Abstract
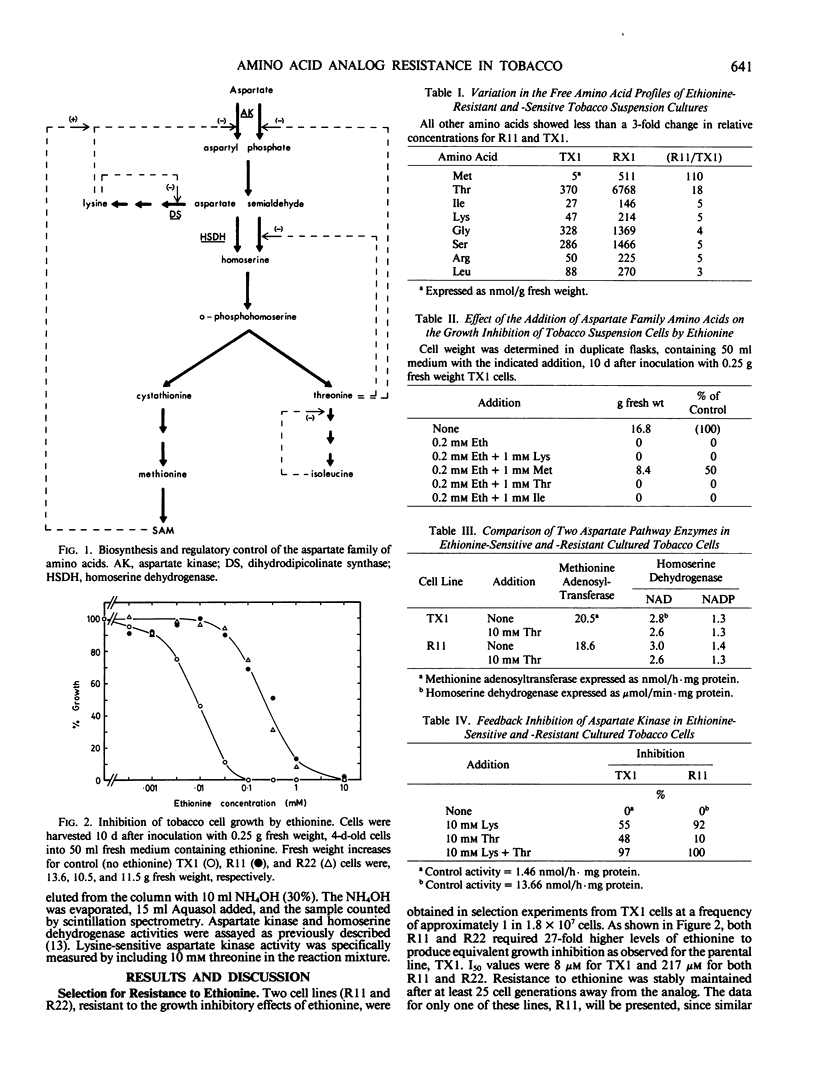
Two cultured tobacco cell lines (Nicotiana tabacum L. cv Xanthi) were selected for resistance to growth inhibition by the methionine analog ethionine. Comparison of the free amino acid pool levels in these lines with those of the ethionine-sensitive parental line showed substantial accumulation of methionine (110×), threonine (18×), and lysine (5×). In vitro enzymic analysis of lysine-sensitive aspartate kinase activity showed the resistant lines to contain 16 times that found in the sensitive line. The lysine-sensitive enzymes from both resistant and sensitive lines coeluted from DEAE-cellulose and exhibited similar Km values. Both showed identical lysine plus S-adenosylmethionine inhibition profiles suggesting that the elevated activity in the resistant lines is not due to a structural change in the lysine-sensitive enzyme but possibly to the level of its expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alix J. H. Molecular aspects of the in vivo and in vitro effects of ethionine, an analog of methionine. Microbiol Rev. 1982 Sep;46(3):281–295. doi: 10.1128/mr.46.3.281-295.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright S. W., Miflin B. J., Rognes S. E. Threonine accumulation in the seeds of a barley mutant with an altered aspartate kinase. Biochem Genet. 1982 Apr;20(3-4):229–243. doi: 10.1007/BF00484421. [DOI] [PubMed] [Google Scholar]
- Hibberd K. A., Green C. E. Inheritance and expression of lysine plus threonine resistance selected in maize tissue culture. Proc Natl Acad Sci U S A. 1982 Jan;79(2):559–563. doi: 10.1073/pnas.79.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. C., Smith D. A. S-adenosylmethionine synthetase in methionine regulatory mutants of Salmonella typhimurium. Mol Gen Genet. 1973 Oct 16;126(1):7–18. doi: 10.1007/BF00333477. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- SORSOLI W. A., SPENCE K. D., PARKS L. W. AMINO ACID ACCUMULATION IN ETHIONINE-RESISTANT SACCHAROMYCES CEREVISIAE. J Bacteriol. 1964 Jul;88:20–24. doi: 10.1128/jb.88.1.20-24.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano K., Komamine A. Change in the proportion of two aspartokinases in carrot root tissue in response to in vitro culture. Plant Physiol. 1978 Jan;61(1):115–118. doi: 10.1104/pp.61.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]


