regulator as it was originally identified. During the last decade, it has been shown to carry out versatile functions during the last decade. From cell cycle control to gene expression regulation and apoptosis, CDK1 is intimately involved in many cellular events that are vital for cell survival. Here, we provide a comprehensive catalogue of the CDK1 upstream regulators and substrates, describing how this kinase is implicated in the control of key ‘cell cycle-unrelated’ biological processes. Finally, we describe how deregulation of CDK1 expression and activation has been closely associated with cancer progression and drug resistance.
Subject terms: Bioinformatics, Phosphorylation, Regulatory networks, Cellular signalling networks
Introduction
Progression throughout the cell cycle requires complex regulatory mechanisms that mainly rely on the oscillation of protein level and activity of cyclins and cyclin-dependent kinases (CDKs), respectively. Widespread compensation among approximately 20 CDKs and 30 cyclins has been reported in mammals [1]. Interestingly, knock-out mice of several CDK genes (e.g., CDK2, CDK3, CDK4, CDK6) have been generated and found to be viable. In contrast, CDK1 conditional knockout mice are embryonic lethal, suggesting an essential role of this gene in cell cycle progression [2]. CDK1, the first identified member of the Cdk family, is conserved in all organisms and regulates the transition between the G2 phase and mitosis. The activity of CDK1 is modulated by its binding to cyclin B1 and by its phosphorylation on crucial residues. Specifically, during the late G2 phase, the gradual accumulation of cyclin B1 promotes the formation of the cyclin B1-CDK1 complex, the pre-Mitosis Promoting Factor (or pre-MPF), which is maintained in an inactive state in the cytoplasm by the phosphorylation of CDK1 on Tyr15 and Thr14 mediated by the WEE1 and MYT1 kinases, respectively [3, 4]. This preparatory step prevents premature entry into mitosis, allowing the cells to check for DNA replication errors through the G2/M checkpoint control governed by the ATM/ATR kinases and grants a ready-to-use pool of cyclin B1-CDK1 complex to use if the cell successfully passes the checkpoints. At the end of the G2 phase, the MPF is activated by two consequent events: the dephosphorylation of Tyr15 and Thr14 residues mediated by the CDC25B/C phosphatases and the phosphorylation of Thr161 mediated by the cyclin H-CDK7 complex [5]. Interestingly, although more than 13,000 reports have been published in the last decades, many questions about CDK1 are still open. By taking advantage of manually annotated signalling resources and recently reported findings, here we provide a comprehensive catalogue of CDK1 upstream regulators and substrates. Our literature screening confirmed that CDK1 is more than a cell cycle regulator, as it was originally identified, and it is involved in a variety of crucial biological processes. Interestingly, these functions are controlled by CDK1 alone or in complex with cyclin B1 and additional cyclins such as cyclin A and cyclin E, suggesting alternative modalities of activation [6]. Finally, alteration of CDK1 expression level has been widely associated to cancer progression, as already extensively reviewed [7, 8]. Here we exploited pan-cancer (phospho)proteomic dataset stored in different databases to clarify the correlation between the phosphorylation of specific regulatory sites of CDK1 and consequently its activation with tumorigenesis.
The CDK1 upstream kinome
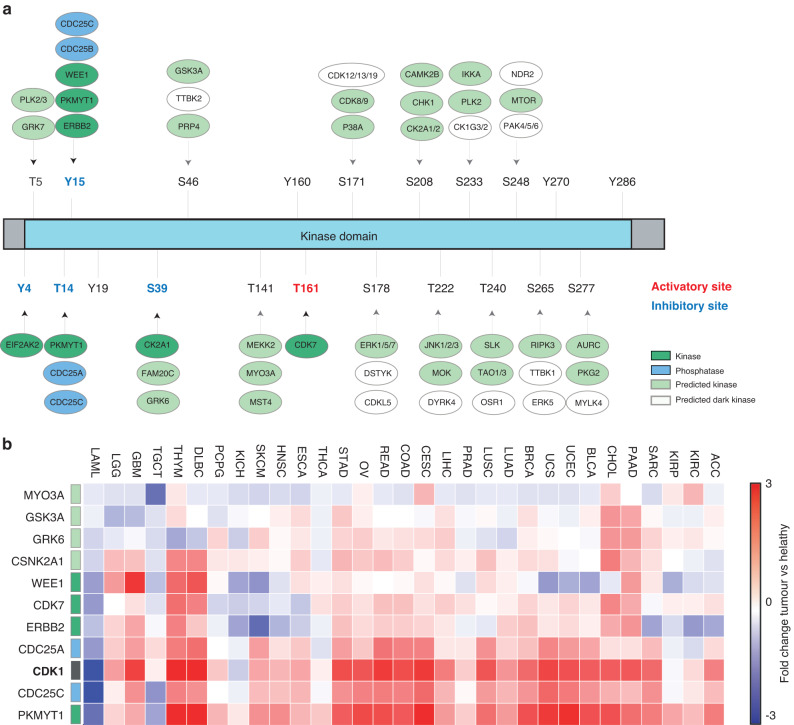
Phosphorylation is the fundamental mechanism controlling CDK1 kinase activity. The concerted activity of WEE1 and PKMYT1 kinases and CDC25A, CDC25B and CDC25C phosphatases controls the phosphorylation level of the two inhibitory Thr14 and Tyr15 residues whereas CDK7 phosphorylates the activatory Thr361 (Fig. 1a). Many others phosphorylation sites, mainly located on the kinase domain of CDK1, have been identified in large-scale high-throughput experiments [9]. Apart from Tyr4 and Ser39 phosphorylated by EIF2AK2 and CK2, respectively, the upstream kinases as well as the functional role of the remaining 16 phosphorylation sites are still unknown (Table 1). Interestingly, Johnson and collaborators recently embarked on the characterisation of the human kinome atlas, a very recent tour-de-force study to profile the substrate specificities for 300 human serine/threonine kinases, and were able to identify high-confidence kinases capable of phosphorylating every reported phosphorylation site in the human Ser/Thr phosphoproteome [10]. In Fig. 1a we display for each uncharacterised phosphosite of CDK1 the top predicted kinases (with a percentile score greater than 90%) in the kinome atlas (Fig. 1a). As shown, 12 out of 33 predicted CDK1 kinases are understudied and classified as dark genes [11] GO biological processes enrichment analysis of the CDK1 kinases reveals that many kinases are involved in RTK signalling pathways, including MAPKs, AKT-mTOR as well as DNA repair. By taking advantage of GEPIA, a database of RNA-seq expression data from tumour samples and normal tissues derived from the Cancer Genome Atlas (TCGA) and the Genotype-Tissue Expression (GTEx), upstream CDK1 regulators sharing a highly correlated expression profile across cancer tissues were reported. As shown in Fig. 1b, correlated genes include both well-characterised modulators of the CDK1 activity (eg. PKMYT1, CDC25A/C and WEE1) and some of the kinome atlas-predicted kinases (GSK3A and GRK6). Interestingly, the receptor tyrosine kinase ERBB2 was also found among the CDK1-related genes. This is in line with the study of Tan and colleagues which demonstrates that ERBB2 binds to and colocalizes with cyclin B-CDK1 complexes and phosphorylates Tyr15 of CDK1 in breast cancer cells [12]. These observations suggest that CDK1 may be activated by alternative pathways.
