Abstract
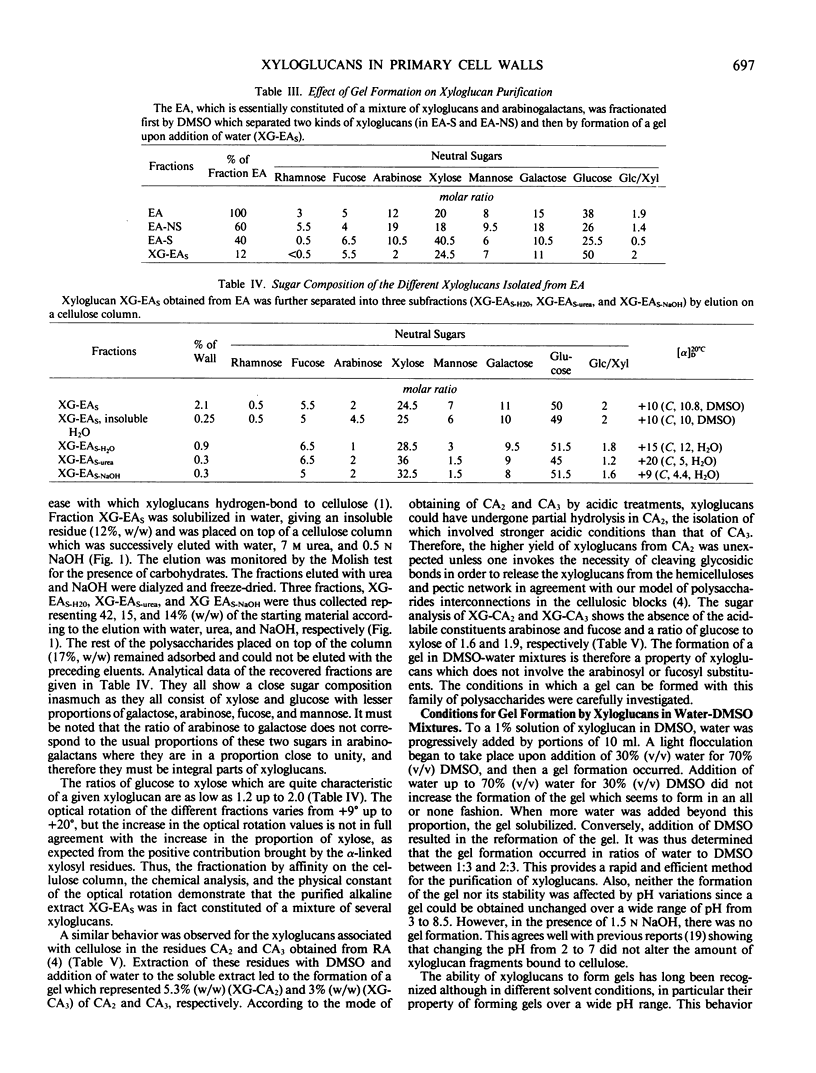
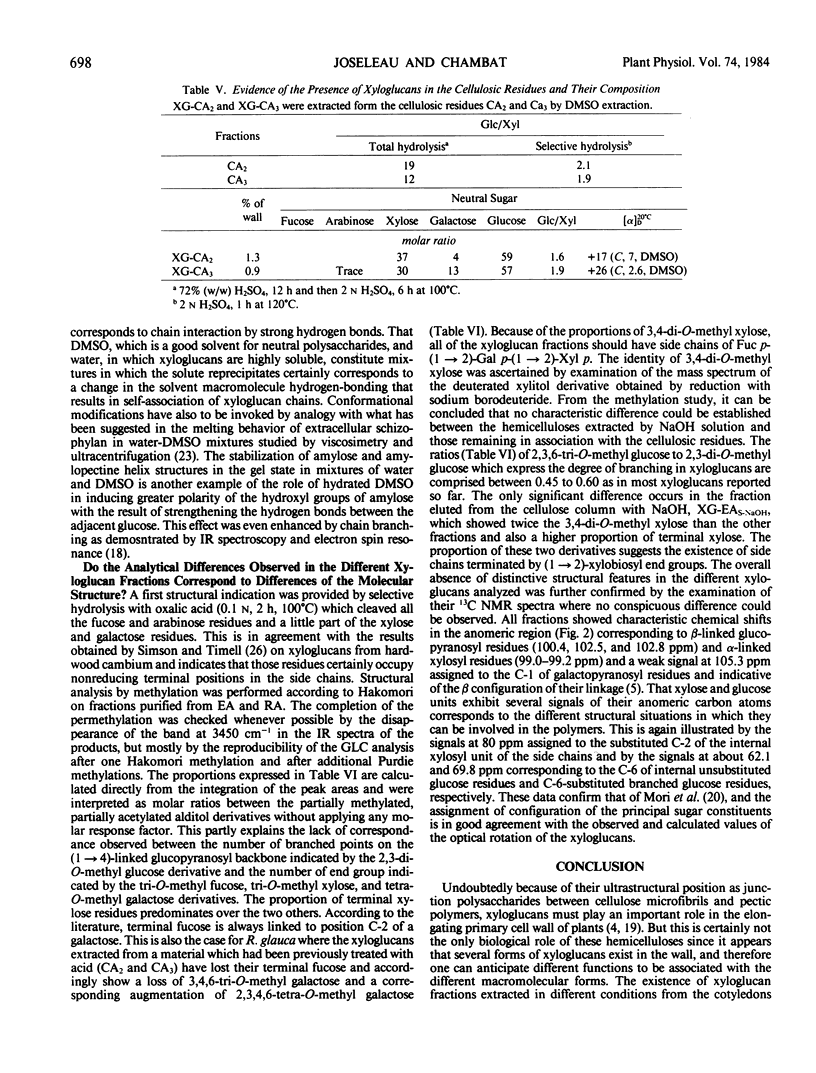
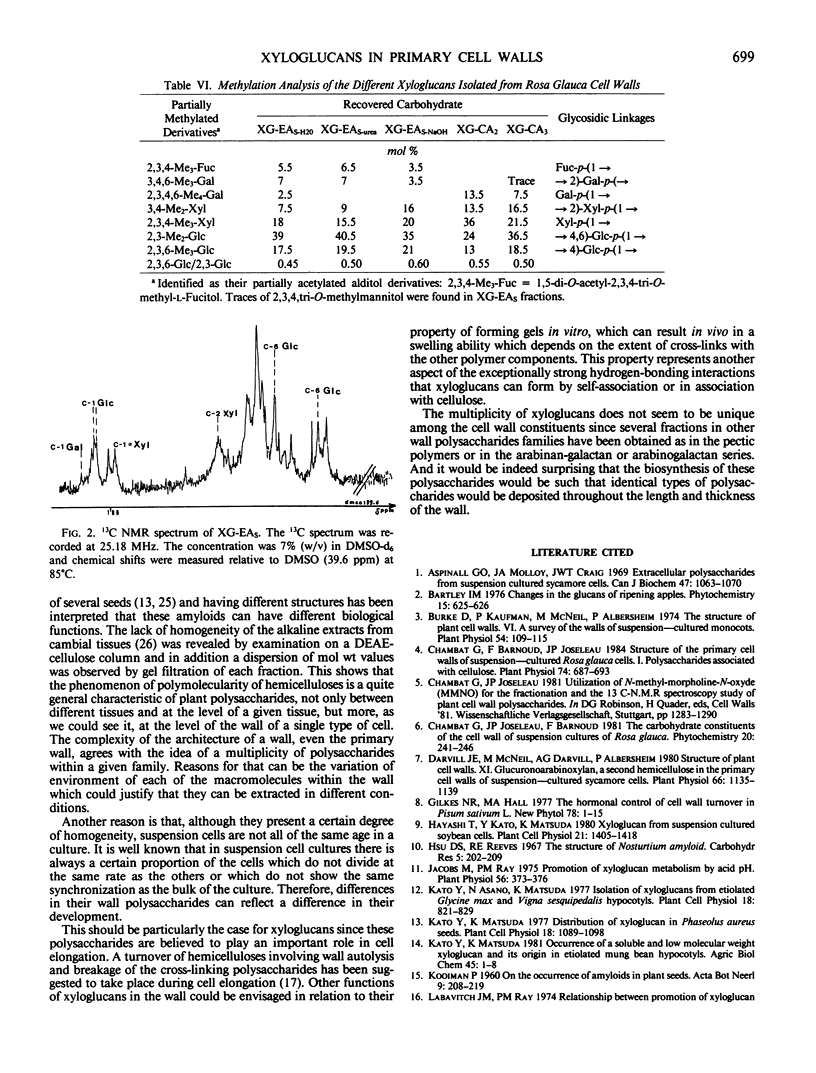
Xyloglucans, characteristic hemicellulosic polysaccharides of plant primary walls, have been isolated from Rosa glauca suspension-cultured cells. The cell wall material was fractionated by two sequences of extraction based on solubilization of the hemicelluloses in alkaline and organic solvent systems, respectively. In both cases, only a part (about 50%) of the total xyloglucan could be extracted, the rest remaining tightly associated with cellulose and necessitating the use of acid to be solubilized. Purification of xyloglucans was effected by formation of a gel in appropriate mixtures of dimethyl sulfoxide and water. Further fractionation could be achieved on a cellulose column eluted with chaotropic solvents. This demonstrated the heterogeneity of xyloglucans in the primary cell walls. Analytical data show that all fractions are constituted with the same sugars: l-arabinose, l-fucose, d-galactose, d-xylose, and d-glucose, but their relative proportions differ, particularly the ratio of glucose to xylose which varies from 1.2 to 2 within the different xyloglucans. The structure of these hemicelluloses was established by methylation analysis and shown to consist of a (1 → 4)-linked glucan backbone which carries substituents on the O-6 of glucose. Here again, the multiple forms of xyloglucans was suggested by the various patterns of substitutions found on the different fractions. The configuration of the linkages were established by 13C nuclear magnetic resonance spectroscopy and shown to be β for the glucan backbone, α for the xylosyl and fucosyl substituents, and β for the galactosyl substituents. These configurations agree with the specific rotation of the xyloglucan.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall G. O., Molloy J. A., Craig J. W. Extracellular polysaccharides from suspension-cultured sycamore cells. Can J Biochem. 1969 Nov;47(11):1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambat G., Barnoud F., Joseleau J. P. Structure of the Primary Cell Walls of Suspension-Cultured Rosa glauca Cells: I. Polysaccharides Associated with Cellulose. Plant Physiol. 1984 Mar;74(3):687–693. doi: 10.1104/pp.74.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill J. E., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: XI. GLUCURONOARABINOXYLAN, A SECOND HEMICELLULOSE IN THE PRIMARY CELL WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Promotion of Xyloglucan Metabolism by Acid pH. Plant Physiol. 1975 Sep;56(3):373–376. doi: 10.1104/pp.56.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Kivilaan A., Bandurski R. S. In vitro autolysis of plant cell walls. Plant Physiol. 1967 Jul;42(7):968–972. doi: 10.1104/pp.42.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]


