Abstract
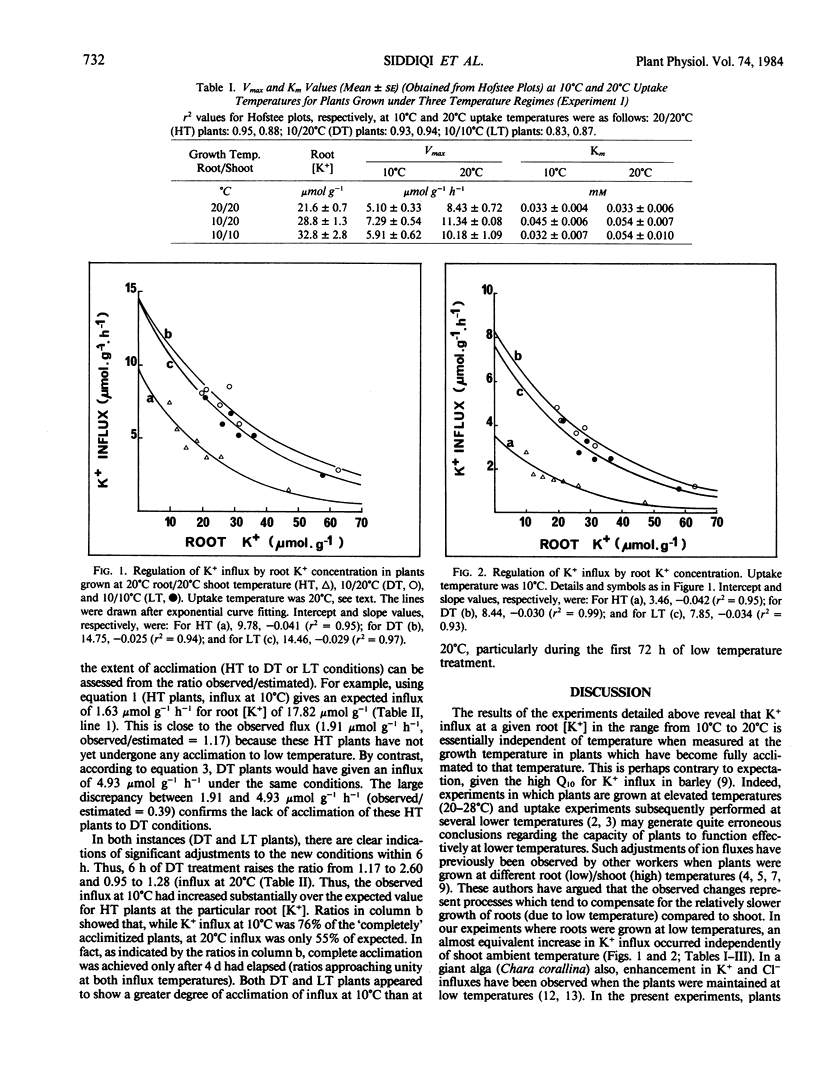
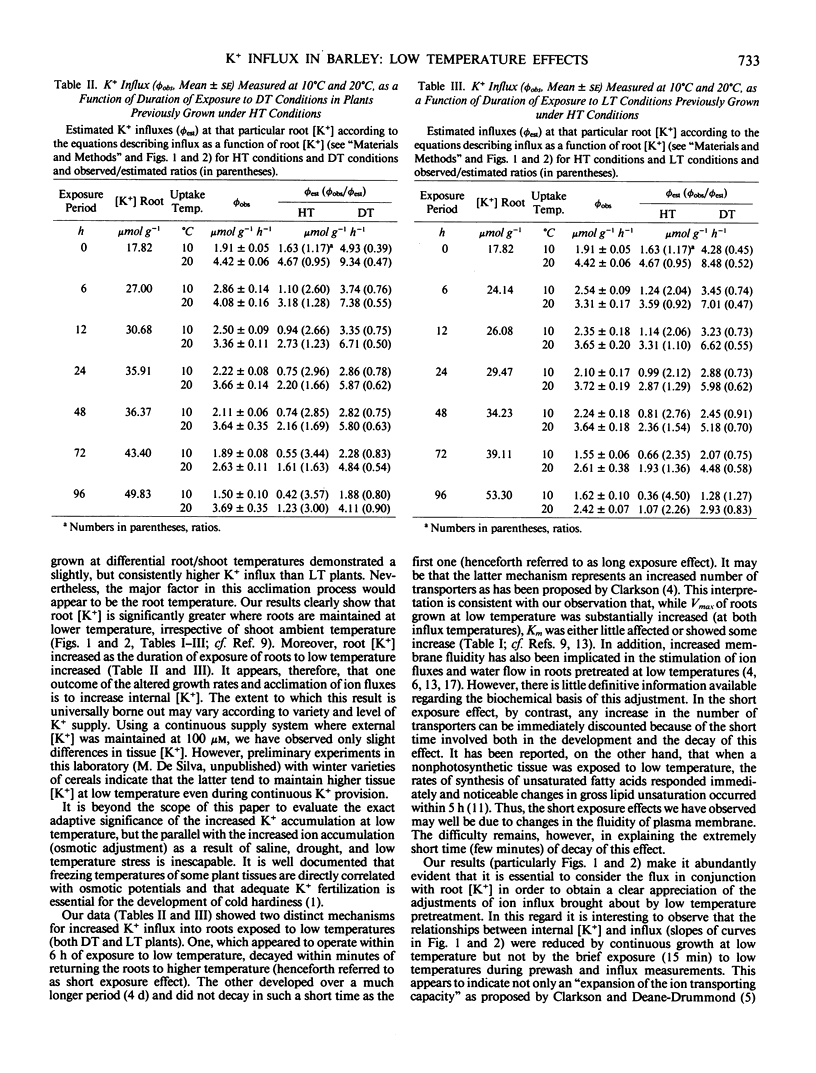
Influx and accumulation of K+ in barley (Hordeum vulgare L. cv Fergus) roots were measured at two temperatures (10°C and 20°C) in plants which had been grown with roots and shoots at 20°C (HT plants), with roots and shoots at 10°C (LT plants), and with roots at 10°C and shoots at 20°C (DT plants). Under conditions where K+ was in limited supply during the prior growth period, K+ influx and accumulation were consistently higher in roots of DT and LT plants than in those of HT plants. Thus, it would appear that this low temperature response is not limited specifically to conditions in which temperature differentials are maintained between roots and shoots. Nevertheless, it was generally the case that increases of influx were larger in DT and LT plants so that the temperature differentials may intensify the low temperature response. When K+ influx was examined over a wide range of root [K+], it was seen that the characteristic reduction of influx associated with increased internal [K+] was substantially greater in HT than DT or LT plants. Transfer of plants grown under HT conditions to DT or LT regimes led to both short-term and long-term adjustments of influx. The former became apparent within 6 hours of exposure to the new conditions and decayed within minutes of transfer back to 20°C. The long-term adjustments were only apparent after prolonged exposure (days) to the lower root temperature and these did not decay as rapidly. Regardless of shoot temperature, the transfer of roots from 20°C to 10°C caused a gradual increase of root [K+] so that 4 days later LT and