Fig. 1. The CDK1 upstream kinome.
a Schematic representation of the CDK1 regulatory sites and upstream regulators. Known regulatory kinases and phosphatases are represented in green and blue, respectively. Predicted Ser/Thr kinases are represented in light green and white (dark kinases). b Heatmap displaying the Log2 fold-change of gene expression level between tumour and healthy tissues of CDK1 and its upstream regulators according to The Cancer Genome Atlas (TCGA) GEPIA database.
Table 1.
The CDK1 regulatory kinome.
| Gene name | CDK1 phosphosite | Effect | Evidence (PMID) |
|---|---|---|---|
| CDK7 | Thr161 | up-regulates activity | 8344251 |
| CDC25A | Thr14-Tyr15 | up-regulates activity | 10454565 |
| CDC25C | Thr14-Tyr15 | up-regulates activity | 19574738 |
| EIF2AK2 | Tyr4 | down-regulates | 20395957 |
| PKMYT1 | Thr14-Tyr15 | down-regulates | 9001210 |
| ERBB2 | Tyr15 | down-regulates activity | 12049736 |
| WEE1 | Tyr15 | down-regulates activity | 16096060 |
| GRK7 | Thr5 | unknown | 36631611 |
| PLK2/3 | Thr5 | unknown | 36631611 |
| OSR1 | Thr240 | unknown | 36631611 |
| SLK | Thr240 | unknown | 36631611 |
| TAO1/3 | Thr240 | unknown | 36631611 |
| DYRK4 | Thr222 | unknown | 36631611 |
| JNK1/2/3 | Thr222 | unknown | 36631611 |
| MOK | Thr222 | unknown | 36631611 |
| MEKK2 | Thr141 | unknown | 36631611 |
| MST4 | Thr141 | unknown | 36631611 |
| MYO3A | Thr141 | unknown | 36631611 |
| GSK3A | Ser46 | unknown | 36631611 |
| PRP4 | Ser46 | unknown | 36631611 |
| TTBK2 | Ser46 | unknown | 36631611 |
| FAM20C | Ser39 | unknown | 36631611 |
| GRK6 | Ser39 | unknown | 36631611 |
| AURC | Ser277 | unknown | 36631611 |
| MYLK4 | Ser277 | unknown | 36631611 |
| PKG2 | Ser277 | unknown | 36631611 |
| ERK5 | Ser265 | unknown | 36631611 |
| TTBK1 | Ser265 | unknown | 36631611 |
| NDR2 | Ser2498 | unknown | 36631611 |
| MTOR | Ser248 | unknown | 36631611 |
| PAK4/5/6 | Ser248 | unknown | 36631611 |
| CK1G3/2 | Ser233 | unknown | 36631611 |
| IKKA | Ser233 | unknown | 36631611 |
| PLK2 | Ser233 | unknown | 36631611 |
| CAMK2B | Ser208 | unknown | 36631611 |
| CHK1 | Ser208 | unknown | 36631611 |
| CK2A1/2 | Ser208 | unknown | 36631611 |
| CDKL5 | Ser178 | unknown | 36631611 |
| DSTYK | Ser178 | unknown | 36631611 |
| ERK1/5/7 | Ser178 | unknown | 36631611 |
| CDK12/13/19 | Ser171 | unknown | 36631611 |
| CDK8/9 | Ser171 | unknown | 36631611 |
| P38A | Ser171 | unknown | 36631611 |
| CSNK2A1 | Ser39 | up-regulates | 15788687 |
The CDK1 substrates landscape
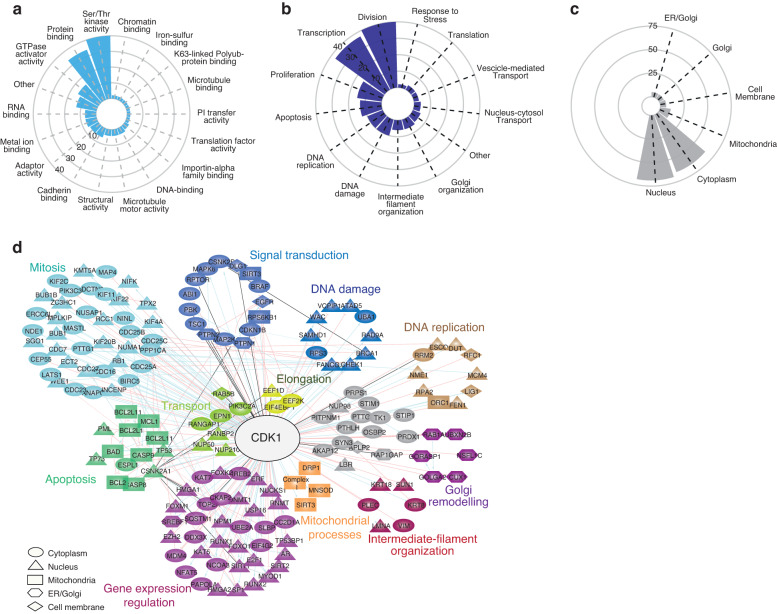
CDK1 is a hub kinase that directly phosphorylates approximately 200 proteins, as reported in the Signor database (Table 2) [13]. GO-term enrichment analysis reveals that CDK1 substrates are significantly associated with molecular functions mostly implicated in signalling propagation (eg. kinase, GTPase and protein binding activities) and gene expression modulation (DNA and RNA binding activities) (Fig. 2a). In addition, many of the CDK1 substrates are implicated in the regulation of transcription and to a lesser extent translation. The analysis reveals that besides its well-characterised role in cell division, CDK1 regulates different biological processes, phosphorylating proteins implicated in apoptosis, Golgi organisation and protein transport (Fig. 2b). Although the canonical cell cycle-dependent activity of CDK1 mainly occurs in the nucleus and in the cytosol [14], the ability to coordinate many different processes relies on the complex and dynamin shuttling of CDK1 through the different subcellular compartments. The subcellular compartmentalisation analysis of CDK1 substrates revealed that some of the CDK1 substrates are located in the mitochondria, ER and Golgi compartments (Fig. 2c). As the functional role of CDK1 in the regulation of mitosis has been already extensively reviewed, here we describe how CDK1 alone or in complex with cyclin B1 controls crucial ‘unconventional’ biological functions through the phosphorylation of a highly connected signalling network (Fig. 2d).
