Abstract
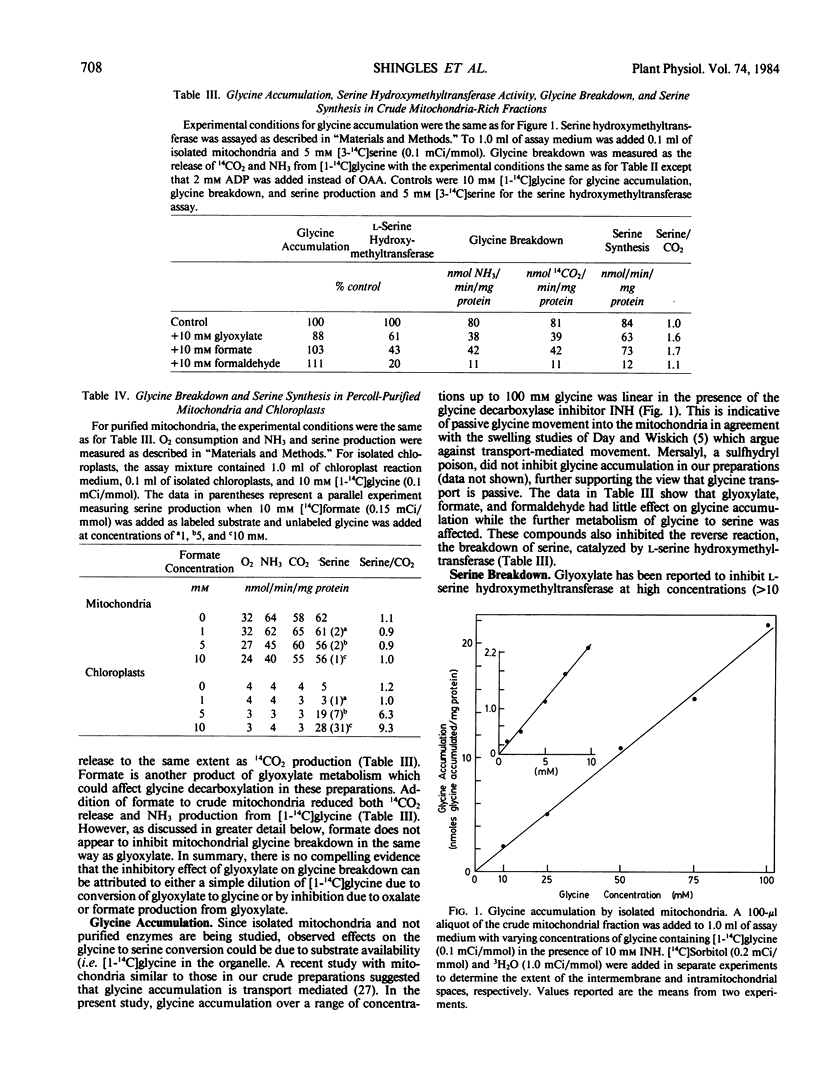
Glycine decarboxylation and serine synthesis were studied in pea (Pisum sativum L.) leaf discs, in metabolically active intact chloroplasts, and in mitochondria isolated both partially by differential centrifugation (i.e. `crude') and by further purification on a Percoll gradient. Glycolate, glyoxylate, and formate reduced glycine decarboxylase activity (14CO2 and NH3 release) in the crude green-colored mitochondrial fractions, and in the leaf discs without markedly altering serine synthesis from [1-14C]glycine. Glycolate acted because it was converted to glyoxylate which behaves as a noncompetitive inhibitor (Ki = 5.1 ± 0.5 millimolar) on the mitochondrial glycine decarboxylation reaction in both crude and Percoll-purified mitochondria. In contrast, formate facilitates glycine to serine conversion by a route which does not involve glycine breakdown in the crude mitochondrial fraction and leaf discs. Formate does not alter the conversion of two molecules of glycine to one CO2, one NH3, and one serine molecule in the Percoll-purified mitochondria. In chloroplasts which were unable to break glycine down to CO2 and NH3, serine was labeled equally from [14C]formate and [1-14C]glycine. The maximum rate of serine synthesis observed in chloroplasts is similar to that in isolated metabolically active mitochondria. Formate does not appear to be able to substitute for the one-carbon unit produced during mitochondrial glycine breakdown but can facilitate serine synthesis from glycine in a chloroplast reaction which is probably a secondary one in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron G. P., Spalding M. H., Edwards G. E. Stoichiometry of carbon dioxide release and oxygen uptake during glycine oxidation in mitochondria isolated from spinach (Spinacia oleracea) leaves. Biochem J. 1979 Nov 15;184(2):457–460. doi: 10.1042/bj1840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Sinha S. K. The interconversion of glycine and serine by plant tissue extracts. Biochem J. 1966 Nov;101(2):542–549. doi: 10.1042/bj1010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Glycine metabolism and oxalacetate transport by pea leaf mitochondria. Plant Physiol. 1981 Aug;68(2):425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski B. A Study of Formate Production and Oxidation in Leaf Peroxisomes during Photorespiration. Plant Physiol. 1979 Feb;63(2):289–293. doi: 10.1104/pp.63.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidation of formate by peroxisomes and mitochondria from spinach leaves. Biochem J. 1974 Jan;138(1):77–85. doi: 10.1042/bj1380077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miflin B. J., Marker A. F., Whittingham C. P. The metabolism of glycine and glycollate by pea leaves in relation to photosynthesis. Biochim Biophys Acta. 1966 Jun 8;120(2):266–273. doi: 10.1016/0926-6585(66)90346-3. [DOI] [PubMed] [Google Scholar]
- Oliver D. J. Formate oxidation and oxygen reduction by leaf mitochondria. Plant Physiol. 1981 Sep;68(3):703–705. doi: 10.1104/pp.68.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Regulation of Glycine Decarboxylase and l-Serine Hydroxymethyltransferase Activities by Glyoxylate in Tobacco Leaf Mitochondrial Preparations. Plant Physiol. 1982 Jul;70(1):61–66. doi: 10.1104/pp.70.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingles R. M., Arron G. P., Hill R. D. Alternative pathway respiration and lipoxygenase activity in aged potato slice mitochondria. Plant Physiol. 1982 Jun;69(6):1435–1438. doi: 10.1104/pp.69.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Walker G. H., Sarojini G., Oliver D. J. Identification of a glycine transporter from pea leaf mitochondria. Biochem Biophys Res Commun. 1982 Aug;107(3):856–861. doi: 10.1016/0006-291x(82)90601-5. [DOI] [PubMed] [Google Scholar]