DT roots contained, respectively, 53.3 and 49.83 micromoles per gram compared to 17.82 micromoles per gram for roots maintained at 20°C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom A. J., Chapin F. S. Differences in steady-state net ammonium and nitrate influx by cold- and warm-adapted barley varieties. Plant Physiol. 1981 Nov;68(5):1064–1067. doi: 10.1104/pp.68.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. W., Berry J. A. Effects of low temperature on respiration and uptake of rubidium ions by excised barley and corn roots. Plant Physiol. 1978 May;61(5):858–860. doi: 10.1104/pp.61.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol. 1976 Jul;58(1):33–37. doi: 10.1104/pp.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., James A. T. The effect of low temperatures on fatty acid biosynthesis in plants. Biochem J. 1969 Apr;112(3):325–330. doi: 10.1042/bj1120325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D. Physiological Control of Chloride Transport in Chara corallina: I. EFFECTS OF LOW TEMPERATURE, CELL TURGOR PRESSURE, AND ANIONS. Plant Physiol. 1981 Jun;67(6):1113–1118. doi: 10.1104/pp.67.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi M. Y., Glass A. D. Simultaneous consideration of tissue and substrate potassium concentration in k uptake kinetics: a model. Plant Physiol. 1982 Jan;69(1):283–285. doi: 10.1104/pp.69.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh L., Horváth I., Horváth L. I., Dudits D., Farkas T. Protoplast plasmalemma fluidity of hardened wheats correlates with frost resistance. FEBS Lett. 1979 Nov 15;107(2):291–294. doi: 10.1016/0014-5793(79)80393-2. [DOI] [PubMed] [Google Scholar]