Table 2.
The CDK1 substrates.
| Gene name | Effect | Phosphosite | Evidence (PMID) |
|---|---|---|---|
| ABI1 | inhibition | S216 | 21900237 |
| AKAP12 | activation | T767 | 23063527 |
| ANAPC1 | activation | S355 | 14657031 |
| APLP2 | unknown | T736 | 9109675 |
| AR | activation | S83 | 21799006 |
| ATAD5 | inhibition | S653 | 31875566 |
| BAD | activation | S91 | 24677263 |
| BCL2 | activation | T56 | 10766756 |
| BCL2L11 | activation | S104 | 22071694 |
| BIRC5 | activation | T34 | 11861764 |
| BRCA1 | activation | S1191-S1189-S1497 | 19683496 |
| BUB1 | activation | T609 | 16760428 |
| BUB1B | activation | T620 | 17785528 |
| CASP8 | inhibition | S387 | 20937773 |
| CASP9 | inhibition | T125 | 16287866 |
| CC2D1A | activation | S208 | 20171170 |
| CDC16 | activation | S560 | 14657031 |
| CDC23 | activation | T565 | 14657031 |
| CDC25A | activation | S116-S18 | 12411508 |
| CDC25B | activation | S321-S160 | 20801879;12107172 |
| CDC25C | opposite effects | S214-S168-T48-T67-S122-T130 | 10864927; 10037602 |
| CDC27 | activation | T446-S426 | 14657031 |
| CDC7 | activation | T376 | 10846177 |
| CDKN1B | inhibition | T187 | 10931950 |
| CEP55 | inhibition | S428-S425 | 16198290 |
| CHEK1 | activation | S301-S286 | 21765472 |
| CKAP2 | activation | T623 | 19369249 |
| CSNK2A1 | activation | S362-S370-T360-T344 | 19941816;7592773 |
| CSNK2B | activation | S209 | 7578274 |
| CUX1 | inhibition | S1237-S1270 | 11584018 |
| DCTN6 | activation | T186 | 23455152 |
| DDX3X | inhibition | T323-T204 | 16280325 |
| DLG1 | unknown | S443-S158 | 19066288 |
| DNMT1 | activation | S154 | 21565170 |
| DUT | activation | S11 | 8631817 |
| E2F1 | activation | S337-S332 | 8087847 |
| ECT2 | opposite effects | T848-T444-T373 | 16247472; 16170345 |
| EEF1D | unknown | S133 | 12551973 |
| EEF2K | inhibition | S359 | 18337751 |
| EGFR | inhibition | S1026 | 8360196 |
| EIF4EBP1 | inhibition | T70 | 11553333 |
| EIF4G2 | activation | T508 | 29530922 |
| EPN1 | inhibition | S382 | 10764745 |
| ERCC6L | activation | T1063 | 17218258 |
| ESPL1 | inhibition | S1126 | 11747808 |
| EZH2 | inhibition | T487-T345 | 21659531 |
| FANCG | activation | S387 | 15367677 |
| FEN1 | inhibition | S187 | 12853968 |
| FOXK2 | activation | S428-S373 | 20810654 |
| FOXM1 | activation | T611-S251 | 19737929 |
| FOXO1 | inhibition | S249 | 18408765 |
| GOLGA2 | inhibition | S37 | 9753325 |
| GORASP1 | inhibition | S274 | 15834132 |
| HMGA1 | inhibition | T53-T78-S36 | 17960875;1939057 |
| HMGA2 | inhibition | S59-S44 | 10636877 |
| INCENP | activation | T412 | 16378098 |
| IREB2 | inhibition | S157 | 18574241 |
| KAT5 | activation | S90-S86 | 16103124 |
| KAT7 | activation | T88-T85 | 18250300 |
| KHDRBS1 | unknown | T317 | 9315091 |
| KIF11 | activation | T926 | 9235942 |
| KIF20B | activation | T1644 | 11470801 |
| KIF22 | activation | T463 | 12727876 |
| KIF2C | inhibition | T537 | 20368358 |
| KIF4A | activation | T1161 | 29771379 |
| KMT5A | activation | S100 | 20966048 |
| KRT18 | activation | S34 | 9524113 |
| KRT8 | activation | S432 | 9524113 |
| LATS1 | activation | S613 | 12372621 |
| LBR | inhibition | S71 | 14718546 |
| LIG1 | activation | S76 | 12851383 |
| LMNA | activation | S390-S392-S22 | 18815303 |
| MAP2K1 | inhibition | T292-T286 | 8114697 |
| MAP4 | inhibition | S696-S787 | 9398320;10791892 |
| MAPK6 | activation | S684-T698-S688-ST05 | 20236090 |
| MASTL | activation | T194-T207 | 22354989 |
| MCL1 | inhibition | T92 | 20526282 |
| MCM4 | inhibition | T19 | 12714602 |
| MDM4 | inhibition | S96 | 15735705 |
| MPLKIP | activation | T120-S104-S93 | 17310276 |
| MYOD1 | inhibition | S200-S5 | 21902831;14749395 |
| NCOA3 | inhibition | S728 | 22163316 |
| NFAT5 | activation | T135 | 21209322 |
| NIFK | activation | T238 | 16244663 |
| NINL | activation | S185 | 20890132 |
| NME1 | activation | S120 | 18234856 |
| NPM1 | inhibition | S70-T237-T234-T199 | 19933706;12058066; |
| NSFL1C | inhibition | S140 | 12810701 |
| NUCKS1 | inhibition | S181 | 12413487 |
| NUMA1 | inhibition | T2055 | 23921553 |
| NUP210 | activation | S1881 | 8672508 |
| NUP50 | inhibition | S221 | 19767751 |
| NUP98 | inhibition | S612-S623-T670 | 21335236 |
| NUSAP1 | inhibition | T338-T300 | 22101338 |
| ORC1 | activation | T375-S258-S273 | 11931757 |
| PAPOLA | activation | S537-S558-S545 | 34048556 |
| PBK | unknown | T9 | 15541388 |
| PIK3C2A | inhibition | S259 | 12719431 |
| PIK3C3 | inhibition | T159 | 20513426 |
| PITPNM1 | activation | S382-T287 | 15125835 |
| PLEC | inhibition | T4539 | 8626512 |
| PPP1CA | inhibition | T320 | 12202491 |
| PRDX1 | inhibition | T90 | 11986303 |
| PRPS1 | activation | S103 | 31253668 |
| PTHLH | inhibition | T121 | 10373465 |
| PTPN1 | unknown | S386 | 8491187 |
| PTPN2 | unknown | S304 | 15030318 |
| PTTG2 | activation | S165 | 10656688 |
| RAB5B | unknown | S123 | 10403367 |
| RAD9A | activation | S277-S328-T355-S336-T292 | 12734188 |
| RANBP2 | activation | S2276-S2251-S2246-S2280 | 26051540 |
| RANGAP1 | activation | S428-T409-S442-T2153 | 15037602 |
| RAP1GAP | unknown | S484 | 1406653 |
| RB1 | inhibition | S249-S811-S807-T252-T373 | 1756735 |
| RCC1 | activation | S2-S381-S11-T274 | 15014043 |
| REPS2 | inhibition | S463 | 10764745 |
| RFC1 | inhibition | T506 | 12930972 |
| RNMT | activation | T77 | 26942677 |
| RPA2 | activation | S23-S29 | 1318195 |
| RPS3 | activation | T221 | 21871177 |
| RPS6KB1 | activation | T444-S434-S394 | 11705993;12586835;9271440 |
| RPTOR | unknown | S696-T706 | 20169205 |
| RRM2 | inhibition | S20-T33 | 9990288;22632967 |
| RUNX1 | opposite effects | S276-S21-T273-S266-S249-S397 | 1705873; 12058866; 11278991 |
| RUNX2 | activation | S465 | 16407259 |
| SAMHD1 | inhibition | T592 | 23602554 |
| SGO1 | activation | T346 | 24055156 |
| SIRT1 | activation | S540 | 19107194 |
| SIRT2 | inhibition | S368 | 17488717 |
| SLBP | inhibition | T62 | 18490441 |
| SP1 | activation | T739 | 20150555 |
| SQSTM1 | activation | T269-S272 | 20974803 |
| SREBF1 | activation | S439 | 16880739 |
| STIM1 | inhibition | S668 | 19881501 |
| STIP1 | inhibition | T332-S189-T198 | 14754904 |
| SUN1 | inhibition | S334-S48 | 25482198 |
| SYN3 | activation | S470 | 14732590 |
| TK1 | inhibition | S13 | 14697231 |
| TOP2A | unknown | S1247-S1361-S1354-S1393 | 7635160 |
| TP53 | activation | S315 | 24173284 |
| TP53BP1 | inhibition | S1678 | 30685087 |
| TP73 | inhibition | T86 | 12676926 |
| TPX2 | inhibition | T72 | 25688093 |
| TSC1 | inhibition | T1047-T417-S584 | 14551205 |
| UBA1 | activation | S835-S4 | 7724583;9099746 |
| UBE2A | activation | S120 | 11953320 |
| UBXN2B | inhibition | S56-T59 | 23500464 |
| USP16 | activation | S552 | 24013421 |
| VCPIP1 | inhibition | T761-S768 | 23500464 |
| VIM | inhibition | S55 | 7983050 |
| WAC | activation | T244-T471-T482-T457 | 30021153 |
| WEE1 | inhibition | S123 | 16085715 |
| ZC3HC1 | inhibition | S395 | 17389604 |
CDK1 direct substrates were extracted from the SIGNOR database [13] and reported.
Fig. 2. The CDK1 substrates landscape.
a–c Circular bar plots showing the GO-term enrichment analysis of the CDK1 substrates in terms of molecular function (a), biological processes (b) and cell compartment (c). d Signalling network of CDK1 substrates extracted from SIGNOR database and connected in Cytoscape. GO-term enrichment analysis was performed to group and classify the substrates according to the biological processes and cell compartment localisation. The edges connecting CDK1 to its substrates are red if the interaction is annotated as inhibitory, blue if activatory, black if unknown.
Gene expression regulation
During mitosis, the nuclear envelope breakdown and the chromatin condensation globally downregulate transcription. Consistently, CDK1 controls a network of transcription factors and chromatin regulators, regulating the expression of about 8,000 mitosis-specific genes [15]. Interestingly, recent studies revealed that CDK1 phosphorylates and modulates the activity of crucial transcription factors, including RUNX2, SIRT1/2, NPM1 and SREBF1 (Fig. 2d). The resultant stabilisation and activation of these transcription factors by CDK1 mediates cell proliferation and apoptosis by modulating differentiation and metabolic processes [16, 17].
Signal transduction
CDK1 plays a crucial role in promoting cell proliferation by directly phosphorylating key signalling proteins, such as MAPK6, or ERK3, and MAP2K2 [18]. The CDK1-mediated phosphorylation of MAPK6 leads to the activation of the cascade of MAPKs signalling pathways. Additionally, CDK1 is known to phosphorylate serine residues on RPTOR and RPS6KB1, indicating a key role of CDK1 in regulating mTORC1 activity [19, 20]. Tendentially, phosphorylation by CDK1 can modulate the activity, localisation, and interactions of signalling kinases, promoting downstream signalling events involved in cell growth, differentiation, and survival.
Apoptosis
The cyclinB1-CDK1 complex localises in the mitochondria, playing a crucial and complex role in the regulation of apoptosis. Contrasting observations have been reported about the pro-apoptotic or anti-apoptotic role of the cyclinB1-CDK1 complex. While it inhibits apoptosis through the phosphorylation of caspase-9 and BIRC5 proteins [21, 22], it has been reported that the complex promotes cell death by directly phosphorylating and activating Bcl-2 family members. Specifically, CDK1 activates BAD by phosphorylating it on Ser128 and impairing its interaction with 14-3-3 proteins. Consequently, BAD can translocate to the mitochondria promoting mitochondrial membrane permeabilization and apoptosis [23, 24]. Moreover, CDK1 phosphorylates BCL2L1, BCL2 and MCL1, suppressing their anti-apoptotic functions. Based on these data, the conflicting role of CDK1 in either protecting cells from apoptosis or inducing apoptosis can be affected by different experimental conditions and specific cellular contexts. From a clinical point of view, understanding the contradictory role of CDK1 in apoptosis could be an important achievement in identifying new therapeutic strategies. However, data from animal models and clinical trials are incomplete and the CDK1-mediated regulation of apoptosis remains still poorly investigated.
Mitochondrial processes
Beyond apoptosis, CDK1 regulates other crucial mitochondrial processes, including mitochondrial dynamics through the phosphorylation of specific proteins involved in mitochondrial fusion and fission. For instance, CDK1 phosphorylates Ser585 of Drp-1, inducing its mitochondrial translocation and triggering fission [25]. CDK1 contributes to maintaining cellular redox balance and protects cells from oxidative stress. Indeed, mitochondria-translocated CDK1 phosphorylates Ser106 of the Manganese Superoxide Dismutase (MnSOD) enzyme, stabilising its protein level and enhancing its antioxidant activity [26]. Additionally, it has been shown that CDK1 mediates the upregulation of the oxidative phosphorylation process by phosphorylating Thr150 and Ser159 of SIRT3 [27] and activating a cluster of subunits of the Complex I, which increases the mitochondrial metabolism and ATP production [28].
Golgi remodelling
Evidence also suggests a role of the CDK1 in modulating Golgi-related topological and structural changes. Golgi-located CDK1 phosphorylates GRASP65, GM130 and the small RAS GTPase RAB1 inducing the disassembly of the Golgi network and blocking the vesicle fusion with the ER. CDK1 can also regulate N-glycosylation enzymes, such as MANI. During mitosis, Golgi fragmentation blocks the intra-Golgi transport causing the accumulation of cargo molecules and enzymes. The inhibitory phosphorylation on S12 by CDK1 inhibits MANI activity to limit the aberrant glycosylation of the molecular entities trapped together in the Golgi compartment [29, 30].
Transport
CDK1 phosphorylates and modulates the activity of different transport-related proteins. CDK1-mediated phosphorylation of Rab5B regulates the dynamics and the maturation of early endosomes, impacting the sorting and recycling of internalised membrane proteins. Moreover, CDK1 phosphorylates EPN1, a key regulator of the endocytic processes [31]. Specifically, the CDK1-dependent phosphorylation EPN1 affects its interaction with clathrin and other endocytic proteins, modulating the assembly and dynamics of clathrin-coated pits and vesicles. Additionally, CDK1 phosphorylates nuclear transport factors, including importins and exportins, which are responsible for the recognition and transport of cargo molecules into and out of the nucleus. For instance, the CDK1-mediated phosphorylation of RANBP2 and NUP50, which are compliant with the nuclear export and import pathways, respectively, influences the efficiency and dynamics of nucleocytoplasmic transport processes.
Intermediate-filament organisation
Finally, CDK1 can phosphorylate several intermediate-filament proteins, including vimentin (VIM), lamin A/C (LMNA), and keratin 8 (KRT8). These phosphorylation events serve as crucial regulatory mechanisms that not only influence cell cycle-related alterations in cell morphology and structure but can also play a pivotal role in facilitating cell migration during immune responses or metastasis [32].
In summary, recent studies have implicated CDK1 in a wide variety of cell cycle-independent roles. Although it was originally believed that CDK1 must partner with cyclin B1 to become active, ample demonstration of functions for CDK1 alone has been reported.
CDK1 in cancer
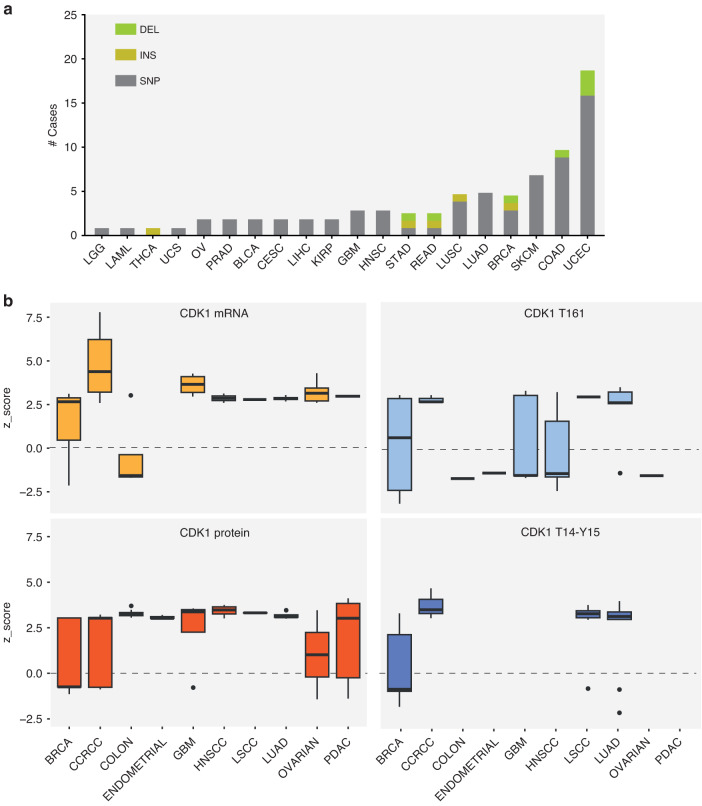
Deregulation of CDK1 has been closely associated with cancer. Interestingly, oncogenic alterations of CDK1 can be considered rare genetic events, suggesting that complex molecular mechanisms contribute to the aberrant regulation of CDK1 in cancer. Indeed, CDK1 mutations (mostly SNPs) were identified in 0.74% of cancer patients, with the highest frequency in Uterine Corpus Endometrial Carcinoma (UCEC), Colon adenocarcinoma (COAD) and Skin Cutaneous Melanoma (SKCM) (Fig. 3a). According to The Cancer Genome Atlas (TCGA) (Fig. 2a) and Clinical Proteomic Tumour Analysis Consortium (CPTAC) (Fig. 3b, c), CDK1 is upregulated in many cancerous tissues compared to normal tissues, at both transcript and protein levels. Its overexpression has been correlated with inferior survival rate and poor clinical outcome [33–38]. Noteworthy, thanks to the growing availability of patient-specific phosphoproteomic data, it is possible to evaluate the activation of the state of CDK1 by monitoring the phosphorylation level of its regulatory residues. Specifically, by comparing Thr14, Tyr15 and Thr161 levels in different cancer types, it appears that CDK1 is likely to be fully active only in breast cancer tissue (BRCA in Fig. 3b), where the two inhibitory residues appear hypo-phosphorylated, whereas it seems to be inactive (with Thr14 and Tyr15 hyper-phosphorylated or Thr161 hypo-phosphorylated) in the remaining cancer types. Altogether, these observations suggest that while the CDK1 protein level is high in most of the cancer tissues, its activity, as revealed by its phosphorylation status on regulatory sites, seems to be suppressed. Although these observations may seem contradictory, it is important to consider that the biological significance of CDK1 phosphorylation on its function is more complex than previously thought. Despite the expected inhibitory effect on CDK1 activity, the phosphorylation of Tyr15 has been found to be increased in several cancer types and has been associated with the development of drug resistance. This discovery suggests that the impact of CDK1 phosphorylation on its function goes beyond simple inhibition. The upregulation of Tyr15 phosphorylation in cancer may contribute to the dysregulation of CDK1 activity and potentially play a role in the acquisition of resistance to anticancer drugs. The receptor tyrosine kinase ERBB2 receptor, SRC kinase and the non-receptor tyrosine kinase Breast Tumour Kinase (BRK) have been shown to phosphorylate Tyr15 of CDK1 [12]. In breast cancer cell lines and primary tumours, the ERRB2-mediated increased phosphorylation of Tyr15 of CDK1 leads to the inactivation of BAD and consequently resistance to taxol-induced apoptosis and drive cells to mitotic slippage and prolonged cell cycle arrest. This allows breast cancer cells to survive microtubule-targeting agent treatment. Additionally, signalling and cell growth and consequently to the onset of drug resistance. Interestingly, the results of our recent study demonstrated a clear link between increased phosphorylation levels of CDK1 at Tyr15 and the development of resistance to FLT3 inhibitors in acute myeloid leukaemia cells carrying FLT3-ITD mutations, the most common genetic alterations [39]. Collectively, this evidence reveals the complex interplay between CDK1 phosphorylation, activation and crucial cancer-related processes such as apoptosis, cell cycle regulation, drug resistance, and invasive potential.
Fig. 3. Pan-cancer analysis of CDK1 alteration.
a Bar plot showing the most common alterations in CDK1 and their frequency in cancer according to the UCSC Genome Browser. b Boxplots representing the mRNA, the total protein and the phosphorylation levels of CDK1 according to data available in The Cancer Genome Atlas (TCGA) GEPIA and CPTAC databases. Data are reported as z-score between primary tissues of the selected tumours and normal tissues.
Despite its unclear activation state in cancer, CDK1 has emerged as an attractive target for therapeutic intervention. Although first-generation pan-CDK inhibitors (e.g., flavopiridol and roscovitine) have demonstrated efficacy in inducing G1/G2 phase arrest and ultimately apoptosis of cancer cells [27–29], their low specificity and high toxicity have hindered their clinical approval. Recently, highly selective, second-generation inhibitors, RO-3306 and NU6102, have been developed [40, 41]. Despite their potential, to date, limited preclinical studies have been performed to assess their efficacy in targeting alterations of CDK1 in cancer. Combination therapy seems to be an effective approach to enhance the efficacy of CDK1-associated inhibitors in clinical trials. The inhibition of CDK1 induces cell cycle arrest at the G2/M phase where cells are most vulnerable to radiation-induced DNA damage (i) and dysfunction of the DNA repair process, leading to the accumulation of DNA damage and increasing the susceptibility to DNA-damaging agents [42, 43].
Conclusions
Over the past 2 decades, growing evidence has shown that CDK1 possesses functions that extend beyond its traditional role in regulating cell cycle progression. In this review, we interrogate signalling databases to obtain a comprehensive catalogue of CDK1 substrates. The “CDK1 substratome” is implicated in a variety of crucial biological processes, ranging from gene expression regulation, apoptosis, mitochondrial fission and fusion and Golgi structural remodelling. Interestingly, these substrates are not localised in the nucleus and cytosol compartments, suggesting the mitochondrial and Golgi translocation of CDK1 in certain conditions. In this review, we also examined proteins controlling the phosphorylation and consequently the activity of CDK1. Besides the well-characterised modulators of CDK1, our analysis highlights a novel potential role for MYO3A, GSK3A and GRK6 kinases, whose expression profile is highly correlated with CDK1 itself and its modulators across cancer tissues. Finally, by taking advantage of cancer patient-specific transcriptomic, proteomic and phosphoproteomic data stored in different databases, we report that while CDK1 protein is clearly upregulated in tumours, its activity seems to be suppressed, as revealed by its phosphorylation status on regulatory sites. Indeed, CDK1-associated inhibitors failed to demonstrate sufficient efficacy in cancer patients. Future studies will be necessary to understand the functional consequences of targeting CDK1 and its upstream modulators in combination with standard chemotherapeutic drugs. Finally, our perspective highlights that the study of the non-canonical functions of CDK1 is certainly a far cry from being a mature field and the continuous pursuit towards identifying the complete repertoire of its modulators can bring many surprises along the way.
Acknowledgements
We thank Dr. Veronica Venafra for her help in the plots showed in Fig. 2A-B-C. This research was funded by the Italian Association for Cancer Research (AIRC) with a grant to FS (Start-Up Grant n. 21815) and by the Italian Minister of University and Research (MUR) with a PRIN grant (n. 2022L8RAKN). GM is supported by the AIRC Start-up grant number 21815.
Author contributions
GM, FS designed the study and wrote the manuscript; GM, FS performed the analyses; LV revised the study; FS provided funding.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martínez-Alonso D, Malumbres M. Mammalian cell cycle cyclins. In: Clotet J, Dodding M, Fagotto F, editors. Seminars in cell and developmental biology. vol. 107. Elsevier Ltd; 2020. p. 1–190. [DOI] [PubMed]
- 2.Santamaría D, Barrière C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–5. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 3.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–43. [DOI] [PMC free article] [PubMed]
- 4.Krek W, Nigg EA. Differential phosphorylation of vertebrate p34(cdc2) kinase at the G1/S and G2/M transitions of the cell cycle: Identification of major phosphorylation sites. EMBO J. 1991;10:305–16. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon MJ, Lee T, Kirschner MW. Role of phosphorylation in p34cdc2 activation: Identification of an activating kinase. Mol Biol Cell. 1992;3:13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development (Cambridge) 2013;140:3079–93. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Liu Q, Guo X, Yuan Q, Nian S, Kang P, et al. Systematic pan-cancer analysis identifies cdk1 as an immunological and prognostic biomarker. J Oncol. 2022;2022:8115474. [DOI] [PMC free article] [PubMed]
- 8.Liu X, Wu H, Liu Z. An integrative human pan-cancer analysis of cyclin-dependent kinase 1 (CDK1). Cancers (Basel). 2022;14:2658. [DOI] [PMC free article] [PubMed]
- 9.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–20. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JL, Yaron TM, Huntsman EM, Kerelsky A, Song J, Regev A, et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature. 2023;613:759–66. doi: 10.1038/s41586-022-05575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berginski ME, Moret N, Liu C, Goldfarb D, Sorger PK, Gomez SM. The dark kinase knowledgebase: an online compendium of knowledge and experimental results of understudied kinases. Nucleic Acids Res. 2021;49:D529–35. doi: 10.1093/nar/gkaa853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan M, Jing T, Lan KH, Neal CL, Li P, Lee S, et al. Phosphorylation on tyrosine-15 of p34Cdc2 by ErbB2 inhibits p34Cdc2 activation and is involved in resistance to taxol-induced apoptosis. Mol Cell. 2002;9:993–1004. doi: 10.1016/S1097-2765(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 13.Lo Surdo P, Iannuccelli M, Contino S, Castagnoli L, Licata L, Cesareni G, et al. SIGNOR 3.0, the SIGnaling network open resource 3.0: 2022 update. Nucleic Acids Res. 2023;51:D631–7. doi: 10.1093/nar/gkac883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavet O, Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J Cell Biol. 2010;189:247–59. doi: 10.1083/jcb.200909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palozola KC, Donahue G, Liu H, Grant GR, Becker JS, Cote A, et al. Mitotic transcription and waves of gene reactivation during mitotic exit. Science. 2017;358:119–22. doi: 10.1126/science.aal4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YJ, Dominguez-Brauer C, Wang Z, Asara JM, Costa RH, Tyner AL, et al. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J Biol Chem. 2009;284:30695–707. doi: 10.1074/jbc.M109.007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slee EA, Lu X. Requirement for phosphorylation of P53 at Ser312 in suppression of chemical carcinogenesis. Sci Rep. 2013;3:3105. doi: 10.1038/srep03105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanguay PL, Rodier G, Meloche S. C-terminal domain phosphorylation of ERK3 controlled by Cdk1 and Cdc14 regulates its stability in mitosis. Biochem J. 2010;428:103–11. doi: 10.1042/BJ20091604. [DOI] [PubMed] [Google Scholar]
- 19.Papst PJ, Sugiyama H, Nagasawa M, Lucas JJ, Maller JL, Terada N. Cdc2-cyclin B phosphorylates p70 S6 kinase on Ser411 at mitosis. J Biol Chem. 1998;273:15077–84. doi: 10.1074/jbc.273.24.15077. [DOI] [PubMed] [Google Scholar]
- 20.Gwinn DM, Asara JM, Shaw RJ. Raptor is phosphorylated by cdc2 during mitosis. PLoS One. 2010;5:e9197. doi: 10.1371/journal.pone.0009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’ Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, et al. Regulation of apoptosis at cell division by p34 cdc2 phosphorylation of survivin [Internet]. Available from: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed]
- 22.Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/Cyclin B1 protects mitotic cells against apoptosis. Mol Cell. 2007;26:301–10. doi: 10.1016/j.molcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Cai X, Han X, Xu N, Chang DC. CDK1 switches mitotic arrest to apoptosis by phosphorylating Bcl-2/Bax family proteins during treatment with microtubule interfering agents. Cell Biol Int. 2014;38:737–46. doi: 10.1002/cbin.10259. [DOI] [PubMed] [Google Scholar]
- 24.Konishi Y, Lehtinen M, Donovan N, Bonni A. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell. 2002;9:1005–16. doi: 10.1016/S1097-2765(02)00524-5. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–9. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 26.Candas D, Fan M, Nantajit D, Vaughan AT, Murley JS, Woloschak GE, et al. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J Mol Cell Biol. 2013;5:166–75. doi: 10.1093/jmcb/mjs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Fan M, Candas D, Qin L, Zhang X, Eldridge A, et al. CDK1-mediated SIRT3 activation enhances mitochondrial function and tumor radioresistance. Mol Cancer Ther. 2015;14:2090–102. doi: 10.1158/1535-7163.MCT-15-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Fan M, Candas D, Zhang TQ, Qin L, Eldridge A, et al. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev Cell. 2014;29:217–32. doi: 10.1016/j.devcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22:3279–90. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang s, Haga y, Li J, Zhang J, Kweon HK, Seino J, et al. Mitotic phosphorylation inhibits the Golgi mannosidase MAN1A1. Cell Rep. 2022;41:111679. doi: 10.1016/j.celrep.2022.111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kariya K, Koyama S, Nakashima S, Oshiro T, Morinaka K, Kikuchi A. Regulation of complex formation of POB1/Epsin/adaptor protein complex 2 by mitotic phosphorylation. J Biol Chem. 2000;275:18399–406. doi: 10.1074/jbc.M000521200. [DOI] [PubMed] [Google Scholar]
- 32.Kraxner J, Köster S. Influence of phosphorylation on intermediate filaments. Biol Chem. 2023; 404:821–7. [DOI] [PMC free article] [PubMed]
- 33.Xing Z, Wang X, Liu J, Zhang M, Feng K, Wang X. Expression and prognostic value of CDK1, CCNA2, and CCNB1 gene clusters in human breast cancer. J Int Med Res. 2021;49:300060520980647. [DOI] [PMC free article] [PubMed]
- 34.Bednarek K, Kiwerska K, Szaumkessel M, Bodnar M, Kostrzewska-Poczekaj M, Marszalek A, et al. Recurrent CDK1 overexpression in laryngeal squamous cell carcinoma. Tumor Biol. 2016;37:11115–26. doi: 10.1007/s13277-016-4991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, He F, Zhang Z, Xiang Z, Hu D. CDK1 serves as a potential prognostic biomarker and target for lung cancer. J Int Med Res. 2020;48:0300060519897508. doi: 10.1177/0300060519897508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung WW, Lin YM, Wu PR, Yen HH, Lai HW, Su TC, et al. High nuclear/cytoplasmic ratio of cdk1 expression predicts poor prognosis in colorectal cancer patients. BMC Cancer. 2014;14:1–7. doi: 10.1186/1471-2407-14-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong S, Huang F, Zhang H, Chen Q. Overexpression of BUB1B, CCNA2, CDC20, and CDK1 in tumor tissues predicts poor survival in pancreatic ductal adenocarcinoma. Biosci Rep. 2019;39:BSR20182306. [DOI] [PMC free article] [PubMed]
- 38.Yang Y, Liu Q, Guo X, Yuan Q, Nian S, Kang P, et al. Systematic pan-cancer analysis identifies CDK1 as an immunological and prognostic biomarker. 2022;2022:8115474. 10.1155/2022/8115474 [DOI] [PMC free article] [PubMed]
- 39.Massacci G, Venafra V, Latini S, Bica V, Pugliese GM, Graziosi S, et al. A key role of the WEE1-CDK1 axis in mediating TKI-therapy resistance in FLT3-ITD positive acute myeloid leukemia patients. Leukemia. 2023;37:288–97. doi: 10.1038/s41375-022-01785-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies TG, Bentley J, Arris CE, Boyle FT, Curtin NJ, Endicott JA, et al. Structure-based design of a potent purine-based cyclin-dependent kinase inhibitor. Nat Struct Biol. 2002;9:745–9. doi: 10.1038/nsb842. [DOI] [PubMed] [Google Scholar]
- 41.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1 [Internet]. Proc Natl Acad Sci USA. 2006;103:10660-5. [DOI] [PMC free article] [PubMed]
- 42.Liao H, Ji F, Geng X, Xing M, Li W, Chen Z, et al. CDK1 promotes nascent DNA synthesis and induces resistance of cancer cells to DNA-damaging therapeutic agents [Internet]. Oncotarget. 2017;8: 90662–73. [DOI] [PMC free article] [PubMed]
- 43.Prevo R, Pirovano G, Puliyadi R, Herbert KJ, Rodriguez-Berriguete G, O’Docherty A, et al. CDK1 inhibition sensitizes normal cells to DNA damage in a cell cycle dependent manner. Cell Cycle. 2018;17:1513–23. doi: 10.1080/15384101.2018.1491236. [DOI] [PMC free article] [PubMed] [Google Scholar]